Abstract
An early stage in thymocyte development, after rearrangement of the β chain genes of the T cell receptor (TCR), involves expression of the pre-TCR complex and accompanying differentiation of CD4−CD8− double negative (DN) cells to CD4+CD8+ double positive (DP) cells. The ZAP-70 and Syk tyrosine kinases each contain two N-terminal SH2 domains that bind phosphorylated motifs in antigen receptor subunits and are implicated in pre-T receptor signaling. However, mice deficient in either ZAP-70 or Syk have no defect in the formation of DP thymocytes. Here we show that, in mice lacking both Syk and ZAP-70, DN thymocytes undergo β chain gene rearrangement but fail to initiate clonal expansion and are incapable of differentiating into DP cells after expression of the pre-TCR. These data suggest that the ZAP-70 and Syk tyrosine kinases have crucial but overlapping functions in signaling from the pre-TCR and hence in early thymocyte development.
T cells develop within the thymus, proceeding from a CD4−CD8− double negative (DN) stage through a CD4+CD8+ double positive (DP) stage to become mature CD4+ or CD8+ single positive T cells. In the αβ lineage, transition from the DN to the DP stage requires the expression of the pre-T cell receptor (pre-TCR), comprised of a TCR β chain, the pTα transmembrane protein, and associated signaling subunits such as TCR ζ and the CD3 polypeptides (1–3). ZAP-70, a cytoplasmic tyrosine kinase with two SH2 domains, associates with tyrosine phosphorylated CD3 and TCR ζ chains and is implicated in signaling from the mature TCR (4).
A scheme for signaling from the TCR suggests that antigen-binding leads to the activation of Src family kinases, such as Lck and Fyn, and to the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAM), with the consensus YXXLX(6–8)YXXL, found in the CD3 and ζ subunits of the TCR (5). Phosphorylation of both tyrosine residues within an ITAM induces binding of the tandem SH2 domains of ZAP-70 and thus recruitment of ZAP-70 into the TCR complex (4). This leads to the stimulation of ZAP-70 tyrosine kinase activity and consequently to phosphorylation of downstream targets. Although ZAP-70 is implicated in signaling from the mature TCR, a closely related kinase, Syk, is apparently required for signaling from the B cell antigen receptor (BCR) (6), and from specific Fc receptors (7), in a process that closely matches the pathway defined for ZAP-70 in T cells.
In addition to their functions in transmitting signals from mature antigen receptors, both ZAP-70 and Syk are candidates for signaling molecules that act downstream of the pre-TCR and pre-BCR in immature T cells and B cells, respectively, and might therefore be involved in early steps of lymphoid development. Indeed, mice deficient in Syk show an early block in B cell development, which is likely due to a defect in pre-BCR signaling (8, 9). Such Syk mutant mice show no major abnormalities in αβ T cell development, raising the possibility that ZAP-70 might act to play an analogous role in thymocyte development after rearrangement of the β chain gene at the DN stage. However, mice deficient in ZAP-70 do not exhibit any arrest in T cell development before the DP stage (10). Similarly, immunodeficient human patients lacking functional ZAP-70 also produce normal numbers of DP thymocytes and indeed accumulate CD4+ SP cells although these cells are not responsive to stimulation (11, 12). One possible interpretation of these data is that neither Syk nor ZAP-70 plays a role in early DN thymocyte development. Alternatively, it is possible that Syk and ZAP-70 may act jointly during the early stages of thymocyte maturation and can therefore functionally compensate for one another in thymocytes lacking only one of these two tyrosine kinases. This raises the possibility that Syk and ZAP-70 may indeed have a role in the development of DN thymocytes to DP cells, which was not revealed in mice lacking either Syk or ZAP-70 alone. To address this issue, we analyzed thymocyte development in neonatal mice deficient for both Syk and ZAP-70.
EXPERIMENTAL PROCEDURES
Thymocyte Analysis.
The generations of ZAP-70-deficient and Syk-deficient mice have been reported (8, 10). All mice were kept in the 129 genetic background. Mice were killed for analysis at 1-day-old. Total thymocytes were extracted by gentle passing of the thymus through a syringe with a 22-guage needle, followed by a single pass of the suspension through a 25-guage needle. The numbers of thymocytes were counted using a hematocytometer. Between 0.2 × 106 and 0.5 × 106 cells were used for flow cytometry analysis. In all cases, staining with propidium iodide was used to gate out the dead cells. All analyses were limited to live, nonerythrocytes. All antibodies used were purchased from PharMingen.
For cell size analyses, cells stained with CD44 and CD25 were first analyzed on a two-dimensional plot. A region gate was applied to all of the cells stained positive for CD25, including both the CD44+ and the CD44− subsets. With regions gated also for live and nonerythrocytes, the CD25+ population was analyzed on a histogram for forward scatter values. Results from the double mutant and wild type were plotted together, after adjusting for individual maximal value in relative cell numbers.
TCR β Chain Gene Rearrangements.
Gene rearrangement activity of the β chain locus was evaluated with PCR using DNA primers specific for three different Vβ families (Vβ5, Vβ8, and Vβ12). Sequences of the PCR primers were identical to those as reported (13, 14). The Dβ2-specific primer was used together with the Jβ2.6-specific primers to generate Dβ–Jβ arrangement products as controls. The PCR cycle profile was: 94°C, 8-min denaturation, followed by a 20-cycle program, 94°C, 1 min; 63°C, 2 min; and 72°C 3 min and was finished with a final 72°C, 10-min extension step. Samples were allowed to cool to room temperature, and one-third of each was analyzed on a 1.5% agarose gel and subsequently transferred to a nitrocellulose membrane. Specific rearrangement products were detected with a 32P-end labeled (with T4 kinase) primer specific for the Jβ2.6 gene segment in a 6× SSC-based hybridization solution at 37°C. The membrane was washed at room temperature with 6 × SSC, 0.1% SDS, followed by two washes at 37°C, 15 min each.
RESULTS
Severe Reduction of Thymocyte Population in Mice Lacking both Syk and ZAP-70.
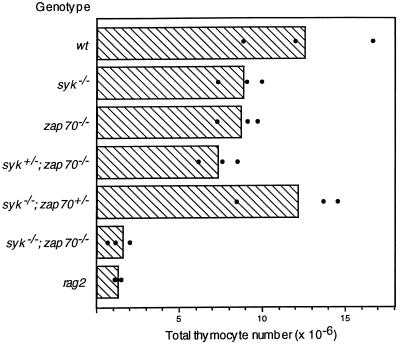
To test the possibility that Syk and ZAP-70 play a role in the differentiation of DN thymocytes, we generated mice deficient in both of these kinases. Mutant mice doubly heterozygous for gene-targeted alleles of syk (8) and zap-70 (10) were intercrossed to generate double homozygous mutants. Because Syk deficiency leads to perinatal lethality, we analyzed thymocyte development in mice 1 day after birth. Thymuses from neonatal mice that were doubly homozygous for the syk and zap-70 mutations contained ≈8-fold fewer total thymocytes than their wild-type litter mates (Fig. 1). Similar to the defect observed in rag-2 mutant mice, most of the smaller thymocyte subset was deleted in syk−/−; zap-70−/− double mutant neonates (data not shown). In compound mutant mice carrying at least one wild-type allele of syk or zap-70, the numbers of thymocytes were comparable to, or only slightly less than, those of the wild-type neonates. Hence, the generation of thymocytes is dependent on the expression of either Syk or ZAP-70.
Figure 1.
Thymocyte numbers in neonatal mice. The averaged numbers of thymocytes from 1-day-old wild-type (wt) and mutant neonates are plotted in the bar graph as shown. Black dots indicate the actual thymocyte count of each sample.
Arrested Development of CD4−CD8− Thymocytes in Syk and ZAP-70 Double Mutant.
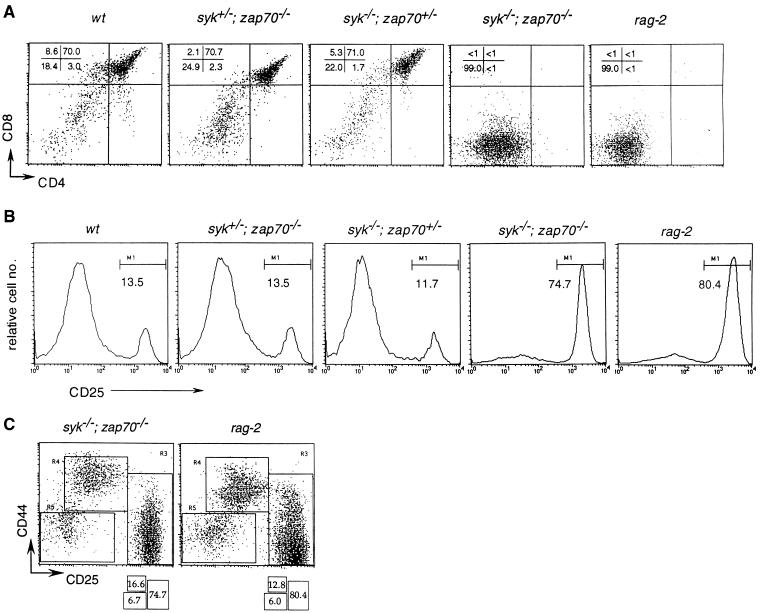
Surface phenotype analysis revealed that the CD4+CD8+ DP population was completely missing in the double homozygous mutants. This contrasts with the prominent DP population (70% of total thymocytes) observed in the single Syk-deficient or ZAP-70-deficient mutants (Fig. 2A). Neither a heterozygous syk mutation in a zap-70−/− background nor a heterozygous zap-70 mutation in a syk−/− background induced any substantial defect in DP thymocyte development (Fig. 2A), suggesting that these two kinases can carry out similar functions in DN cells.
Figure 2.
Arrested thymocyte development in syk−/−; zap-70−/− double mutants. (A) FACScan analysis of thymocytes from 1-day-old neonates with anti-CD4 (fluorescein isothiocyanate) and anti-CD8 (phycoerythrin) antibodies. The percentage of cells in the four quadrants are indicated in the top right-hand corner. (B) FACScan analysis of thymocytes with anti-CD25 (fluorescein isothiocyanate) and anti-CD44 (phycoerythrin) antibodies. Only histograms of CD25 expression are depicted, and the percentage of the CD25+ population in each group is shown under the bar denoted M1. (C) FACScan analysis of thymocytes with anti-CD25 (fluorescein isothiocyanate) and anti-CD44 (phycoerythrin) antibodies. The percentage of cells in the three regions, R3, R4, and R5, are shown in the boxes beneath.
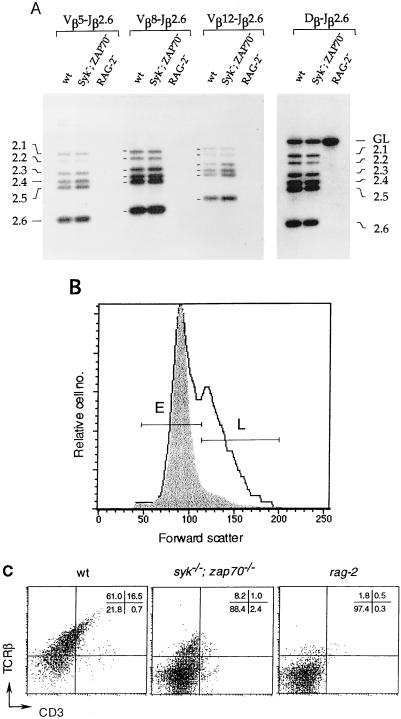
The differentiation of thymocytes is marked by the transient expression of CD44 and CD25 (15). DN thymocytes advance progressively through CD44+CD25−, CD44+CD25+, and CD44−CD25+ stages and finally emerge as CD44−CD25− cells, which are the immediate precursors of DP thymocytes (15). In syk−/−; zap-70−/− mutants, between 75 and 80% of thymocytes were arrested at the CD25+ stage whereas only ≈3% of wild-type thymocytes were found in the CD25+ population (Fig. 2B). Progression of DN thymocytes from the CD44+CD25+ to the CD44−CD25+ stage is accompanied by the initiation of Vβ–DβJβ gene rearrangement (15) and the expression of productively rearranged β gene products as a component of the pre-TCR complex. In wild-type neonates, >75% of the thymocytes developed into DP cells at the CD25− stage (data not shown). In contrast, the Syk; ZAP-70-deficient neonates exhibited an accumulation of DN thymocytes at the CD25+ stage (Fig. 2B). Comparison of thymocytes stained for CD44 and CD25 revealed that both the Syk;ZAP-70-deficient and the rag-2 neonates exhibited a very similar block in development, with most cells arrested in the CD44−CD25+ stage. Using PCR primers specific for several β gene families (Vβ5, Vβ8, and Vβ12), we were able to detect Vβ–DβJβ rearrangement activity in syk−/−; zap-70−/− mutant and wild-type thymocytes (Fig. 3A). Thus, the lack of both Syk and ZAP-70 does not impair the development of DN thymocytes through the stage of β chain gene rearrangement. This suggests that the developmental arrest of αβ lineage T cells induced by combined Syk and ZAP-70 deficiencies occurs after expression of the pre-TCR.
Figure 3.
Arrested development of DN thymocytes in syk−/−; zap-70−/− mutant mice. (A) Comparison of the gene rearrangement activity of the TCR β chain locus in wild-type (wt), syk−/−; zap-70−/− mutant, and rag-2 mice. The products of rearrangement, as detected by PCR, between the Vβ or the Dβ gene segments and the Jβ2.1–Jβ2.6 gene segments are indicated. GL marks the PCR products of germ line sequence between Dβ and Jβ2.6 segments. (B) Cell size distribution of CD25+ thymocytes. Total thymocytes stained with CD25 and CD44 antibodies (as described in Fig. 2B) were gated for CD25 expression and analyzed for cell-size distribution base on the forward scatter values. The results from a wild-type (solid line) mouse and a syk−/−; zap-70−/− mutant mouse (shaded area) are overlaid in the histogram as shown. The smaller “E” and the larger “L” subsets are marked. (C) FACScan analysis of thymocytes with anti-CD3 (fluorescein isothiocyanate) and anti-TCR β antibodies. The percentages of thymocytes in the four quadrants are shown in the top right-hand corner.
Thymocytes Lacking Syk and ZAP-70 Fail to Proliferate After pre-TCR Expression.
An immediate consequence of pre-TCR signaling in the CD44−CD25+ population is the induction of cell cycle progression, leading to the expansion of cells that have achieved productive rearrangement of β chain genes (16)—a process also termed “β selection.” As a result, the CD25+ population can be further categorized, according to their cell sizes, into two subsets: the E and the L subsets. Although the E subset contains mainly smaller resting cells, which are virtually indistinguishable from those in rag-2 mutants, the majority of cells in the larger L subset have entered the cell cycle, as reflected by their increased cell size, high levels of positive cell cycle regulators (cyclins A and B), and the presence of the predominantly hyperphosphorylated form of Rb (16). Thus, cell size analysis of the CD25+ population can reveal the progress of these cells after pre-TCR formation. For this purpose, the CD25+ population from syk−/−; zap-70−/− mutants was compared with the corresponding population from the wild-type or rag-2 mutant mice. syk−/−; zap-70−/− mutant thymocytes gated for CD25 expression were essentially identical in cell size distribution, as measured by forward scatter, to CD25+ cells from rag-2 neonates (data not shown). However, comparison with the wild-type thymocytes showed that the CD25+ population from the syk−/−; zap-70−/− mutants specifically lacked the L subset of larger, cycling cells (Fig. 3B). Thus, thymocytes lacking both Syk and ZAP-70 are apparently defective in their capacity to undergo clonal expansion even though these cells have completed β chain rearrangement and are therefore competent to express the pre-TCR. In contrast to the wild-type thymocytes that are capable of advancing to the DP stage and therefore exhibit up-regulated levels of TCR β and CD3 expression, only ≈10% of total thymocytes from syk−/−; zap-70−/− mice expressed β chain at a relatively low level (Fig. 3C). This surface expression of the β chain in syk−/−; zap-70−/− thymocytes is probably in the form of the pre-TCR.
DISCUSSION
We have identified a critical role for Syk and ZAP-70 in early thymocyte development, a function that was not revealed in mutant mice lacking only one of these kinases. These results show an essential role for Syk and ZAP-70 in pre-TCR signaling and suggest that Syk and ZAP-70 have overlapping activities in the differentiation of DN thymocytes to DP cells. Our data, therefore, are consistent with the suggestion that these two kinases can perform very similar biological functions (17–19) although Syk and ZAP-70 clearly have somewhat distinct modes of activation downstream of the CD3 and TCR ζ signaling complex (20). Because the thymocytes in syk−/−; zap-70−/− mice show rearrangement of TCR β genes but fail to undergo down-regulation of CD25 and expansion into a population of cycling cells, our data suggest that Syk and ZAP-70 function interchangeably in signaling downstream of the pre-TCR. According to this scheme, phosphorylation of the CD3 and TCR ζ signaling subunits of the pre-TCR by Src family kinases leads to the recruitment and activation of Syk/ZAP-70, phosphorylation of intracellular targets (4, 21), and subsequent proliferation and differentiation of DN thymocytes. Consistent with this view, disruption of the TCR β (22), the pTα (1), or the CD3 invariant genes (23–25) impairs the formation of DP thymocytes, while combined mutations in both Lck and Fyn (26, 27) induce a complete block in the differentiation of DN to DP thymocytes.
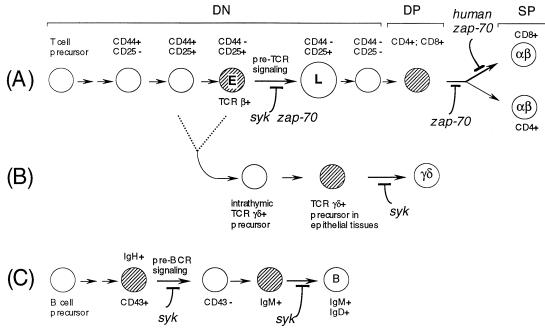
Taken with earlier data, these results suggest that Syk and ZAP-70 act at multiple checkpoints during T and B cell development (Fig. 4). ZAP-70 is not significantly expressed in B cells (28); therefore Syk appears to be uniquely required for signaling from the pre-BCR and thus for the clonal expansion of pre-B cells that have productively rearranged the Ig heavy chain genes (8). Syk also has separate functions later in B cell development, such as in the maturation of B cells to the IgM+; IgD+ stage (8) and in antigen-induced B cell activation, likely due to signaling downstream of the mature BCR (6). During the later stages of αβ T cell development, ZAP-70 alone is required for the differentiation of DP thymocytes to the CD4 and CD8 lineages in the mouse (10), the differentiation of the CD8 lineage in humans, and the activation of CD4+ peripheral T cells (11, 12). This may be due either to a unique biochemical activity of ZAP-70 in signaling from the mature TCR or to the fact that Syk expression is down-regulated during T cell development (28). In contrast, Syk plays a specific role in regulating extrathymic development of γδ T lineage cells (29). The data reported here indicate that, in αβ lineage T cells, Syk and ZAP-70 act jointly in the differentiation of DN to DP cells, most probably due to a role in signaling by the pre-TCR. These SH2-containing tyrosine kinases therefore appear to act at an early checkpoint during thymocyte development that monitors productive rearrangement of β chain genes.
Figure 4.
The roles of Syk and ZAP-70 in B and T cell development. The roles of Syk and ZAP-70 at different points in the development of the αβ T lineage (A), the γδ T lineage (B), and the B cell lineage (C) are depicted. The development of the γδ lineage is derived from the common intermediate T cell precursors as shown in A at a point before pre-TCR signaling (CD44−CD25+ “E”). The cell types whose development is most obviously affected by mutations in syk or zap-70 are shaded. This summary is based on the present study and others reported (8, 10–12, 29).
Acknowledgments
We thank Cindy Guidos and Adrian Hayday for helpful discussion. A.C.C. is the recipient of a Pew Scholars Award. This work was supported by a grant from Bristol-Myers-Squibb and by an International Research Scholar Award from the Howard Hughes Medical Institute to T.P. T.P. is a Terry Fox Cancer Research Scientist of the National Cancer Institute of Canada.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: DN, double negative; DP, double positive; TCR, T cell receptor; BCR, B cell antigen receptor.
References
- 1.Fehling H J, Krotkova A, Saint-Ruf C, von Boehmer H. Nature (London) 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 2.Groettrup M, Ungewiss K, Azogui O, Palacios R, Owen M J, Hayday A C, von Boehmer H. Cell. 1993;75:283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling H J, von Boehmer H. Science. 1994;266:1208–1212. . O. N. [PubMed] [Google Scholar]
- 4.Iwashima M, Irving B A, Chan A C, Weiss A. Science. 1994;263:1136–1139. [PubMed] [Google Scholar]
- 5.Chan, A. C. & Shaw, A. S. (1996) Curr. Opin. Immunol. 394–401. [DOI] [PubMed]
- 6.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharenberg A M, Lin S, Cuenod B, Yamamura H, Kinet J P. EMBO J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Nature (London) 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 9.Turner M, Mee P J, Costello P S, Williams P, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L J. Nature (London) 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 10.Negishi I, Motoyama N, Nakayama K-I, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan A C, Loh D Y. Nature (London) 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 11.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman C M. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 12.Chan A C, Kadlecek T A, Elder M E, Filipovich A H, Kuo W-L, Iwashima M, Parslow T G, Weiss A. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 13.Anderson S J, Abraham K M, Nakayama T, Singer A, Perlmutter R M. EMBO J. 1992;11:4877–4866. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson S J, Levin S D, Perlmutter R M. Nature (London) 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey D I, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 16.Hoffman E S, Passoni L, Crompton T, Leu T M J, Schatz D G, Koff A, Owen M J, Hayday A C. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand E W, Weinberg K, Mazer B D, Kadlecek T A, Weiss A. J Exp Med. 1995;182:1057–1066. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolanus W, Romeo C, Seed B. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- 19.Kong G-H, Bu J-Y, Kurosaki T, Shaw A S, Chan A C. Immunity. 1995;2:485–492. doi: 10.1016/1074-7613(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 20.Chu D H, Spits H, Peyron J-F, Rowley R B, Bolen J B, Weiss A. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 21.van Oers N S C, van Boehmer H, Weiss A. J Exp Med. 1995;182:1585–1590. doi: 10.1084/jem.182.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaemisch R, Hooper M L, Tonegawa S. Nature (London) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 23.Ohno H, Aoe T, Taki S, Kitamura D, Ishida Y, Rajewsky K, Saito T. EMBO J. 1993;12:4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malissen M, Gillet A, Rocha B, Trucy J, Viver E, Boyer C, Kontgen F, Brun N, Mazza G, Spanopoulou E, Guy-Grand D, Malissen B. EMBO J. 1993;12:4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C P, Ueda R, She J, Sancho J, Wand B, Weddell G, Loring J, Kurahara C, Dudley E C, Hayday H, Terhorst C, Huang M. EMBO J. 1993;12:4863–4875. doi: 10.1002/j.1460-2075.1993.tb06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groves T, Smiley P, Cooke M P, Forbush K, Perlmutter R M, Guidos C J. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 27.van Oers N S C, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 28.Chan A C, van Oers N S C, Tran A, Turka L, Law C L, Ryan J C, Clark E A, Weiss A. J Immunol. 1994;152:4758–4766. [PubMed] [Google Scholar]
- 29.Mallick-Wood C A, Pao W, Cheng A M, Lewis J M, Kulkarni S, Bolen J B, Rowley R B, Tigelaar R E, Pawson T, Hayday A C. Proc Natl Acad Sci USA. 1996;93:9704–9709. doi: 10.1073/pnas.93.18.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]