Abstract
Chemokine production at the blood–retina barrier probably plays a critical role in determining the influx of tissue-damaging cells from the circulation into the retina during inflammation. The blood–retina barrier comprises the retinal microvascular endothelium and the retinal pigment epithelium. Chemokine expression and production by human retinal microvascular endothelial cells (REC) have never been reported previously, so we examined the in vitro expression and production of monocyte chemoattractant protein-1 (MCP-1), regulated on activation of normal T-cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, MIP-1β, interleukin (IL)-8, epithelial cell-derived neutrophil activating protein-78 (ENA-78) and growth related oncogene α (GROα) in these cells, both unstimulated and stimulated by cytokines likely to be present during the evolution of an inflammatory response. We compared this to expression and production of these chemokines in vitro in human retinal pigment epithelial cells (RPE). MCP-1 was expressed and produced constitutively by REC but all the chemokines were produced in greater amounts upon stimulation with the proinflammatory cytokines IL-1β and tumour necrosis factor-α (TNF-α). MCP-1 and IL-8 were produced at much higher levels than the other chemokines tested. MIP-1α and MIP-1β were present only at low levels, even after stimulation with IL-1β and TNF-α. Cytokines with greater anti-inflammatory activity, such as IL-4, IL-10, IL-13, transforming growth factor-β (TGF-β) and IL-6, had little effect on chemokine production either by REC alone or after stimulation with IL-1β and TNF-α. RPE, although a very different cell type, showed a similar pattern of expression and production of chemokines, indicating the site-specific nature of chemokine production. Chemokine production by REC and RPE is probably significant in selective leucocyte recruitment during the development of inflammation in the retina.
Introduction
Under normal circumstances the blood–retina barrier, comprising the retinal vascular endothelium and the retinal pigment epithelium, forms a barrier between the retina and the circulation. During the inflammatory eye disease, endogenous posterior uveoretinitis (EPU), this barrier breaks down, allowing leucocytes, particularly monocytes and T lymphocytes, to move into the retina, causing tissue damage and leading to impaired vision and, in some cases, blindness.1 In general, the underlying cause of EPU is thought to be an autoimmune response to ocular antigens, particularly retinal antigens.
Chemokines, which act primarily as chemoattractants and activators of specific leucocytes at the site of inflammation,2 may be involved in this influx of inflammatory cells. On the basis of differences in the positions of cysteines within a conserved four-cysteine motif, the chemokine family is divided into two major classes: CXC and CC.3 The CXC chemokines include interleukin(IL)-8, and those such as IL-8 that contain the ELR motif are particularly important in the attraction of neutrophils.3,4 In comparison, the CC chemokines such as macrophage inflammatory protein-1α (MIP-1α), MIP-1β, regulated on activation of normal T-cell expressed and secreted (RANTES) and monocyte chemoattractant protein-1/2/3 (MCP-1/2/3), have powerful chemoattractant and activator properties for monocytes and T cells.5
The retinal microvascular endothelial cells (REC) and human retinal pigment epithelial cells (RPE), which form the blood–retina barrier, probably produce chemokines and influence the infiltration of inflammatory cells in uveitis.2 RPE have been shown to have the ability to produce the chemokines MCP-1, IL-8, RANTES and growth related oncogene α (GROα)6–10in vitro.
Because of the limited availability of human REC and the difficulty of culturing them, nothing is known about the ability of these microvascular cells to produce chemokines. Studies carried out on microvascular cells from dermis, brain and nasal mucosa have shown production of the chemokines RANTES11 and MCP-1,12,13 and their involvement in leucocyte recruitment during disease has been postulated. However, for REC the only study on chemokine production has been in bovine cells where MCP-1 has been shown to be stimulated by vascular endothelial cell growth factor.14
We have examined, for the first time, the expression and production of the CC chemokines MCP-1, RANTES, MIP-1α, MIP-1β and the CXC chemokines IL-8, GROα and epithelial-cell derived neutrophil activating protein-78 (ENA-78) in REC, both as constitutive products and in response to both proinflammatory and anti-inflammatory cytokines, which are probably present in the microenvironment of the cells in vivo as inflammatory disease develops and resolves. We compared chemokine production by REC with that by RPE to determine whether these cells differ in their regulation of trans-barrier leucocyte traffic.
Materials and methods
Cell isolation and culture
REC were cultured, from eyes obtained from five different donors, using a modified method15 that included initial culture in microvascular endothelial cell basal medium plus microvascular endothelial cell growth supplement, gentamicin 50 µg/ml and amphotericin B (50 ng/ml) (TCS Biologicals Ltd, Botolph Claydon, Bucks, UK). Cells from two retinae from the same donor were used to seed a fibronectin-coated (50 µg/ml) 15-mm diameter tissue-culture well. Positive immunohistochemical detection of Von Willebrand factor confirmed that the cultures consisted of endothelial cells alone. After two passages, cells were cultured in REC complete medium [Glucose-free Glasgow minimal essential medium (GMEM) supplemented with 0·225% (v/v) sodium bicarbonate, 10 mm HEPES, 10% (v/v) tryptose phosphate broth, 1 mm sodium pyruvate, MEM non-essential amino acids at single strength, 4 mm l-glutamine, 100 IU/ml of penicillin, 100 µg/ml of streptomycin, 2·5% (v/v) human platelet-deprived serum and 5 mm d-glucose] in fibronectin-coated (50 µg/ml) flasks.
RPE from eight different donors were isolated, cultured and ascertained to be 100% epithelial in nature, as previously described.9 Human umbilical vein endothelial cells (HUVEC) were cultured in REC complete medium but with 10% fetal bovine serum (FBS) in place of human platelet-deprived serum.
Treatment of cells with cytokines
For analysis of chemokine production or mRNA expression, healthy dividing cells: REC (from passages 4 and 5), RPE (from passages 4–7) and HUVEC, were cultured to near confluency. Medium was aspirated, the cultures washed gently three times with Hanks' balanced salt solution (HBSS) and replaced with serum-free medium for 16 hr before cytokines were added and culture continued for up to 48 hr for chemokine production or 6 hr for mRNA expression. Control cultures were treated in the same manner but no cytokines were added. Previous dose–response studies had shown that the concentrations of cytokines used were appropriate for both REC and RPE. Cell-free conditioned medium was collected and stored at −80° until assay. The protein content of the cells remaining at this stage was measured using a protein–Coomassie Blue binding method.16
Enzyme-linked immunosorbent assay (ELISA)
Immunoreactive chemokine produced by REC or RPE was quantified using ELISAs according to the manufacturer's instructions (R & D Systems Europe Ltd, Abingdon, Oxford, UK). The minimum amount of chemokine detectable using these assays was below 15 pg/ml. Owing to the scarcity of REC, which do not grow as readily in culture, the ELISAs on REC supernatants were carried out using the same kits and standards as for the RPE, but sequentially, for up to four assays.17 Standards were combined at the start of a sequential set of ELISAs and taken through each assay with the samples. There was no detrimental effect upon the standard curves for each assay using this method and the difference between results obtained at the end of a serial ELISA run and for a solo ELISA was a decrease of 14·97% ± 4·158, which is similar to that found by Steffen & Ebersole17 and compares with 18·09% ± 3·56 for reproducibility between two individual assays. This was also the case when RPE supernatants were analysed using both methods.
RNA extraction
RNA was extracted using a modification of the method of Chomczynski & Sacchi.18 Briefly, cultures were washed and resuspended in 4 m guanidinium isothiocyanate, 25 mm sodium citrate (pH 7·0), 0·5% sarkosyl and 0·1 m 2-mercaptoethanol. The RNA was extracted using phenol–chloroform–isoamyl alcohol.
Reverse transcription–polymerase chain reaction (RT–PCR)
Poly(A)+ RNA from 1·6 µg of total RNA was reverse transcribed with 200 U reverse transcriptase (Superscript; GibcoBRL, Life Technologies Ltd, Paisley, Renfrewshire, UK). One microlitre or 5 µl of this cDNA was used in the PCR. Each PCR was carried out in a total volume of 25 µl containing 0·8 mm each of dATP, dCTP, dGTP and dTTP, 5 µl of Taq buffer, 0·125 µl of Taq polymerase (Bioline, London, UK) and 2·5 µl of primer mix. An end-point dilution method,19 using 10-fold serial dilutions of each cDNA, was used to compare levels of the more abundant mRNAs with greater accuracy.
The following β-actin, MCP-1, IL-8, ENA-78 and GROα primers were obtained from Oswel (Southampton, Hampshire, UK): β-actin, 5′-GTCCTTAATGTCACGCACGATTTC-3′, 5′-GTGGGGCGCCCCAGGCACCA-3′; MCP-1, 5′-GATGCAATCAATGCCCCAGTC-3′,5′-TTGCTTGTCCAGGTGG TCCAT-3′; IL-8, 5′-CCCTCTGCACCCAGTTTTCCT-3′, 5′-ACTCCAAACCTTTCCACCCCA-3′; GROα, 5′-GAAAAGATGCTGAACAGTGACA-3′, 5′-TAACTTGGGGTTGACATTTCA-3′; ENA-78, 5′-CTATGAGCCTCCTGTCCAGC-3′, 5′-ATCATTTTGGGATGAACTCCC-3′. RANTES,20 MIP-1α21 and MIP-1β21 primers were from Applied Bio-systems Ltd (Warrington, Cheshire, UK).
Twenty-eight cycles of amplification were performed. Each cycle consisted of a denaturation step at 94° for 50 seconds, annealing at 55° for 1 min and polymerization at 72° for 1 min 30 seconds. In the first cycle, denaturation was carried out for 2 min and in the final cycle polymerization was for 5 min. After amplification, samples were run on a 1·8% agarose gel (molecular biology grade; Sigma, Poole, Dorset, UK) in TBE (0·045 m Tris-borate, 0·001 m EDTA) containing 0·4 µg/ml of ethidium bromide. Relative abundance of product was assessed by calculating the ratios of the chemokine band to the β-actin band for each sample using the genegenius software (Syngene, Cambridge, Cambridgeshire, UK). For sequencing, 1 µl of PCR product was reamplified, as described above, and sequenced (Applied Biosystems, Warrington, Cheshire, UK).
Statistical analysis
Assays were repeated a minimum of three times and using cultures from at least three different donors. Data are presented as the mean ± SEM. The statistical significance of the results was assessed using a one-way analysis of variance (anova) with Newman-Keuls post-test when multiple treatments were compared, and Student's unpaired two-tailed t-test when two columns alone were compared (GraphPad Software Inc., San Diego, California, USA).
Results
Chemokine production
Production of each chemokine by both REC and RPE was examined at time-points up to 48 hr and showed similar time courses for both REC and RPE (Fig. 1).
Figure 1.
Time-course of chemokine production in response to interleukin-1β (IL-1β) (0·25 ng/ml, 50 IU/ml) over 48 hr for human retinal microvascular endothelial cells (REC) and human retinal pigment epithelial cells (RPE). The chemokine concentration (pg/ml) in culture supernatants was detected using enzyme-linked immunosorbent assay (ELISA). Monocyte chemoattractant protein-1 (MCP-1), closed squares; regulated on activation of normal T-cell expressed and secreted (RANTES), open squares; interleukin-8 (IL-8), closed circles; growth related oncogene α (GROα), open circles; and epithelial cell-derived neutrophil activating protein-78 (ENA-78), closed triangles. Results shown are from one representative experiment.
Figure 2 shows chemokine production by REC and RPE in response to the proinflammatory cytokines IL-1β, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), as measured using ELISA. MCP-1 was produced constitutively (P < 0·002), and increased concentrations of this chemokine were found in the supernatant upon stimulation with both IL-1β (P < 0·001) and TNF-α (P < 0·01). MCP-1 was also increased significantly (P < 0·05) in REC, but not in RPE, upon stimulation with IFN-γ. Increased RANTES production in response to IL-1β and TNF-α (P < 0·05) was further increased by TNF-α and IFN-γ in combination (P < 0·01). MIP-1α and MIP-1β (data not shown) were detectable, but at less than 50 ng/mg/ml, and only when the duration of incubation was lengthened to 48 hr, and the concentration of IL-1β and TNF-α increased (IL-1β to 2·5 ng/ml and TNF-α to 20 ng/ml) and the two cytokines combined.
Figure 2.
Chemokine production by human retinal microvascular endothelial cells (REC) (open bars) and human retinal pigment epithelial cells (RPE) (hatched bars) in response to interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). Chemokine was detected in supernatants after 24 hr of culture using enzyme-linked immunosorbent assay (ELISA). IL-1β was used at 0·25 ng/ml (50 IU/ml), TNF-α at 10 ng/ml (1100 IU/ml) and IFN-γ at 20 ng/ml (136 IU/ml). Results shown are combined from at least three experiments using REC from different donors. Error bars indicate the standard error of the mean (SEM).
IL-8, GROα and ENA-78 were not produced constitutively but responded to stimulation with IL-1β and TNF-α. IL-8 was increased to a greater extent in response to IL-1β (P < 0·001) than to TNF-α, as were GROα (P < 0·001) and ENA-78 (P < 0·01). Levels of GROα and ENA-78, however, were lower than that of IL-8, which was comparable with MCP-1. IFN-γ had little effect, either alone or in combination with IL-1β and TNF-α. ENA-78 production by RPE, in response to IL-1β or TNF-α, was lower than that of REC although this was not significant. IFN-γ decreased ENA-78 production in RPE in response to IL-1β and TNF-α (P < 0·05).
IL-4, IL-6, IL-10 and IL-13 had no effect on the production of MCP-1, RANTES, MIP-1α or MIP-1β either unstimulated or stimulated with IL-1β or TNF-α in either REC or RPE (Fig. 3). Data are not shown for MIP-1α or MIP-1β. Transforming growth factor β (TGF-β), however, affected RANTES production in response to TNF-α in RPE by down-regulating it significantly (P = 0·014).
Figure 3.
Chemokine production by human retinal microvascular endothelial cells (REC) (left column) and human retinal pigment epithelial cells (RPE) (right column) in response to interleukin (IL)-4, IL-6, IL-10, IL-13 and transforming growth factor-β (TGF-β) alone (open bars) or in combination with interleukin-1β (IL-1β) (hatched bars) or tumour necrosis factor-α (TNF-α) (cross-hatched bars). Chemokine was detected by enzyme-linked immunosorbent assay (ELISA) in RPE supernatants after 24 hr of culture. IL-1β was used at 0·25 ng/ml, and IL-4, IL-6, IL-10, IL-13, TGF-β and TNF-α all at 10 ng/ml. Results shown are combined from at least three experiments using REC and RPE from different donors. Error bars indicate the standard error of the mean (SEM).
Production of IL-8, GROα and ENA-78 (Fig. 3), either unstimulated or stimulated with IL-1β or TNF-α, were not altered significantly in response to IL-6, IL-10 or IL-13 in either REC or RPE. In REC, however, IL-4 significantly (P < 0·05) down-regulated GROα and ENA-78 in response to IL-1β. TGF-β was ineffective except for ENA-78 production by RPE where the response to IL-1β was decreased by TGF-β (P < 0·05).
Chemokine mRNA expression
cDNA samples from REC and RPE of different donors showed similar efficiency of reverse transcription when β-actin was amplified. With 1·6 µg of total RNA initially and 1 µl of cDNA used as template, β-actin could be detected down to a 1 : 100 dilution of cDNA.
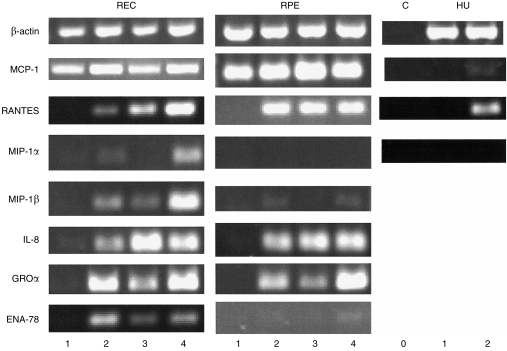
Figure 4 shows representative results of RT–PCR on RNA samples prepared from REC, RPE and HUVEC, and Table 1 shows the expression of the chemokine mRNAs as a ratio to β-actin. In general, the mRNA expression of chemokines follows the patterns, both unstimulated and in response to IL-1β and/or TNF-α, determined in the production studies. In all cases IL-1β and TNF-α markedly stimulated chemokine production.
Figure 4.
Chemokine mRNA expression as detected by reverse transcription–polymerase chain reaction (RT–PCR). RNA was extracted from cells 6 hr after stimulation of human retinal microvascular endothelial cells (REC), human retinal pigment epithelial cells (RPE) and human umbilical vein endothelial cells (HUVEC) (HU) cultures (C, no cDNA). Lane 0, (control) no cDNA; lane 1, unstimulated cultures; lane 2, 0·25 ng/ml of interleukin-1β (IL-1β); lane 3, 10 ng/ml of tumour necrosis factor-α (TNF-α); lane 4, IL-1β (0·25 ng/ml) + TNF-α (10 ng/ml). Results shown for macrophage inflammatory protein-1α (MIP-1α), MIP-1β, regulated on activation of normal T-cell expressed and secreted (RANTES) and epithelial cell-derived neutrophil activating protein-78 (ENA-78) had 5 µl of cDNA in the PCR reaction whereas those for β-actin, monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8) and growth related oncogene α (GROα) had 1 µl of cDNA.
Table 1.
Chemokine mRNA expression (ratio to β-actin) in human retinal microvascular endothelial cells (REC) and human retinal pigment epithelial cells (RPE) in response to interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α)
| No cytokine | IL-1β | TNF-α | IL-1β + TNF-α | |
|---|---|---|---|---|
| REC cells | ||||
| MCP-1 | 0·591 ± 0·319 | 1·289 ± 0·045 | 1·736 ± 0·063 | 1·981 ± 0·364 |
| RANTES | 0·000 | 0·011 ± 0·011 | 0·158 ± 0·158 | 0·095 ± 0·095 |
| MIP-1α | 0·000 | 0·000 | 0·000 | 0·349 ± 0·349 |
| MIP-1β | 0·000 | 0·041 ± 0·021 | 0·008 ± 0·008 | 0·161 ± 0·043 |
| IL-8 | 0·048 ± 0·021 | 0·460 ± 0·344 | 1·015 ± 0·461 | 0·404 ± 0·155 |
| GROα | 0·140 ± 0·140 | 0·460 ± 0·194 | 0·381 ± 0·207 | 1·040 ± 0·079 |
| ENA-78 | 0·000 | 0·137 ± 0·137 | 0·253 ± 0·253 | 0·069 ± 0·069 |
| RPE cells | ||||
| MCP-1 | 0·479 ± 0·323 | 0·907 ± 0·457 | 0·789 ± 0·385 | 0·807 ± 0·323 |
| RANTES | 0·000 | 0·074 ± 0·074 | 0·061 ± 0·061 | 0·206 ± 0·154 |
| MIP-1α | 0·000 | 0·000 | 0·000 | 0·000 |
| MIP-1β | 0·000 | 0·090 ± 0·090 | 0·000 | 0·142 ± 0·142 |
| IL-8 | 0·077 ± 0·077 | 0·423 ± 0·192 | 0·598 ± 0·181 | 0·890 ± 0·183 |
| GROα | 0·042 ± 0·042 | 0·607 ± 0·286 | 0·253 ± 0·100 | 0·828 ± 0·418 |
| ENA-78 | 0·000 | 0·000 | 0·000 | 0·000 |
ENA-78, epithelial cell-derived neutrophil activating protein-78; GROα, growth related oncogene α; IL-8, interleukin-8; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; MIP-1β, macrophage inflammatory protein-1β; RANTES, regulated on activation of normal T-cell expressed and secreted.
Although the PCR was optimized to be in the geometric phase for most samples, the levels of MCP-1 were so high that the PCR was also carried out using serial dilutions of the cDNA (data not shown). RANTES, MIP-1α, MIP-1β and ENA-78 were not detectable when the cDNA was diluted. For REC and RPE the dilution end-points were mostly the same when the same chemokines and cell treatments were compared.
Discussion
These studies show for the first time that REC have the ability to produce significant amounts of some of the major inflammatory chemokines, particularly MCP-1, RANTES, IL-8 and GROα, and as such will probably attract T lymphocytes, monocytes and neutrophils across the blood–retina barrier. Chemokine mRNA expression and production responded similarly to cytokines, suggesting that control of chemokine production in both cell types is mainly at the level of transcription, not translation.
Constitutive expression and production of MCP-1 in both REC and RPE, but not in HUVEC, was increased significantly in response to IL-1β. This pattern of expression and production by RPE has been found by other groups22 with the RPE-produced MCP-1 shown to be almost entirely secreted,23 predominantly to the basal side towards the choroidal vessels.24 This higher MCP-1 concentration at the choroidal side of the blood–retina barrier, rather than at the retinal side, has led to the suggestion that although MCP-1-responsive cells will be attracted from the choroidal vessels they will be unable to cross the blood–retina barrier into the retina and so damage to the retina will be limited.24 An alternative explanation is that once attracted from the circulation to the high concentration of MCP-1 at the REC or RPE, the MCP-1 receptor on these leucocytes will be internalized and other chemokine gradients will come into effect. It has been suggested that for a particular leucocyte to move to its final position in the tissue there will be a complex sequence of sequential migratory events to a hierarchy of chemoattractants.25
MCP-1 is generally thought of as an inflammatory, inducible chemokine, rather than a regulatory, constitutive chemokine, although it is known to be produced constitutively in neoplasia.26 However, if this significant constitutive production by both REC and RPE is also found in vivo it seems probable that it may have a more immunoregulatory role at the blood–retina barrier, regulating the traffic of leucocytes in normal as well as inflammatory situations.
Levels of MIP-1α and MIP-1β expression and production by both REC and RPE, although low, are present in response to high levels of proinflammatory cytokines and after a longer period of stimulation, suggesting that these chemokines, which will attract both lymphocytes and monocytes,3 may be of greater significance at the blood–retina barrier once a major inflammatory reaction is in progress, amplifying the response.
The production of IL-8, GROα and ENA-78 by both REC and RPE in response to proinflammatory cytokines indicates that neutrophils4 may also be attracted to the blood–retina barrier in an inflammatory situation. In experimental autoimmune uveitis (EAU), an animal model of EPU, neutrophils appear to act as effector cells in the early stage of disease27. This substantial production of IL-8, GROα and ENA-78, which are angiogenic chemokines,28 raises the possibility that they may be involved in the neovascularization that can accompany EPU.29
Chemokine production by both REC and RPE was regulated predominantly by proinflammatory cytokines, of which IL-1β and TNF-α significantly increased production of all the chemokines tested. There was little chemokine down-regulation by the anti-inflammmatory cytokines IL-4, IL-6, IL-10 and IL-13, as described in other cell types,30,31 although IL-4 down-regulated GROα and ENA-78 in REC. IL-4 has been reported to potentiate IL-1β and TNF-α stimulation of IL-8 and MCP-1 in RPE32 but at higher concentrations of IL-4 and IL-1β than those tested in our study. RANTES and ENA-78 production in RPE was down-regulated by TGF-β, which is one of the main cytokines proposed to have an immunosuppressive effect in the eye33 and has been shown to be produced by RPE.34
It has been suggested that in EAU there is a switch to a T helper 2 (Th2)-type response with disease resolution.35 This increased chemokine response to inflammatory cytokines and minor response to anti-inflammatory cytokines by the REC and RPE at the blood–retina barrier indicates that any switch to a Th2-type response, will influence chemokine production, mostly by reduction of IL-1, TNF-α and IFN-γ and less by production of IL-4, IL-10 and IL-13.
Although we have demonstrated (data not shown) that REC production of MCP-1 was similar to that in dermal microvascular endothelial cells,12 in human non-multiple sclerosis brain microvascular endothelial cells, MCP-1 mRNA was only detected after stimulation with IFN-γ.13 Chemokine production in RPE, although similar in some respects to that of epithelial cells at other locations, also showed marked differences, particularly in the response to anti-inflammatory chemokines, which were down-regulatory for MCP-1 and RANTES in intestinal epithelial cells36 and in airway epithelial cells,37 respectively.
Thus, REC probably play an important role in leucocyte recruitment during the development of inflammation in the retina via their chemokine production. This follows a similar pattern of production and expression to that of RPE, despite major differences in the behaviour of REC and RPE in culture, indicating that chemokine responses are determined more by location than by intrinsic cell type and reflect the site-specific recruitment of leucocyte subsets in the retina.
Acknowledgments
The authors wish to thank the Netherlands Ophthalmic Research Institute, Amsterdam, for their valued help in providing material for this study. This work was made possible by a grant from The Wellcome Trust No: 049531 and The Guide Dogs for the Blind Association. I. J. C. is a Wellcome Trust Research Fellow.
Abbreviations
- EAU
experimental autoimmune uveitis
- ENA-78
epithelial cell-derived neutrophil activating protein-78
- EPU
endogenous posterior uveoretinitis
- GROα
growth related oncogene α
- HUVEC
human umbilical vein endothelial cells
- MCP
monocyte chemoattractant protein
- MIP-1
macrophage inflammatory protein-1
- RANTES
regulated on activation of normal T-cell expressed and secreted
- REC
human retinal microvascular endothelial cells
- RPE
human retinal pigment epithelial cells
References
- 1.Forrester JV. Endogenous posterior uveitis. Br J Ophthalmol. 1990;74:620–3. doi: 10.1136/bjo.74.10.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 3.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 5.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–33. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 6.Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64:819–25. [PubMed] [Google Scholar]
- 7.Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Bian ZM, Elner SG, Strieter RM, Glass MB, Lukacs NW, Kunkel SL, Elner VM. Glycated serum albumin induces chemokine gene expression in human retinal pigment epithelial cells. J Leukoc Biol. 1996;60:405–14. doi: 10.1002/jlb.60.3.405. [DOI] [PubMed] [Google Scholar]
- 9.Crane IJ, Kuppner MC, McKillop-Smith S, Knott RM, Forrester JV. Cytokine regulation of RANTES production by human retinal pigment epithelial cells. Cell Immunol. 1998;184:37–44. doi: 10.1006/cimm.1997.1235. 10.1006/cimm.1997.1235. [DOI] [PubMed] [Google Scholar]
- 10.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GROα gene requires both NF-κB and novel constitutive factors. J Biol Chem. 1995;270:30619–26. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 11.Terada N, Maesako KI, Hamano N, Ikeda T, Sai M, Yamashita T, Fukuda S, Konno A. RANTES production in nasal epithelial cells and endothelial cells. J Allergy Clin Immunol. 1996;98:s230–7. doi: 10.1016/s0091-6749(96)70071-4. [DOI] [PubMed] [Google Scholar]
- 12.Goebeler M, Yoshimura T, Toksoy A, Ritter U, Brocker EB, Gillitzer R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol. 1997;108:445–51. doi: 10.1111/1523-1747.ep12289711. [DOI] [PubMed] [Google Scholar]
- 13.Frigerio S, Gelati M, Ciusani E, Corsini E, Dufour A, Massa G, Salmaggi A. Immunocompetence of human microvascular brain endothelial cells: cytokine regulation of IL-1β, MCP-1, IL-10, sICAM-1 and sVCAM-1. J Neurol. 1998;245:727–30. doi: 10.1007/s004150050275. 10.1007/s004150050275. [DOI] [PubMed] [Google Scholar]
- 14.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-κB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–7. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 15.Knott RM, Robertson M, Forrester JV. Regulation of glucose transporter (GLUT 3) and aldose reductase mRNA in bovine retinal endothelial cells and retinal pericytes in high glucose and high galactose culture. Diabetologia. 1993;36:808–12. doi: 10.1007/BF00400354. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Steffen MJ, Ebersole JL. Sequential ELISA for cytokine levels in limited volumes of biological fluids. Biotechniques. 1996;21:504–9. doi: 10.2144/96213rr04. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Hoey S, Grabowski PS, Ralston SH, Forrester JV, Liversidge J. Nitric oxide accelerates the onset and increases the severity of experimental autoimmune uveoretinitis through an IFN-γ-dependent mechanism. J Immunol. 1997;159:5132–42. [PubMed] [Google Scholar]
- 20.Robinson E, Keystone EC, Schall TJ, Gillett N, Fish EN. Chemokine expression in rheumatoid arthritis (RA): evidence of RANTES and macrophage inflammatory protein (MIP)-1β production by synovial T cells. Clin Exp Immunol. 1995;101:398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1α, MIP-1β and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–604. [PubMed] [Google Scholar]
- 22.Elner SG, Elner VM, Bian ZM, Lukacs NW, Kurtz RM, Strieter RM, Kunkel SL. Human retinal pigment epithelial cell interleukin-8 and monocyte chemotactic protein-1 modulation by T-lymphocyte products. Invest Ophthalmol Visual Sci. 1997;38:446–55. [PubMed] [Google Scholar]
- 23.Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res. 1997;65:781–9. doi: 10.1006/exer.1997.0380. 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- 24.Holtkamp GM, De Vos AF, Peek R, Kijlstra A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-β2) by human retinal pigment epithelial cells. Clin Exp Immunol. 1999;118:35–40. doi: 10.1046/j.1365-2249.1999.01016.x. 10.1046/j.1365-2249.1999.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–7. doi: 10.1016/s0167-5699(99)01469-3. 10.1016/S0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 27.McMenamin PG, Broekhuyse RM, Forrester JV. Ultrastructural pathology of experimental autoimmune uveitis: a review. Micron. 1993;24:521–46. doi: 10.3109/08916939308993315. [DOI] [PubMed] [Google Scholar]
- 28.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 29.Dees C, Arnold JJ, Forrester JV, Dick AD. Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol. 1998;116:1456–61. doi: 10.1001/archopht.116.11.1456. [DOI] [PubMed] [Google Scholar]
- 30.Burns MJ, Furie MB. Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms. Infect Immun. 1998;66:4875–83. doi: 10.1128/iai.66.10.4875-4883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parks E, Lukacs NW, Strieter RM, Kunkel SL. Chemokine expression in endothelial cells and monocytes is differentially regulated. Pathobiology. 1998;66:64–70. doi: 10.1159/000027998. [DOI] [PubMed] [Google Scholar]
- 32.Bian Z-M, Elner SG, Strieter RM, Kunkel SL, Lukacs NW, Elner VM. IL-4 potentiates IL-1β- and TNF-α-stimulated IL-8 and MCP-1 protein production in human retinal pigment epithelial cells. Curr Eye Res. 1999;18:349–57. doi: 10.1076/ceyr.18.5.349.5353. [DOI] [PubMed] [Google Scholar]
- 33.Forrester JV, Lumsden L, Liversidge J, Kuppner M, Mesri M. Immunoregulation of uveoretinal inflammation. Prog Retinal Eye Res. 1995;14:393–412. [Google Scholar]
- 34.Jaffe GJ, Roberts WL, Wong HL, Yurochko AD, Cianciolo GJ. Monocyte-induced cytokine expression in cultured human retinal pigment epithelial cells. Exp Eye Res. 1995;60:533–43. doi: 10.1016/s0014-4835(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 35.Saoudi A, Kuhn J, Huygen K, de Kozak Y, Velu T, Goldman M, Druet P, Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23:3096–103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 36.Kucharzik T, Lugering N, Pauels HG, Domschke W, Stoll R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattractant protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin Exp Immunol. 1998;111:152–7. doi: 10.1046/j.1365-2249.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkman N, Robichaud A, Krishnan VL, Roesems G, Robbins R, Jose PJ, Barnes PJ, Chung KF. Expression of RANTES in human airway epithelial cells: effect of corticosteroids and interleukin-4 -10 and -13. Immunology. 1996;87:599–603. doi: 10.1046/j.1365-2567.1996.477579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]