Abstract
Mice transgenic for the human MUC1 carcinoma-associated antigen (MUC1.Tg) are tolerant to immunization with MUC1 antigen. Recent studies, however, have demonstrated that immunization of MUC1.Tg mice with fusions of MUC1-positive tumour and dendritic cells (FC/MUC1) reverses MUC1 unresponsiveness and results in rejection of established MUC1-positive pulmonary metastases. Here we demonstrate that lymph node cells from MUC1.Tg mice immunized with the FC/MUC1 fusion cells proliferate in response to MUC1 antigen by a mechanism dependent on the function of CD4, major histocompatibility complex (MHC) class II, B7-1, B7-2, CD28, CD40 and CD40 ligand. The findings demonstrate that stimulation of lymph node cells with MUC1 results in selection of MUC1-specific CD8+ T cells. We show that the CD8+ T cells exhibit MUC1-specific cytotoxic T lymphocyte (CTL) activity by recognition of MUC1 peptides presented in the context of MHC class I molecules Kb and Db. The MUC1-specific CD8+ T cells also exhibit antitumour activity against MUC1-positive metastases, but with no apparent reactivity against normal tissues. These results indicate that immunization of MUC1.Tg mice with FC/MUC1 reverses immunological unresponsiveness to MUC1 by presentation of MUC1 peptides in the presence of costimulatory signals and generates MHC-restricted MUC1-specific CD8+ T cells.
Introduction
The human DF3/MUC1 glycoprotein is highly overexpressed in breast and other carcinomas.1,2 MUC1 is normally expressed on the apical borders of secretory epithelium. Carcinoma cells, by contrast, exhibit loss of polarization and express MUC1 at high levels over the entire cell surface.1 The MUC1 gene, located on chromosome 1q21–24,3–5 encodes a high-molecular-weight protein with a 72-amino acid cytoplasmic tail and a transmembrane domain.6 In addition, a mucin-like ectodomain includes variable numbers (30–90) of highly conserved 20-amino acid tandem repeats that are rich in serine, threonine and proline (PDTRPAPGSTAPPAHGVTSA).4 Sialyated O-linked glycans attached to the MUC1 tandem repeats differ structurally in normal and transformed epithelium.7 Incomplete glycosylation of MUC1 in carcinoma cells also contributes to expression of tandem repeat epitopes that are normally cryptic.8,9 Specifically, the APDTR and the RPAPG epitopes of the tandem repeat have been identified as being exposed in breast carcinoma cells.8,9 MUC1 participates in differentiation of epithelial sheets and formation of lumens during mouse development.10 Overexpression of human MUC1 in mouse mammary tumour cells affects tumorigenicity.11 Other studies have demonstrated that MUC1 expression inhibits cell–cell and cell–extracellular matrix adhesion.12–14
Sera from patients with breast and other cancers exhibit immunoglobulin M (IgM) antibodies against MUC1.15 Patients with ovarian cancer have anti-MUC1 antibodies that react with the tandem repeat PDTRPAPGSTAP epitope.16 In addition, patients immunized with mannan–MUC1 produce high-titre immunoglobulin G (IgG) anti-MUC1 antibodies.17 Non-major histocompatibility complex (MHC)-restricted cytotoxic T cells (CTLs) directed against the MUC1 APDTR epitope have been identified in patients with breast and other carcinomas.18–20 By contrast, MHC-restricted recognition of MUC1 has been found in multiparous women.21 The findings that MUC1 is overexpressed in carcinomas and is recognized by humoral and cellular immune responses has generated interest in MUC1 as a potential target for active, specific immunotherapy. In this context, preclinical vaccine studies have been conducted in models of transplanted tumours that express human MUC1.22–28 In mice, the human MUC1 xenoantigen is recognized by MHC-restricted CTLs.22,23,26–28 Because human MUC1 is expressed on the apical borders of normal epithelium and tolerance to self-antigens is a potential obstacle to the development of antitumour immunity, mice transgenic for human MUC1 (MUC1.Tg) have provided an appropriate model to assess induction of anti-MUC1 immune responses. Importantly, MUC1.Tg mice express MUC1 in a pattern and at a level similar to that found in humans.29
The MUC1.Tg mice are tolerant to stimulation with MUC1 antigen29 or irradiated MUC1-positive tumour cells.30 By contrast, immunization of MUC1.Tg mice with fusions of dendritic cells (DC) and MUC1-positive MC-38 carcinoma cells (MC-38/MUC1) reverses immunological unresponsiveness to MUC1.30 The present studies demonstrate that fusions of DC and MC-38/MUC1 induce MUC1-specific CD8+ T cells that recognize peptides derived from the tandem repeat. The results also demonstrate that the MUC1-specific CD8+ T cells confer immunity against MUC1-positive tumours without evidence for autoimmunity.
Materials and methods
MUC1 transgenic mice
A C57BL/6 mouse strain transgenic for human MUC1 was investigated for the presence of MUC1 sequences by polymerase chain reaction (PCR) amplification of tail DNA, as described previously.29 The MUC1 transgenic mice express MUC1 at the apical surfaces of epithelium lining of bronchi, mammary gland, pancreas (acinar cells), kidney (distal convoluted tubules and collecting ducts), gall bladder, salivary glands, stomach and uterus.29,31
Cell culture
Murine MC-38 carcinoma (C57BL/6), MB49 (C57BL/6), B16 (C57BL/6), EMT-6 (BALB/c) and C2C12 (C3H) cells, stably expressing a MUC1 cDNA,4,23 were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin. Human MCF-7 and ZR-75-1 breast carcinoma cells were grown in DMEM and RPMI-1640, respectively, containing 10% heat-inactivated FCS and antibiotics.
Immunization with DC–tumour fusion cells
DC isolated from bone marrow culture were fused to carcinoma cells as described previously but with certain modifications.27 Briefly, DC were mixed with MC38/MUC1 cells at a ratio of 10:1 and fused in 50% polyethylene glycol for 3–5 min. After dilution with serum-free RPMI-1640, the cells were washed and then cultured for 7 days in RPMI-1640 supplemented with 10% FCS and 200 U/ml of granulocyte–macrophage colony-stimulating factor (GM-CSF). As detected by flow cytometry for dual expression of MUC1 and MHC class II, fusion cells comprised 30–50% of the initial population. The FC/MUC1 fusion cells (5 × 105) were administered subcutaneously to the MUC1.Tg mice by injection in the flank near the base of the tail.
In vitro T-cell proliferation
Draining inguinal lymph nodes were removed 2 weeks after FC/MUC1 immunization. The whole lymph node cells (LNC; 5 × 105/ml) were pooled and suspended in DMEM supplemented with 10% heat-inactivated FCS, 50 mm 2-mercaptoethanol, 2 mm l-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin, with or without 5 U/ml of purified MUC1 antigen.32 In certain experiments, pooled lymph node T cells (T-LNC) (5 × 104), purified by passage through nylon wool, were co-cultured with MUC1 antigen and irradiated LNC (2·5 × 105) as antigen-presenting cells (APC). After 3–5 days of culture, cells were pulsed with 1 µCi of [3H]thymidine/well for 12 hr and harvested onto filters using a semiautomatic cell harvester. Radioactivity was measured by liquid scintillation. T-cell proliferation was assessed in the presence of antibodies (5 µg/well) against the following antigens: CD3 (145-2C11), CD4 (L3T4), CD8 (Ly-2), MHC I (M1/42/3.9.8), MHC II (25-9-17), CD28 (37.51), B7-1 (16-10A1), B7-2 (GL1), CD40 (3/23), CD40 ligand (CD40L) (MR1) and intracellular adhesion molecule (ICAM) (3E2).
Generation of CD8+ T-cell lines
LNC were suspended in RPMI-1640 supplemented with 10% heat-inactivated FCS, 50 mm 2-mercaptoethanol, 2 mm l-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin. The cells were incubated with 5 U/ml of MUC1 antigen. Murine interleukin-2 (IL-2; 10 U/ml) was added after 5 days of culture. On days 10 and 15, the LNC were restimulated with 5 U/ml of MUC1 antigen, 10 U/ml of IL-2 and 1:5 irradiated (30 Gy) syngeneic spleen cells. After Ficoll centrifugation and passage through nylon wool, an aliquot of T cells was incubated with phycoerythrin (PE) -conjugated anti-CD8 and fluorescein isothiocyanate (FITC)-conjugated anti-CD4 for 1 hr on ice. The cells were then washed, fixed and analysed using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, San Jose, CA, USA).
Cytotoxicity assays
Cells were labelled with 51Cr for 60 min at 37° as described previously.30 The cell targets (1 × 104) were added to 96-well V-bottom plates and incubated with effector cells for 5 hr at 37°. The supernatants were assayed for 51Cr in a gamma counter. Spontaneous release of 51Cr was assessed by incubation of targets in the absence of effectors. Maximum or total 51Cr release was determined by incubation of targets in 0·1% Triton-X-100. Percentage of specific 51Cr release was determined by the equation:
Epitope-mapping studies
Twenty overlapping 9-mer peptides spanning the 20-amino acid MUC1 tandem repeat were prepared as described previously.33 MC38 cells (C57BL/6; H-2b), or L cells stably expressing H2-Kb (L-Kb) or H2-Db (L-Db), were incubated with 20 µm peptide for 16 hr at 37°. The peptide-pulsed cells (106) were labelled with 100 µCi of Na251CrO4 for 1 hr at 37°. After washing, the radiolabelled cells were incubated with immune CD8+ T cells or naive anti-CD3-stimulated T cells in 96-well V-bottom plates for 5 hr at 37°. Cytotoxicity was determined as the percentage of specific 51Cr release.
Antitumour studies
Groups of 10 MUC1.Tg mice were injected intravenously (in the tail vein) with 1 × 106 MC38/MUC1 cells. The mice were then treated intravenously with 5 × 106 MUC1-specific CD8+ T cells or anti-CD3 monoclonal antibody (mAb)-stimulated T cells on days 4 and 11 after injection of tumour. In certain experiments, the mice were killed at 28 days for enumeration of pulmonary metastases by staining the lungs with India ink.
Immunohistology
Freshly removed tissues were frozen in liquid nitrogen. Tissue sections (5 µm) were prepared in a cryostat and fixed in acetone for 10 min. Sections were incubated with mAb DF3 (anti-MUC1), anti-CD4 (L3T4), or anti-CD8 (Ly-2) for 30 min at room temperature and then subjected to indirect immunoperoxidase staining using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA).
Results
Proliferation of T cells from MUC1.Tg mice immunized with FC/MUC1
To study the response of LNC to FC/MUC1 immunization, LNC from naive and FC/MUC1-immunized mice were assayed for proliferation in response to MUC1 stimulation. LNC from the FC/MUC1-immunized mice, but not naive mice, proliferated in the presence of MUC1 antigen (Fig. 1). Furthermore, purified T-LNC from the FC/MUC1-immunized mice that were incubated with MUC1 antigen alone were associated with a pronounced decrease in proliferation (Fig. 1). The addition of MUC1 and irradiated syngeneic splenocytes to T-LNC from immunized mice was sufficient to substantially restore the proliferative response (Fig. 1). By contrast, there was little effect on lymph node T-cell proliferation after immunization with DC or irradiated MC-38/MUC1 cells (data not shown). These findings indicate that in vitro T-cell proliferation in response to MUC1 is dependent on immunization of the MUC1.Tg mice with FC/MUC1 and the presence of APC.
Figure 1.
Proliferation of lymph node cells in response to MUC1 antigen in vitro. MUC1 transgenic (MUC1.Tg) mice were immunized with 5 × 105 MUC1-positive tumour and dendritic cells (FC/MUC1) on days 0 and 7. Inguinal lymph nodes were harvested on day 14. Pooled, purified lymph node T cells (T-LNC) were isolated by passage through nylon wool. Whole LNC and T-LNC from naive (hatched bars) and FC/MUC1-immunized (solid bars) mice were compared after cultured for 5 days with or without 5 U/ml of MUC1 antigen. Irradiated lymph node cells were added as antigen-presenting cells (APCs) to T-LNC. The cells were pulsed with 1 µCi of [3H]thymidine and harvested onto filters. Radioactivity was measured by liquid scintillation. Similar results were obtained in three separate experiments. (c.p.m., counts per minute.)
Role for costimulation in the proliferative response of immune T cells to MUC1
To study the role of costimulatory receptors in the induction of T-cell proliferation to MUC1 antigen, antibodies were used to block specific accessory interactions. Antibodies against CD4 and MHC class II, but not against CD8 and MHC class I, blocked the proliferative response of immune LNC to MUC1 (Fig. 2a). Antibodies against B7-1, B7-2 and CD28 also inhibited MUC1-induced T-cell proliferation (Fig. 2a). Similarly, addition of antibodies to ICAM-1, CD40 or CD40L abrogated the T-cell response (Fig. 2a). By contrast, LNC exposed to MUC1 for 5 days before the addition of antibodies failed to exhibit sensitivity to the blocking of accessory interactions (Fig. 2b). These findings indicate that once engagement of costimulatory molecules occurs, the proliferative response is irreversible.
Figure 2.
Involvement of specific cell surface molecules in the stimulation of lymph node cells to MUC1 antigen. Lymph node cells (LNC) from mice immunized with MUC1-positive tumour and dendritic (FC/MUC1) cells were incubated with 5 U/ml of MUC1 antigen. Antibodies (5 µg/0·2 ml/well) against the indicated antigens were added at the beginning (a) and after 5 days (b) of culture. The cells were pulsed with [3H]thymidine, and incorporated radioactivity was measured (in counts per minute [c.p.m.] by liquid scintillation. The results (mean ± SE) represent data from one experiment performed in triplicate. Similar results were obtained in three separate experiments. (CD40L, CD40 ligand; ICAM, intracellular adhesion molecule; IgG, immunoglobulin G; MHC, major histocomptibility complex.)
Selection of CD8+ T cells from MUC1.Tg mice immunized with FC/MUC1
LNC from naive MUC1.Tg mice exhibited distinct populations of CD4+ and CD8+ cells after 1 and 10 days of MUC1 stimulation (Fig. 3a). When analysing LNC from FC/MUC1-immunized MUC1.Tg mice, similar patterns were observed after 1 and 3 days of exposure to MUC1 antigen (Fig. 3b). By contrast, analysis at days 5–10 of MUC1 stimulation demonstrated a progressive selection of CD8+ T cells (Fig. 3b). On day 10, nearly 90% of the cells expressed CD8 (Fig. 3b). Splenocytes isolated from naive and FC/MUC1-immunized MUC1.Tg mice were similarly exposed to MUC1 antigen in vitro. As observed with LNC, there was no apparent selection of splenic T cells from naive mice after 10 days of MUC1 stimulation (Fig. 3c). Splenic T cells from the FC/MUC1-immunized mice, however, responded to MUC1 with selection of a population of T cells that was predominantly positive for CD8 (Fig. 3d). These findings demonstrate that immunization with FC/MUC1 induces a T-cell response in vivo that, upon stimulation with MUC1 antigen in vitro, is manifested by selection of a population of CD8+ cells.
Figure 3.
Phenotype of T cells from control and MUC1 transgenic (MUC1.Tg) mice, immunized with fused MUC1-positive tumour and dendritic (FC/MUC1) cells, after in vitro culture with MUC1 antigen. MUC1.Tg mice were immunized with phosphate-buffered saline (PBS) (a) and (c) or with 5 × 105 FC/MUC1 (b) and (d) on days 0 and 7. Inguinal lymph nodes and spleens were harvested on day 14. Lymph node cells (LNC) (a) and (b) and splenocytes (c) and (d) were incubated with 5 U/ml of MUC1 antigen. On the indicated days of in vitro culture, the T cells were purified by passage through nylon wool before incubation with fluorescein isothiocyanate (FITC)-labelled anti-CD4 and phycoerythrin (PE) -labelled anti-CD8. The cells were analysed using a fluorescence-activated cell sorter (FACScan). Similar results were obtained in three separate experiments.
CD8+ T cells stimulated with MUC1 in vitro exhibit MUC1-specific CTL activity
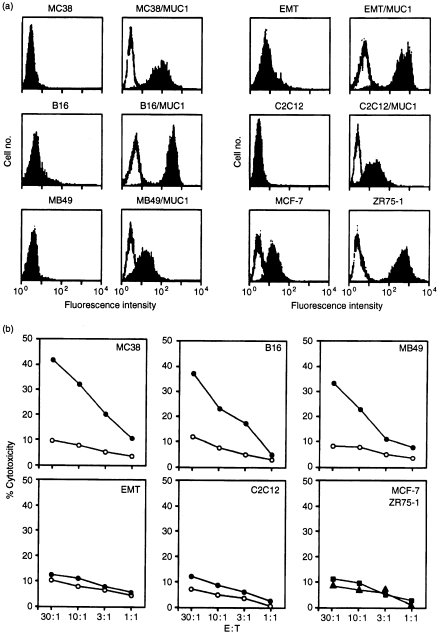
To further characterize the CD8+ T cells selected in the presence of MUC1 in vitro, CTL assays were performed to assess lysis of cells negative and positive for MUC1, as defined by flow cytometry (Fig. 4a). The stimulated CD8+ T cells lysed syngeneic MC-38/MUC1 cells, but not MC-38 cells (Fig. 4b). Lysis of syngeneic B16/MUC1 and MB49/MUC1 cells, but not their MUC1-negative counterparts, was also found with the CD8+ T cells (Fig. 4b). By contrast, there was no detectable lysis of these targets when incubated with T cells from naive MUC1.Tg mice that were exposed to MUC1 antigen in vitro (data not shown). In addition, using the selected CD8+ T cells, there was no apparent lysis of allogeneic EMT6/MUC1 (BALB/c) or C2C12/MUC1 (C3H) cells (Fig. 4b). The CD8+ T cells also failed to lyse human MUC1-positive MCF-7 and ZR-75-1 cells (Fig. 4b). These findings demonstrate that the CD8+ T cells induce lysis of targets by a MUC1-specific, MHC-restricted mechanism.
Figure 4.
Major histocompatibility complex (MHC)-restricted lysis by MUC1-specific CD8+ cytotoxic T lymphocytes (CTLs). (a) The indicated wild-type and MUC1 stable transfectants were incubated with 10 µg/ml of monoclonal antibody (mAb) DF3 (solid areas) and control mouse immunoglobulin G (IgG) (open areas). The cells were analysed by flow cytometry. (b) The CD8+ CTLs were incubated with wild-type tumour cells (○), MUC1-positive transfectants (•), human MUC1-positive MCF-7 (▪) and MUC1-positive ZR-75-1 (▴) cells at the indicated effector to target (E:T) ratios. CTL activity was determined by 51Cr release.
Identification of epitopes recognized by the MUC1-specific CD8+ T cells
To identify epitopes recognized by the MUC1-specific CD8+ T cells, overlapping 9-mer synthetic peptides derived from the MUC1 20-amino acid tandem repeat were incubated with MC-38 carcinoma cells. The peptide-pulsed MC-38 cells were assayed for lysis by the MUC1-specific CD8+ CTLs. The results demonstrate that peptides SAPDTRPAP, SAPDTRPA, TSAPDTRPA, RPAPGSTAP, APGSTAPPA, PAPGSTAPP are recognized by the CD8+ CTLs (Fig. 5a) and not by naive T cells activated with anti-CD3 antibody (Fig. 5b). To determine which C57BL/6 MHC class I molecules (H-2b) are required for recognition, the peptides were pulsed onto L cells that stably express H2-Kb or H2-Db (Fig. 5c). The results demonstrate that the MUC1-specific CTLs lyse L-Kb targets pulsed with SAPDTRPAP, SAPDTRPA and TSAPDTRPA peptides (Fig. 5d, left panel) and L-Db cells pulsed with RPAPGSTAP, APGSTAPPA and PAPGSTAPP peptides (Fig. 5d, right panel). There was no apparent lysis of STAPPAHGV peptide-pulsed cells as compared to unpulsed L-Db or L-Kb cells (Fig. 5c, 5d). As additional controls, incubation of pulsed L-Db cells with anti-Db and pulsed L-Kb cells with anti-Kb abrogated the CTL activity (data not shown). Taken together, these results demonstrate that the MUC1-specific CTLs recognize both H2-Db (RPAPGSTAP)- and H2-Kb (SAPDTRPAP)-containing peptides. We have previously shown that the epitope is clustered around three peptides recognized by H2-Db and H2-Kb.33,34
Figure 5.
Mapping of epitopes recognized by the MUC1-specific CD8+ cytotoxic T lymphocytes (CTLs). (a) and (b), MC-38 carcinoma cells were pulsed with the indicated overlapping 9-mer peptides spanning the MUC1 tandem repeat. The pulsed cells were incubated with MUC1-specific CD8+ CTLs (a) or with anti-CD3-activated T cells from naive MUC1 transgenic (MUC1.Tg) mice (b) at an effector to target (E:T) ratio of 30:1. MC38 and MC38/MUC1 targets were incubated with the T cells as non-pulsed controls. CTL activity was determined by 51Cr release. Similar results were obtained in two separate experiments. (c) L cells stably transfected with H-2 Kb or H-2 Db were incubated with 5 µg/ml of anti-major histocompatibility complex (MHC) class II (25-9-17), anti-MHC class I (M1/42/3.9.8), anti-H2Kb (AF6-88.5) or anti-H2Db (KH95), or with immunoglobulin G (IgG) (open areas) as a control. The cells were analysed by flow cytometry. (d) L-Kb and L-Db cells were pulsed with the indicated MUC1 peptides. The pulsed cells were incubated with MUC1-specific CTLs at an E:T ratio of 30:1. CTL activity was determined by 51Cr release. Similar results were obtained in two separate experiments.
Antitumour activity of MUC1-specific CTLs
To assess the function of MUC1-specific CTLs in vivo, MC-38/MUC1 pulmonary metastases were established by tail vein injection of MC-38/MUC1 cells into the MUC1.Tg mice. Treatment with naive anti-CD3-stimulated T cells had no effect on progression of the pulmonary tumours (Fig. 6a, 6b). Significantly, mice treated with the MUC1-specific CTL had no detectable metastases (Fig. 6a, 6b). Mice treated with MUC1-specific CTL, but not with naive anti-CD3-stimulated T cells, exhibited long-term survival (Fig. 6c). In addition, splenocytes from mice treated with MUC1-specific CTL, but not with naive anti-CD3-stimulated T cells, exhibited lysis of MC38/MUC1 tumour cells (Fig. 6d). Analysis of MUC1-positive bronchi and mammary tissue in mice treated with the MUC1-specific CTL demonstrated persistent expression of MUC1 antigen and no evidence for destruction of ductal epithelium (Fig. 6e). Also, staining of the bronchi and mammary tissue with an anti-CD8 antibody failed to demonstrate T-cell infiltration (data not shown). These findings indicate that treatment of MUC1.Tg mice with MUC1-specific CTLs confers antitumour activity against MUC1-positive metastases without evidence of autoimmunity against normal tissues.
Figure 6.
Treatment of established MC-38/MUC1 pulmonary metastases by MUC1-specific cytotoxic T lymphocytes (CTLs). MUC1 transgenic (MUC1.Tg) mice were injected intravenously via the tail vein with 1 × 106 MC-38/MUC1 cells. The mice were treated with 5 × 106 MUC1-specific CTLs or anti-CD3-activated naive T cells on days 4 and 11. (a) Mice were killed on day 28 and the lungs stained with India ink. (b) Number of pulmonary metastases in mice receiving the indicated treatments. The results (mean ± SE) represent data from 10 mice in each group. (c) Survival of mice (five per group) with pulmonary metastases treated with phosphate-buffered saline (PBS) (○), anti-CD3-activated naive T cells (▴) or MUC1-specific CTLs (•). (d) Mice treated with PBS (○), anti-CD3-activated naive T cells (▴) or MUC1-specific CTLs (•), were killed on day 30. Splenocytes were incubated with MC-38/MUC1 targets at the indicated effector to target (E:T) ratios. Lysis was determined by 51Cr release. Similar results were obtained in repeat experiments. (e) Sections of bronchi and mammary tissue from mice treated with PBS (left panel) or MUC1-specific CTLs (right panel) and killed on day 30. The sections were stained with monoclonal antibody (mAb) DF3 (anti-MUC1). MUC1 antigen is detectable along the apical borders of the secretory epithelium.
Discussion
Immunization of MUC1.Tg mice with FC/MUC1, but not with MUC1 antigen or irradiated MC-38/MUC1 cells, induces MUC1-specific CTLs.30 The in vivo priming of CD8+ CTLs requires the participation of CD4+ T-helper lymphocytes.35,36 Recent work suggests that help is delivered through activation of APCs and thereby direct priming of CTLs.37–39 In the present studies, LNC from the FC/MUC1-immunized MUC1.Tg mice proliferate in response to stimulation with MUC1 antigen. By contrast, there was little, if any, proliferative response of LNC from naive MUC1.Tg mice to MUC1. These results suggest that FC/MUC1 cells prime LNC for subsequent stimulation with MUC1. Stimulation of sensitized LNC in cultures with MUC1 requires the presence of APCs. In this context, T cells purified from the lymph nodes of immunized mice failed to proliferate in response to MUC1 antigen. The finding that the MUC1-induced proliferative response of T-LNC cells is restored by irradiated LNC supports a requirement for APCs. CD40L is expressed on the surface of activated CD4+ T-helper cells, while ligation of CD40 on the surface of APCs increases their capacity for antigen presentation and costimulation.40–43 Moreover, CD40–CD40L interactions are essential in the delivery of T-cell help in the priming of CTLs.37–39,44 The present results demonstrate that the proliferative response of the FC/MUC1-immunized lymph node cells to MUC1 is dependent on the functions of CD4, MHC class II, costimulatory (B7-1, B7-2, CD28) and adhesion (ICAM-1) molecules. In addition, the demonstration that blocking CD40–CD40L interactions inhibits the proliferative response to MUC1 is in concert with involvement of antigen-specific CD4+ T cells in activation of APCs and thereby priming of CTLs. These findings thus suggest that: (i) blocking T-helper interactions with APCs; (ii) CD40L-mediated activation of APCs; and/or (iii) costimulation, inhibit the induction of a proliferative response to MUC1 antigen.
The culture of FC/MUC1-immunized LNC in the presence of MUC1 results in the selection of MUC1-specific CD8+ T cells. These findings indicate that MUC1 peptides are presented by MHC class I molecules. Peptides presented as complexes with MHC class I molecules are generally processed from antigens localized to the cytoplasm of the target cell.45 Nonetheless, exogenous antigens not expected to localize in the cytoplasm are also presented by MHC class I molecules.46–48 Indeed, in cross-priming, antigens from donor cells are acquired by dendritic cells and presented by MHC class I molecules.49 Other studies have demonstrated that induction of CTLs by cross-priming with cell-associated antigens requires the involvement of CD4+ helper T cells.50 Thus, uptake of MUC1 antigen by antigen-presenting LNC apparently results in the presentation of MUC1 peptides by MHC class I molecules and selection of CD8+ MUC1-specific CTLs. Epitope-mapping studies demonstrate that the MUC1-specific CTLs recognize peptides from the MUC1 tandem repeat. Previous studies have shown that MUC1 peptides containing the PDTRPAP epitope bind to MHC class I Kb, while those containing APGSTAP bind to Db.33 The MUC1 epitopes are not consensus motifs for the particular MHC class I alleles and bind with low affinity compared with known high-affinity peptides.33 The FC/MUC1-induced CTLs recognize MUC1 peptide epitopes in the context of H-2 Kb and Db molecules. In addition, the FC/MUC1-induced CTLs, as demonstrated for CTL in wild-type mice immunized with MUC1, exhibit MHC-restricted lysis of MUC1-positive targets.22 Thus, while immunization with MUC1 antigen is insufficient to induce MUC1-specific CTLs in MUC1.Tg mice, the reversal of tolerance to MUC1 in these mice with FC/MUC1 results in CTLs that function like those in wild-type mice.
In the present studies, we showed that treatment with the MUC1-specific CTLs is effective in the rejection of established MC-38/MUC1 metastases. By contrast, the CD3-activated CTLs failed to eliminate MUC1-positive tumours. On the basis of these findings, we conclude that the MUC1-specific CTLs are sufficient to treat MUC1-positive MC-38 metastases. Significantly, treatment with the MUC1-specific CTLs, whilst conferring antitumour immunity in the MUC1.Tg mice, had no apparent effect on normal secretory epithelia that express MUC1. There was no evidence for infiltration of MUC1-positive normal tissues by the MUC1-specific CTLs or destruction of the epithelia that express MUC1 along ducts. One potential explanation for selectivity of the anti-MUC1 response against tumour could be lower levels of MHC class I/MUC1 peptide complexes on the normal secretory cells that express MUC1 and constitute the lining of ducts. While further studies are needed to define the basis for such selectivity, these findings suggest that induction of anti-MUC1 immunity may provide an effective clinical approach for the treatment of MUC1-positive tumours.
Acknowledgments
This investigation was supported by Grant CA78378 and CA87057 awarded by the National Cancer Institute, DHHS; and by Komen Breast grant 9825 awarded by the Susan G. Komen breast cancer foundation.
REFERENCES
- 1.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–32. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 2.Hilkens J, Buijs F, Hilgers J, Hageman P, Calafat J, Sonnenberg A, van der Valk M. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer. 1984;34:197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]
- 3.Gendler SJ, Burchell JM, Duhig T, Lamport D, White R, Parker M, Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci USA. 1987;84:6060–4. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–3. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swallow D, Gendler S, Griffiths B, Corney G, Taylor-Papadimitriou J, Bramwell M. The human tumor associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature. 1987;328:82–4. doi: 10.1038/328082a0. [DOI] [PubMed] [Google Scholar]
- 6.Merlo GR, Siddiqui J, Cropp CS, Liscia DS, Lidereau R, Callahan R, Kufe DW. Frequent alteration of the DF3 tumor-associated antigen gene in primary human breast carcinomas. Cancer Res. 1989;49:6966–71. [PubMed] [Google Scholar]
- 7.Hull S, Bright A, Carraway K, Abe M, Hayes D, Kufe DW. Oligosaccharide differences in the DF3 sialomucin antigen from normal human milk and the BT-20 human breast carcinoma cell line. Cancer Commun. 1989;1:261–7. [PubMed] [Google Scholar]
- 8.Perey L, Hayes DF, Maimonis P, Abe M, O'hara C, Kufe DW. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res. 1992;52:2563–8. [PubMed] [Google Scholar]
- 9.Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987;47:5476–82. [PubMed] [Google Scholar]
- 10.Braga VMM, Pemberton LF, Duhig T, Gendler SJ. Spatial and temporal expression of an epithelial mucin, Muc-1, during mouse development. Development. 1992;115:427–37. doi: 10.1242/dev.115.2.427. [DOI] [PubMed] [Google Scholar]
- 11.Lalani EN, Berdichevsky F, Boshell M, Shearer M, Wilson D, Stauss H, Gendler SJ, Taylor-Papadimitriou J. Expression of the gene coding for a human mucin in mouse mammary tumor cells can affect their tumorigenicity. J Biol Chem. 1991;266:15420–6. [PubMed] [Google Scholar]
- 12.Ligtenberg MJL, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–24. [PubMed] [Google Scholar]
- 13.Wesseling J, van der Valk SW, Vos H, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibitions integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC1 in sera from breast, pancreatic and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 16.Rughetti A, Turchi V, Ghetti CA, et al. Human B-cell immune response to the polymorphic epithelial mucin. Cancer Res. 1993;53:2457–9. [PubMed] [Google Scholar]
- 17.Karanikas V, Hwang L-A, Pearson J, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan–MUC1 fusion protein. J Clin Invest. 1997;100:2783–92. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA. 1989;86:7159–63. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerome KR, Barnd DL, Bendt KM, et al. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–16. [PubMed] [Google Scholar]
- 20.Ioannides CG, Fisk B, Jerome KR, Irimura T, Wharton JT, Finn OJ. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993;151:3693–703. [PubMed] [Google Scholar]
- 21.Agrawal B, Reddish MA, Longenecker BM. In vitro induction of MUC-1 peptide specific type-1 lymphocyte and CTL responses from healthy multiparous donors. J Immunol. 1996;157:2089–2095. [PubMed] [Google Scholar]
- 22.Apostolopoulos V, Loveland BE, Pietersz GA, McKenzie IFC. CTL in mice immunized with human mucin 1 are MHC-restricted. J Immunol. 1995;155:5089–94. [PubMed] [Google Scholar]
- 23.Akagi J, Hodge JV, McLaughlin JP, et al. Therapeutic antitumor response after immunization with an admixture of recombinant vaccinia viruses expressing a modified MUC1 gene and the murine T-cell costimulatory molecule B7. J Immunotherapy. 1997;20:38–47. doi: 10.1097/00002371-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Acres RB, Hareuveni M, Balloul JM, Kieny MP. Vaccinia virus MUC1 immunization of mice: immune response and protection against the growth of murine tumors bearing the MUC1 antigen. J Immunother. 1993;14:136–43. [PubMed] [Google Scholar]
- 25.Hareuveni M, Gautier C, Kieny M-P, Wreschner D, Chambon P, Lathe R. Vaccination against tumor cells expressing breast cancer epithelial tumor antigen. Proc Natl Acad Sci USA. 1990;87:9498–502. doi: 10.1073/pnas.87.23.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J, Chen L, Chen D, Kashiwaba M, Manome Y, Tanaka T, Kufe D. Induction of antigen-specific antitumor immunity with adenoviral-transduced dendritic cells. Gene Ther. 1997;4:1023–28. doi: 10.1038/sj.gt.3300496. [DOI] [PubMed] [Google Scholar]
- 27.Gong J, Chen D, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–61. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 28.Apostolopoulos V, Popovski V, McKenzie IFC. Cyclo-phosphamide enhances the CTL precursor frequency in mice immunized with MUC1–mannan fusion protein (M–FP) J Immunol. 1998;21:109–13. doi: 10.1097/00002371-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 30.Gong J, Chen D, Kashiwaba M, et al. Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells. Proc Natl Acad Sci USA. 1998;95:6279–83. doi: 10.1073/pnas.95.11.6279. 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peat N, Gendler SJ, Lalani E-N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–60. [PubMed] [Google Scholar]
- 32.Sekine H, Hayes DF, Ohno T. Circulating DF3 and CA125 antigen levels in serum from patients with epithelial ovarian cancer. J Clin Oncol. 1985;3:1355–63. doi: 10.1200/JCO.1985.3.10.1355. [DOI] [PubMed] [Google Scholar]
- 33.Apostolopoulos V, Haurum JS, McKenzie IFC. MUC1 peptide epitopes associated with five different H-2 class I molecules. Eur J Immunol. 1997;27:2579–87. doi: 10.1002/eji.1830271017. [DOI] [PubMed] [Google Scholar]
- 34.Apostolopoulos V, Chelvanayagam G, Xing PX, McKenzie IFC. Anti-MUC1 antibodies react directly with MUC1 peptides presented by class I H2 and HLA molecules. J Immunol. 1998;161:767–75. [PubMed] [Google Scholar]
- 35.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–64. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–82. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett SRM, Carbone FR, Karamalis RF, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature. 1998;393:476–7. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 38.Ridge JP, Di Rosa F, Matzinger P. A conditional dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 39.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 40.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Gottfried A. Ligation of CD40 on dendritic cells triggers production of high level of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 42.Stout RD, Suttles J, Xu J, Grewal IS, Flavell RA. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 43.Van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–3. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 44.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T cell tolerance to T cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 45.Heemels MT, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–91. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 46.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 47.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 48.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–50. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 49.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 50.Bennett SRM, Carbone FR, Karamalis F, Miller JFAP, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by crossing-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]