Abstract
Xenogeneic islets could provide an unlimited source of tissue for the treatment of diabetes, and could in theory be transplanted repeatedly in a recipient. However, little is known on the consequences of islet re-transplantation in a recipient who has rejected a first graft. In this study, we investigated the functional consequence of xeno islet re-transplantation in mice sensitized with islets from different species. Sprague–Dawley (SD)-rat islets transplanted in sensitized C57/Bl6 mice that rejected either SD- or Lewis-rat islets underwent accelerated rejection. However, accelerated rejection was not found in mice sensitized with pig or human islets, suggesting that accelerated rejection was species specific. Immunohistochemistry showed increased binding of antibodies and accelerated leucocyte infiltration on re-grafted islets in sensitized mice. In situ apoptosis detection indicated that islet cell apoptosis was correlated with the time of leucocyte infiltration, but not with the time of antibody binding. In vitro experiments with cultured islet cells showed that although antibody binding was increased after incubation with sensitized mouse serum, islet cell cytotoxicity was not increased, suggesting that humoral immunity did not play a direct role in islet destruction. These results indicate that there is a cell-mediated, species-specific accelerated rejection after re-transplantation of xenogeneic islets.
Introduction
Transplantation of pancreatic islets is a potential treatment for patients with insulin-dependent (type 1) diabetes. The preliminary results of islet transplantation are encouraging and the number of insulin-independent patients is increasing with improvements in the technique of islet isolation and with the use of new immune-suppressive drugs.1–3
As islet transplantation becomes more successful, the scarcity of human tissue appears as a potential limit to the procedure, and other animal species are being considered as sources of tissue.4–6 However, two major questions have to be addressed in xenogeneic islet transplantation. First, xeno-antigens trigger immune rejection, destroying the grafted islets. Second, animal viruses may be transmitted to humans. It has been reported that immune tolerance can be induced by injection of donor islet antigens into recipient's thymus,7,8 and long-term survival of encapsulated xenogeneic islets has been achieved in a large animal model.9 Also, prolonged survival of genetically engineered islets has been described.10 This progress in immune-tolerance and immune-isolation as well as in genetic engineering make it likely that xenogeneic islets will be transplanted to humans in the future. In addition, the fear that animal viruses can be transmitted to humans has been eased recently.11 Therefore, it is reasonable to believe that the immune-rejection of xenografts will be overcome and animal virus infection may be avoided with genetically engineered animals in the future.
In theory, because of the unrestricted supply of tissues, xenogeneic islet transplantation could be performed repeatedly even if the xenograft has been rejected. However, little is known on the functional consequence of islet re-transplantation in sensitized recipients, although accelerated rejection may be expected. In this study, we investigated the survival of re-grafted rat islets in mice that had been sensitized with rat, pig or human islet grafts, with attention to the rejection mechanisms.
Materials and methods
Islet isolation and purification
Rat islets
Islets were isolated from Sprague–Dawley (SD) and Lewis male rats (350 g; BRL, Basel, Switzerland) using the intraductal collagenase digestion technique described previously.12 Briefly, 10 ml of Hank's balanced salt solution (HBSS) with 2 mg/ml collagenase type XI (Sigma, St Louis, MO) were injected into the pancreatic duct. After pancreatectomy, the pancreata were digested in a 37° water-bath for 19 min. The isolated islets were further purified by Euro-Ficoll (Sigma) gradient centrifugation. The purified islets were washed three times and then resuspended in HBSS for transplantation.
Pig islets
Pancreata were obtained from female large white pigs (more than 200 kg) in a local slaughterhouse (Orbe, Switzerland). Islet isolation was performed within 4 hr after pancreas procurement using a modified automated method described previously.13 Briefly, after preparation of the pancreas and distension with Liberase PI (Roche, Basel, Switzerland), digestion was performed in a modified digestion chamber at 37° until the appearance of free islets. Islet purification was performed by Euro-Ficoll gradient centrifugation on a cell separator (Cobe 2991, Cobe, Lakewood, CO). The purified islets were washed three times and resuspended in HBSS for transplantation.
Human islets
Human pancreata were obtained from multiorgan heart-beating donors and kept in University of Wisconsin solution at 4° for transport (cold ischaemia time <8 hr). Islet digestion and isolation were performed using a semiautomated method adapted from Ricordi et al.14 Briefly, after preparation of the pancreas and distension with Liberase HI (Roche), digestion and purification were performed as described above for pig islet isolation.
Sensitization of diabetic mice by rat, pig or human islets
Experimental diabetes was induced in C57Bl/6 male mice, 6–8-week-old (25 g, BRL, Basel, Switzerland) by a single intraperitoneal injection of streptozotocin (200 mg/kg, Sigma, Buchs, Switzerland). Diabetes was defined as the presence of blood glucose levels >20 mm on 3 consecutive days. For the sensitization, 200 rat islets, 400 pig islets or 400 human islets were transplanted beneath the left kidney capsule of diabetic mice. Graft function was evaluated by non-fasting blood glucose levels. Values >11 mm on 3 consecutive days were considered as a sign of rejection.
Comparison of survival of grafted rat islets between control and sensitized mice
The second islet transplantation was performed in sensitized mice between 3 and 5 days after rejection of the first islet graft. 200 SD-rat islets were implanted under the right kidney capsule of sensitized mice using the same procedure as described above. Only mice which had a previously functioning islet graft (glycaemia <11 mm for more than 3 days) were used for a second transplantation. Six mice were used in each group for the comparison of the graft survival time.
Immunohistochemistry
Nephrectomy was performed on three control mice and three sensitized mice on days 1, 2, 3, 4, and following rejection after islet transplantation. Kidneys were embedded in tissue-tek (Miles Inc., Elkhart, IN) frozen in liquid methylbutane pre-equilibrated with liquid nitrogen and stored at −80°. Serial frozen sections were cut at 4 µm intervals using a cryostat (Leica, Glattbrugg, Switzerland). Tissue sections were fixed with absolute ethanol for 1 min and then incubated with phosphate-buffered saline (PBS) plus 0·5% bovine serum albumin (BSA; Sigma) for 15 min to block non-specific binding. Further staining was performed for antibody binding, complement deposition and leucocyte infiltration as described previously.15 The slides were examined under a fluorescence microscope (Zeiss Axiophot, Germany).
In situ apoptosis detection in grafted islets
To detect apoptotic cells, commercial kits were used according to the manufacturer's protocol (TACS terminal deoxynucleotide (TdT) apoptosis detection kit, R & D, Minneapolis, MN). Briefly, serial frozen sections were cut at 4 µm intervals, fixed with 3·7% formaldehyde and permeabilized with proteinase K solution. Samples were then incubated in the quenching solution and then in the TdT labelling buffer. Samples were incubated with 50 µl of labelling reaction mix at 37° for 1 hr. The slides were incubated in the TdT stop buffer and then were incubated with 50 µl of streptavidin–horseradish peroxidase (HRP) solution for 10 min and counterstained in methyl green solution. The slides were then sequentially washed with increasing concentrations of ethanol and with xylene. The slides were then mounted and examined under light microscopy. After incubation with proteinase K solution, one sample of each group was treated with TACS-nuclease for 30 min to generate DNA breaks and was used as positive control.
Measurement of anti-rat islet antibodies
Single cell suspension from rat islets was obtained using the ethylenediamine tetra-acetic acid (EDTA)–dispase technique, as described previously.16 Isolated islet cells were seeded in gelatin precoated 96-well plates at a concentration of 2 × 105 cells/well. After overnight culture, cells were washed three times with PBS and fixed with −20° absolute methanol (Fluka, Buchs, Switzerland) for 10 min. The cells were washed and then incubated in PBS containing 0·05% BSA, 0·5% fetal calf serum (FCS; Life Technologies, Basel, Switzerland) and 0·02% Tween 20 (Fluka) for 15 min to block non-specific binding. Cells were incubated in PBS containing 20% of serum from mice unsensitized or sensitized with rat, pig or human islets, for 60 min at room temperature. The cells were washed and then incubated with diluted HRP-conjugated goat anti-mouse immunoglobulin M (IgM), IgG or IgA antibody (Sigma) for 30 min at room temperature. Islet cells incubated with PBS containing 20% inactivated FCS were used as negative controls. Cells were washed and tetramethylbenzidine (TMB; Medgenix, Fleurus, Belgium) was added into each well (100 µl/well). The colour reaction was stopped with 50 µl/well 2 n H2SO4. The optical density was measured in an enzyme-linked immunosorbent assay (ELISA) reader (Bioconcept, Allschwil, Switzerland) at 405 nm. The results were expressed as mean ±SD of three experiments.
Measurement of serum-mediated cytotoxicity on cultured rat islet cells
In order to evaluate the role of antibodies on accelerated rejection of xeno-islets, serum-mediated islet cell cytotoxicity was measured by a 51Cr release assay. Briefly, 10 × 106 freshly isolated rat islet cells were labelled with 200 µCi 51Cr (Amersham, Amersham, UK) for 2 hr at 37° in CMRL (Sigma) containing 10% FCS. After three washings, the cells were seeded in a 96-well plate (2 × 105 cells/well) and incubated in PBS containing 20% of serum from mice unsensitized or sensitized with rat, pig or human islets. Cells incubated with 20% normal rat serum or with 1% Triton-X-100 in PBS were used as negative or positive controls, respectively. After 2 or 4 hr incubation, the plates were centrifuged and supernatants were collected. The 51Cr release in the supernatant was measured by a gamma-counter (Packard, Zurich, Switzerland). The results were expressed as percentage of 51Cr release.
Statistical analysis
Survival curves were calculated with by the Kaplan–Meier method. Wilcoxon test for independent samples was used to evaluate the significance of differences between groups. Bonferoni correction was used to correct for multiple tests.
Results
Animals sensitized by an islets xenograft experienced accelerated rejection of a subsequent islet graft from the same species
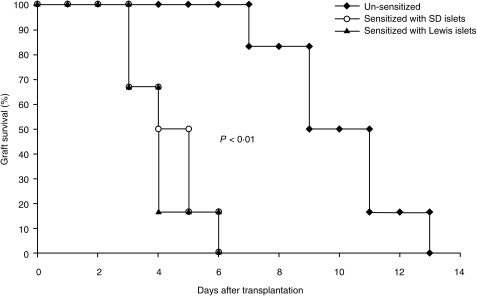
SD-rat islets were transplanted to mice that had been sensitized by SD- or Lewis-rat islets. As shown in Fig. 1, the mean survival time (MST) of the second rat islet graft was significantly shortened as compared to the MST of the first rat islet graft (4·3 ± 1·2 days versus 10 ± 2·1 days and 4·0 ± 1·1 versus 10 ± 2·1 days). This result indicated that islet re-transplanted in mice presensitized by an islet graft from the same species underwent an accelerated rejection.
Figure 1.
Comparison of grafted islet survival between normal and sensitized mice. SD-rat islets of Langerhans were transplanted in diabetic mice that had been presensitized with either SD or Lewis-rat islets. Transplantation of rat islets in unsensitized mice was used as control. Glycaemia >11 mm persisting for 3 days was defined as rejection. The first day of glycaemia >11 mm was considered as the beginning of the rejection.
Accelerated rejection was species specific
After the first xenogeneic islet transplantation (sensitization), all diabetic mice became normoglycaemic for the time before an immune rejection destroyed the grafted islets. The MST of each sensitization group was 10·0 ± 2·1 days (SD rat), 11·2 ± 2·5 days (Lewis rat), 8·3 ± 2·4 days (human) and 7·3 ± 1·7 days (pig).
As show in Fig. 2, the graft of SD-rat islets underwent an accelerated rejection in mice presensitized with either SD- or Lewis-rat islets. No difference of MST of the grafted SD-rat islets was found between mice presensitized with SD-rat islets and mice presensitized with Lewis-rat islets. This result indicated that the accelerated rejection did not depend on differences in xeno-antigens between strains of the same species. An accelerated rejection of rat islets was not seen in mice that were presensitized with islets from pigs or humans. The MST of the grafted SD-rat islets was similar between the sensitized and the unsensitized mice (9·3 ± 1·8 days for human sensitized mice and 10·3 ± 2·3 days for pig sensitized mice islets versus 10 ± 2·1 days for controls). These results indicated that the accelerated rejection was species specific.
Figure 2.
Effect of sensitization with islet of different species on the survival of rat islet. Rat islets of Langerhans were transplanted in diabetic mice presensitized with rat islets, human islets or pig islets. Transplantation of rat islets in unsensitized mice was used as control. Glycaemia was measured every two days and the results were expressed as mean ± SD. Glycaemia >11 mm was defined as the rejection threshold (represented as the line between the two asterisks).
Humoral and cell immune participated in accelerated rejection
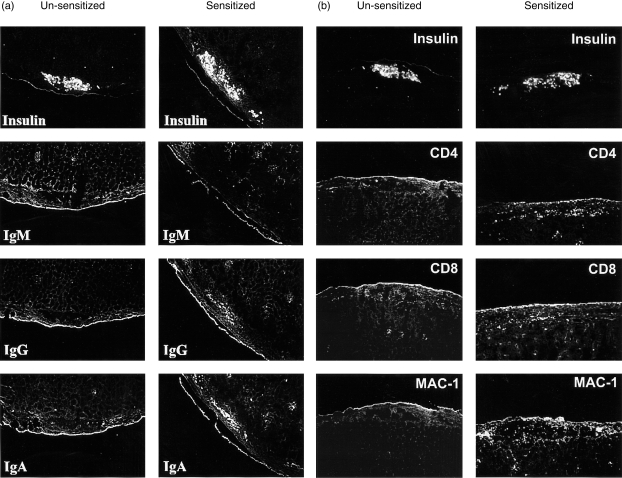
To evaluate the mechanisms of accelerated rejection in mice sensitized with SD-rat islets, the deposition of complement and the leucocyte infiltration in grafted islets at different times were investigated by immunohistochemistry. As summarized in Table 1, two main differences were found on grafted islets between unsensitized and sensitized mice. First, there were increased deposits of IgA and IgG on grafted islets in sensitized mice from day 1 after transplantation, which persisted until rejection. Second, CD4-, CD8- and Mac-1-positive leucocytes infiltrated the graft in sensitized mice as early as day 2 after islet transplantation (and as early as day 1 for Mac-1-positive cells), and persisted until rejection. These changes were not seen or were delayed in unsensitized mice. However, the accelerated leucocyte infiltration was not observed in mice presensitized with human islets, although a similar antibody deposition was found (data not shown). As shown in Fig. 3(a), anti-islet IgG and IgA antibodies were present in the sensitized mice on day 3 after transplantation, whereas these antibodies were absent in unsensitized mice. At this stage, infiltration of CD4-, CD8- and Mac-1-positive cells was observed in the sensitized but not in the unsensitized mice (Fig. 3b).
Table 1.
Comparison of immunohistological characteristics at different times after SD-rat islet transplantation between normal and sensitized mice
| Day 1 | Day 2 | Day 3 | Day 4 | Rejection | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | c n=3 | s n=3 | c n=3 | s n=3 | c n=3 | s n=3 | c n=3 | s n=3 | c n=3 | s n=3 |
| IgM | + | + | + | + | + | + | + | + | + | + |
| IgG | ± | ++ | ± | ++ | ± | ++ | − | + | − | + |
| IgA | ± | ++ | ± | ++ | ± | ++ | + | ++ | ± | ++ |
| C3 | ++ | ++ | ++ | ++ | + | + | ± | + | − | ± |
| CD4 | − | − | − | + | − | ++ | + | ++ | ++ | ++ |
| CD8 | − | − | − | + | − | + | + | ++ | ++ | ++ |
| Mac-1 | ± | ++ | ± | ++ | ± | ++ | + | ++ | ++ | ++ |
Nephrectomy was performed at days 1, 2, 3, 4 after islet transplantation and at rejection in control (c) and sensitized (s) mice. Serial frozen kidney sections were cut at 4 µm intervals and stained with anti-IgM, IgG, IgA, C3, CD4, CD8, Mac-1 antibody and corresponding fluoroscein isothiocyanate-conjugated second antibody as described in Materials and methods. The results were expressed as negative (−), weak (±), positive (+) and strongly positive (++) depending on the fluorescence intensity.
Figure 3.
Comparison of antibody binding and leucocyte infiltration between unsensitized and sensitized mice. (a) Antibody deposit on grafted islets on day 1 after islet transplantation: comparison between control and sensitized mice. (b) CD4-, CD8- and Mac-1-positive leucocyte infiltration on grafted islets on day 3 after islet transplantation: comparison between control and sensitized mice.
Islet cell apoptosis was correlated to the timing of leucocyte infiltration
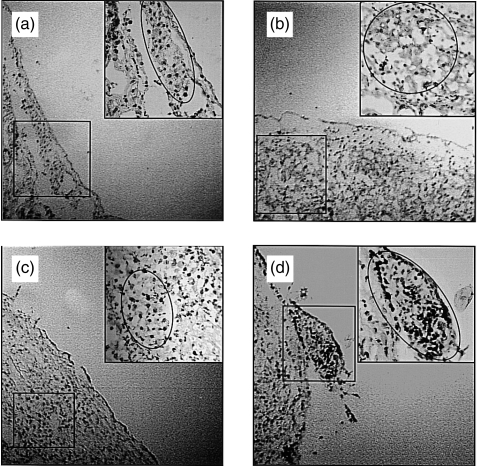
In order to evaluate the impact of antibody deposit and leucocyte infiltration in accelerated rejection, in situ apoptosis detection tests were performed on grafted islets. As shown in Fig. 4, islet cell apoptosis was found from day 2 and was marked on day 4 after islet re-transplantation. The timing of islet apoptosis was correlated with the timing of leucocyte infiltration, but not with the timing of antibody binding, suggesting that islet cell apoptosis was induced by infiltrated leucocytes rather than by the bound antibodies.
Figure 4.
Islet cell apoptosis in sensitized mice. In situ islet cell apoptosis detection in mice presensitized with rat islets on day 1 (a), 2 (b), 3 (c) and 4 (d) after islet transplantation.
Antibody binding and serum-mediated cytotoxicity on isolated rat islet cells
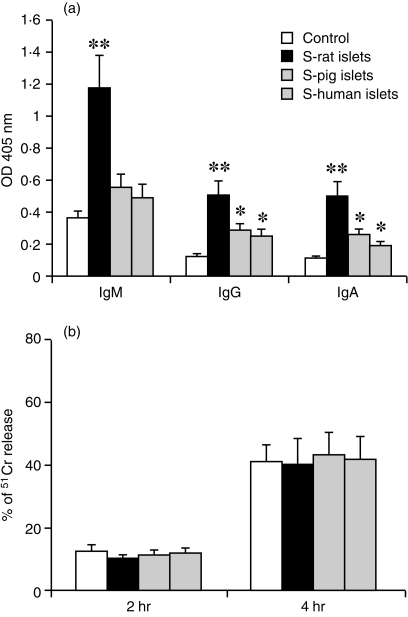
To evaluate further the role of antibodies in accelerated rejection, cultured rat islet cells were incubated with sera from sensitized or unsensitized mice. Antibody binding was measured by cell-based ELISA and serum-mediated islet cell cytotoxicity was investigated by the 51Cr release assay. As shown in Fig. 5(a), antibody binding was increased on islet cells incubated with serum from presensitized mice, as compared with incubation with serum from unsensitized mice. The increased antibody binding was more marked in the group incubated with serum from mice sensitized with rat islets, while the antibody binding after incubation with serum of mice presensitized with pig or with human islets was only marginally increased.
Figure 5.
Comparison of antibody binding and serum-mediated cytotoxicity on cultured rat islet cells. (a) Antibody binding on isolated rat islet cells after incubation with serum from mice unsensitized or sensitized with rat, pig or human islets (measured by cell-based ELISA). (b) Serum-mediated cytotoxicity on isolated rat islet cells after 2 hr or 4 hr incubation with serum from mice unsensitized or sensitized with rat, pig or human islets (measured by 51Cr release test). The results are expressed as mean ± SD of three experiments. **P<0·01, *P<0·05.
However, serum-induced islet cell cytotoxicity was similar at 2 and 4 hr incubation with serum from mice unsensitized and sensitized with rat, pig or human islets as shown in Fig. 5(b). These results suggested that the increased antibody binding on islet cells did not directly contribute to the cytotoxicity in the accelerated rejection.
Discussion
In this study, we show that repeat xenogeneic transplantation of islets of Langerhans in mice that have been previously sensitized with islets from the same species results an accelerated rejection. The accelerated rejection was present after sensitization with islets from a major histocompatibility complex (MHC)-mismatched strain within the same species, but was absent in mice that had been sensitized with islets from a different species. These results suggest that: (1) the accelerated rejection is species specific; (2) the antigens involved in accelerated rejection are shared by different strains within the same species, and (3) there are no antigens that are specific to the islets rather than to the species and that contribute – in this xenograft model – to the accelerated rejection.
Although the accelerated rejection was expected, the species specificity was surprising, even if cross-reactivity between xeno-grafts has been reported previously. The cross-species reactivity of a panel of antibodies has been demonstrated between monkey and pig tissue17 and in a mouse-to-hamster model.18 However, although the species cross-reactivity has been reported in humoral immune reaction, there is still no evidence for species cross-reactivity in cell-mediated immune response. In our study, increased binding of antibodies was found on grafted rat islets in mice presensitized with pig or human islets, but accelerated leucocyte infiltration was absent in these mice. These results imply that the species specificity of accelerated rejection is mainly exhibited in cell-mediated immune reaction. The importance of lymphocytes in the immune rejection of xenografts was demonstrated by the fact that that mice presensitized with rat islets and anti-lymphocyte serum were unresponsive to a second islet graft.19
The immune reactions in islet xenograft rejection were also different according to the species combination.20 However, the two experimental models used in our study, human-to-rat and pig-to-rat are zoologically distant, and the species-specificity of the accelerated rejection in our model can not rule out the possibility of cross-reaction in other models with different species combination. Our results suggest that, if xenogeneic islet re-transplantation is considered after a previous graft has been lost to rejection, a different donor species should be used.
Hyperacute rejection of vascularized xenografts is mediated by preformed antibodies recognizing antigenic determinants on endothelial cells, leading to complement activation.21 However, this reaction is absent in xeno transplantation of devascularized organs, such as islets of Langerhans, suggesting that there is a different rejection mechanism. It has been shown that, while xenogeneic islets can trigger both the recipient's humoral and cellular immune response, the cellular immune response is more critical to the process of rejection.22–24 Little was known whether a similar rejection mechanism occurred when xenogeneic islets were transplanted in a sensitized recipient. To answer this question, an immunohistology of grafted islets was performed in unsensitized and in sensitized mice to investigate the antibody binding, complement deposition and leucocyte infiltration during accelerated rejection. In unsensitized mice, early binding of IgM antibodies and complement were found on grafted islets, and CD4/CD8- or Mac-1-positive cells infiltrated the graft from day 4 after transplantation and persisted to the time of rejection, a finding consistent with our previous studies.25 Two main features characterized the rejection reaction in sensitized mice as compared to unsensitized mice: first, an increased binding of antibodies (IgG and IgA) was found on day 0 after transplantation and persisted to the time of rejection. Second, a rapid leucocyte infiltration was seen from day 2 after islet transplantation and persisted through the time of rejection. However, no differences in IgM and complement deposition were found between both groups.
In situ apoptosis detection in the sensitized mice showed that islet cell apoptosis was present from day 2 and was prominent on day 4 after islet re-transplantation. This timing of islet cell apoptosis was correlated with the timing of leucocyte infiltration, but not with the timing of antibody binding, suggesting that apoptosis may have been induced by infiltrated leucocytes, and not by bound antibodies.
In order to evaluate further the roles of humoral and of cellular immunity in accelerated rejection, the antibody binding and serum-mediated cytotoxicity were measured in isolated rat islet cells after incubation with serum derived from sensitized mice. We observed increased antibody binding on islet cells after incubation with serum of sensitized mice. The increase in antibody binding was more marked for IgG and IgA than for IgM, which is consistent with the findings on grafted islets in vivo. Preformed recipient's antibodies are mostly of IgM type that binds to grafted islets in vivo, but only on microvessels of whole islets in vitro,25,26 while most inducible antibodies belong to the IgG or IgA type. The binding of IgG and IgA on islet cells in vivo and in vitro was found only in sensitized mice, suggesting that these antibodies may be specifically induced by the previous islet graft. We found that serum-mediated islet cell cytotoxicity was similar between unsensitized and the sensitized groups, although the antibody binding was significantly increased. This result indicates that the increased antibody levels in the sensitized mice did not exert a direct toxic role against islet cells. It has been found that serum from a different species may directly destroy islet cells, but not whole islets in vitro.27,28 This serum-induced cytotoxicity is antibody- and complement-dependent, because serum derived from B-cell deficient mice or from decomplementized mice does not have such cytotoxicity.25
Xeno-grafted islets survive at least 3 months in T-cell deficient nude mice and approximately 20 days in B-cell deficient mice,29,30 indicating the prominent role of T-cell-mediated immunity in xeno islet rejection. However, the participation of humoral immunity in the accelerated rejection can not be ruled out even if no direct cytotoxicity to islet cell was found in our in vitro experiments. In fact, the deposited antibodies could promote cell-mediated immune response by chemotaxis or by antibody-dependent cell-mediated cytotoxicity, thus contributing to the accelerated rejection, as shown in previous studies.31–33
In summary, the results described above make us conclude that: (1) there is an accelerated rejection when xenogeneic islets are re-transplanted in sensitized recipient; (2) the accelerated rejection is species specific; (3) the accelerated rejection occurs mainly by mechanisms of cell-mediated immunity.
Acknowledgments
This study was supported by Swiss National Science Foundation (No: 32–50865·97 to P. Morel, J. Philippe and J. Lou). We thank Mr David Matthey-Doret, Mrs Corinne Sinigaglia. Mr Raymond Mage and Mrs Elisabeth Bernoulli for their skilful assistance, Dr B. Mermillod for the help in the statistical analysis and Dr D. Burger for helpful comments in the manuscript preparation.
Abbreviations
- MST
mean survival time
- SD-rat
Sprague–Dawley rat
References
- 1.Alejandro R, Lehmann R, Ricordi C, et al. Long-term function (6 years) of islet allografts in type 1 diabetes. Diabetes. 1997;46:1983–89. doi: 10.2337/diab.46.12.1983. [DOI] [PubMed] [Google Scholar]
- 2.Oberholzer J, Triponez F, Mage R, et al. Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation. 2000;69(6):1115–23. doi: 10.1097/00007890-200003270-00016. [DOI] [PubMed] [Google Scholar]
- 3.Oberholzer J, Triponez F, Lou J, Morel P. Clinical islet transplantation: a review. Ann N Y Acad Sci. 1999;875:189–99. doi: 10.1111/j.1749-6632.1999.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 4.Weir GC, Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes. 1997;48:1247–56. doi: 10.2337/diab.46.8.1247. [DOI] [PubMed] [Google Scholar]
- 5.Platt JL. Islet xenotransplantation: how sweet it is. J Clin Invest. 1996;98(6):1273–74. doi: 10.1172/JCI118911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorling A, Riesbeck K, Warrens A, Lechler R. Clinical xenotransplantation of solid organs. Lancet. 1997;349:867–71. doi: 10.1016/S0140-6736(96)09404-4. 10.1016/s0140-6736(96)09404-4. [DOI] [PubMed] [Google Scholar]
- 7.Posselt AM, Barker CF, Tomaszewski JE, Markmann JF, Choti MA, Naji A. Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science. 1990;249:1293–5. doi: 10.1126/science.2119056. [DOI] [PubMed] [Google Scholar]
- 8.Qian T, Schachner R, Brendel M, Kong SS, Alejandro R. Induction of donor-specific tolerance to rat islet allografts by intrathymic inoculation of solubilized spleen cell membrane antigens. Diabetes. 1993;42:1544–6. doi: 10.2337/diab.42.10.1544. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417–22. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efrat S, Fejer G, Brownlee M, Horwitz MS. Prolonged survival of pancreatic islet allografts mediated by adenovirus immunoregulatory transgenes. Proc Natl Acad Sci USA. 1995;92:6947–51. doi: 10.1073/pnas.92.15.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibell A, Groth CG. No viral disease after xenotransplantation. Nature. 1998;392:646. doi: 10.1038/33517. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro AM, Hao E, Rajotte RV, Kneteman NM. High yield of rodent islets with intraductal collagenase and stationary digestion: a comparison with standard technique. Cell Transplant. 1996;5:631–8. doi: 10.1177/096368979600500606. 10.1016/s0963-6897(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C, Socci C, Davalli AM, et al. Isolation of the elusive pig islet. Surgery. 1990;107:688–94. [PubMed] [Google Scholar]
- 14.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 15.Carter AS, Cerundolo L, Koo DD, Rust NA, Morris PJ, Fuggle SV. Cross-species reactivity of a panel of antibodies with monkey and porcine tissue. Xenotransplantation. 1999;6:123–30. doi: 10.1034/j.1399-3089.1999.00014.x. [DOI] [PubMed] [Google Scholar]
- 16.Gannedahl G, Karlsson-Parra A, Wallgren A, Roos-Engstrand E, Nilsson B, Totterman TH, Tufveson G. Role of antibody synthesis and complement activation in concordant xenograft retransplantation. Transplantation. 1994;58:337–44. [PubMed] [Google Scholar]
- 17.Goss JA, Nakafusa Y, Finke EH, Flye MW, Lacy PE. Induction of tolerance to islet xenografts in a concordant rat-to-mouse model. Diabetes. 1994;43:16–23. doi: 10.2337/diab.43.1.16. [DOI] [PubMed] [Google Scholar]
- 18.Medbury HJ, Hibbins M, Lehnert AM, Hawthorne WJ, Chapman JR, Mandel TE, O'connell PJ. The cytokine and histological response in islet xenograft rejection is dependent upon species combination. Transplantation. 1997;64:1307–14. doi: 10.1097/00007890-199711150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721–8. doi: 10.1016/s0952-7915(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JD, Simeonovic CJ, Ting JH, Ceredig R. Role of CD4+ T-lymphocytes in rejection by mice of fetal pig proislet xenografts. Diabetes. 1989;38:217–19. doi: 10.2337/diab.38.1.s217. Suppl. [DOI] [PubMed] [Google Scholar]
- 21.Simeonovic CJ, Ceredig R, Wilson JD. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell-depleted mice. Transplantation. 1999;49:849–56. doi: 10.1097/00007890-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Gill RG, Rosenberg AS, Lafferty KJ, Singer A. Characterization of primary T cell subsets mediating rejection of pancreatic islet grafts. J Immunol. 1989;143(7):2176–8. [PubMed] [Google Scholar]
- 23.Oberholzer J, Yu D, Triponez F, et al. Decomplementation with cobra venom factor prolongs survival of xenografted islets in a rat to mouse model. Immunology. 1999;97:173–80. doi: 10.1046/j.1365-2567.1999.00742.x. 10.1046/j.1365-2567.1999.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirenda V, Le Mauff B, Boeffard F, Cassard A, Jugeau N, Soulillou J P, Anegon I. Intact pancreatic islet function despite humoral xenorecognition in the pig-to-monkey combination. Transplantation. 1998;66:1485–95. doi: 10.1097/00007890-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Schaapherder AF, Wolvekamp MC, te Bulte MT, Bouwman E, Gooszen HG, Daha MR. Porcine islet cells of Langerhans are destroyed by human complement and not by antibody-dependent cell-mediated mechanisms. Transplantation. 1996;62:29–33. doi: 10.1097/00007890-199607150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kin T, Nakajima Y, Kanehiro H, et al. Effect of complement activation in human serum on isolated porcine islets. Cell Transplant. 1996;5(Suppl. 1):S45–47. doi: 10.1016/0963-6897(96)00038-3. 10.1016/0963-6897(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 27.Gourlay WA, O'neil JJ, Hancock WW, Monaco AP, Maki T. Resistance of established porcine islet xenografts to humoral rejection by hyperimmune sera. Transplantation. 1999;68:888–93. doi: 10.1097/00007890-199909270-00023. [DOI] [PubMed] [Google Scholar]
- 28.Mirenda V, Sigalla J, Fiche M, et al. Pig pancreatic islet xenografts in a B-cell-deficient mouse model. Transplant Proc. 1997;29:762–3. doi: 10.1016/s0041-1345(96)00472-1. 10.1016/s0041-1345(96)00472-1. [DOI] [PubMed] [Google Scholar]
- 29.Thomas FT. Experimental pancreatic islet cell xenotransplantation: isolated pancreatic islet xenografting. In: Cooper DKC, Kemp E, Platt JL, White DJG, editors. Xenotransplantation. Berlin: Springer-Verlag; 1997. pp. 545–60. [Google Scholar]
- 30.Kumagai-Braesch M, Satake M, Qian Y, Holgersson J, Moller E. Human NK cell and ADCC reactivity against xenogeneic porcine target cells including fetal porcine islet cells. Xenotransplantation. 1998;5:132–45. doi: 10.1111/j.1399-3089.1998.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 31.Schroder D, Wanka H, Hehmke B, Kloting I, Kohler E, Knospe S, Schmidt S. Beta cell destruction of pancreatic islets transplanted into diabetic BB/OK rats may be reflected by increased antibody-mediated anti-islet cytotoxicity. Diabetes Res. 1990;15:27–32. [PubMed] [Google Scholar]