Abstract
Acute macrophage (Mφ) depletion, using a liposome-mediated ‘suicide technique’, markedly suppressed priming of splenic CD4+ and CD8+ T-cell responses to vesicular stomatitis virus (VSV). However, phagocytic marginal dendritic cells (MDC), but not interdigitating dendritic cells (IDC), are now known to be also depleted by this technique. To clarify the role splenic dendritic cell (DC) subsets and Mφ play in priming for a virus-specific T-cell-mediated immune response, DC and Mφ were purified from VSV-infected mice and assayed for the presence of epitopes recognized by VSV helper T (Th) cells and cytotoxic T lymphocytes (CTL). Antigen pulse experiments performed in situ demonstrated that VSV Th cell and CTL epitopes became transiently associated only with DC, but not Mφ or B cells, indicating that DC represent the critical antigen-presenting cell (APC) population in vivo for this virus. The failure of MDC/Mφ-deficient mice to become primed was not due to the complete elimination of antigen-presenting DC because VSV peptide/class I and II complexes were detected on IDC following lipsome-mediated elimination of phagocytic cells. However, the VSV-induced chemokine response was dramatically suppressed in these mice. Thus, despite the expression of VSV peptide/class I and II complexes, IDC are not sufficient to prime VSV Th cells in the absence of MDC and/or splenic Mφ.
Introduction
Antigen-presenting cells (APC) play a critical role in the initiation of an immune response. Within the spleen, several cell types could function as APC in vivo and therefore regulate cell-mediated immune responses. For example, morphologically distinct and highly compartmentalized macrophage (Mφ) subpopulations have been identified such as red pulp macrophages (RPM), marginal metallophilic macrophages (MMM), marginal zone macrophages (MZM) and white pulp macrophages (reviewed in ref. 1). Depletion of splenic Mφ in vivo by liposome-mediated delivery of dichloromethylene bisphosphonate (Cl2MBP-liposomes)2–5 suppressed immune responses to a variety of antigens6–11 and also revealed that Mφ subsets were functionally distinct.8–10 Marginal dendritic cells (MDC) were also depleted by this procedure and have been shown to migrate to the T-cell-dependent areas of the spleen following antigen capture.12–14
We have studied the role of dendritic cells (DC) and Mφ subsets in regulating the T-cell responses to vesicular stomatitis virus (VSV) in vivo. We chose to study VSV, a member of the rhabdovirus family, because it is biochemically well characterized and elicits a cell-mediated immune response that has been extensively studied by a number of laboratories.15–17 We previously reported that acute depletion of phagocytic cells in vivo markedly suppressed priming for recall cytotoxic T lymphocyte (CTL) and T helper (Th) cell responses in vitro.18 Repopulation kinetics following Cl2MBP-liposome treatment indicated that efficient priming of both of these T-cell subsets was dependent on the presence of either MDC and/or RPM.18 Because interdigitating dendritic cells (IDC) remain following Cl2MBP-liposome treatment and are considered to be APC, it is not clear why IDC do not efficiently prime VSV Th cells and CTL. Differences in antigen capture or chemokine production may reduce the efficacy of IDC to prime VSV T cells. The purpose of the present study was to assess how these different activities may impact IDC and their ability to prime VSV T cells in situ.
Materials and methods
Mice and cell lines
BALB/cByJ mice, obtained from The Jackson Laboratory (Bar Harbor, ME) were used throughout these studies. Standard cell lines used for detecting CTL activity were EL-4 (H-2b thymoma), P815 (H-2d mastocytoma) and YAC-1 (H-2a Moloney sarcoma virus-induced lymphoma). Cells were maintained by bi-weekly passage as previously described.19
Virus preparation, infection and generation of secondary Th-cell and CTL responses
Wild-type VSV, provided by Dr Philip Marcus, University of Connecticut, was grown and assayed as previously described.20 This virus, as well as a temperature-sensitive mutant of VSV (G41), was grown in confluent monolayers of Vero cells and virus titres were determined by standard plaque assays.21 Mice were infected (primed) with VSV by a single intraperitoneal (i.p.) injection of 2 × 107 plaque-forming units (PFU). VSV recall Th-cell and CTL responses were elicited in vitro with either mock or VSV-infected stimulator cells as previously reported.18
MDC/Mφ depletion and assay of T-cell priming
Prior to VSV infection, BALB/c mice were acutely depleted of splenic and peritoneal Mφ using Cl2MBP-liposomes.18 Cl2MBP-liposomes were a kind gift of Boehringer Mannheim, GmbH, Mannheim, Germany. Control animals were treated with either phosphate-buffered saline (PBS) vehicle alone or liposome-entrapped PBS. No differences were noted in animals treated with PBS or liposome-entrapped PBS. The efficacy of splenic MDC/Mφ depletion was verified by acid phosphatase staining22 or immunohistochemistry.23 The ability of T cells, primed in the absence or presence of MDC/Mφ, to mount recall VSV Th-cell (proliferative) and CTL responses in vitro was used as an indicator of the efficiency of T-cell priming in vivo. Efficient priming in vivo against this virus is required to obtain secondary anti-VSV T-cell responses in vitro.24 CTL responses to VSV were generated in macroculture or microculture systems as previously described.25,26 Cytotoxic activity was detected with a standard 5-hr 51Cr release assay using radiolabelled P815 tumour target cells that were either mock or VSV-infected. The specificity of the CTL was verified by demonstrating minimal lysis (<5%) of mock-infected P815 and VSV-infected EL4 target cells. VSV-induced proliferative responses, mediated predominantly by CD4+ cells, were generated as previously reported.24 All assays reflect the response of pooled T cells (minimum three mice/group) and are representative of two or three independent experiments.
Purification and characterization of splenic APC
For assessment of APC function in vitro, resident peritoneal Mφ were obtained by lavage of the peritoneal cavity of non-infected mice with PBS. Cells were cultured overnight and the adherent population was isolated by vigorous pipetting. Established procedures were followed to isolate splenic DC.27 Briefly, spleens were digested with collagenase, centrifuged on a bovine serum albumin (BSA) column and low-density spleen cells (LDSC) were isolated from the top of the column. To obtain DC for analysis of APC function in vitro, LDSC were incubated on tissue culture plates (2 hr), washed to remove non-adherent cells and then incubated overnight. The non-adherent population, enriched for DC (≥80% CDllc+) was then infected with VSV as described above. Similar procedures were used to isolate splenic Mφ, except adherence was performed with the high-density fraction and the adherent fraction was isolated the following day.
To evaluate the ability of DC to function as APC for VSV in vivo, LDSC were separated into Fc receptor-positive (FcR+) and -negative (FcR−) populations by rosette formation with antibody-coated sheep red blood cells (SRBC). Following this isolation procedure allowed us to examine freshly isolated DC and avoided an overnight incubation in vitro where further antigen processing may have been induced artifactually. To obtain highly purified APC populations, we also incubated adherent LDSC isolated from VSV-infected mice overnight and then subjected the non-adherent population (DC-enriched) to rosette formation. The FcR− (non-rosetting) population was highly enriched for DC (>95% CD11c+ I-Ad+). Highly purified splenic Mφ were isolated from the high-density spleen cell (HDSC) fraction using a similar overnight adherence step to remove contaminating T and B cells. The following day, non-adherent cells (DC-enriched) were gently removed with three washes and the FcR+ adherent cells were isolated by rosette formation. The FcR+ adherent population was typically >95% MOMA-2+ <2% CD11c+ and was used as a source of purified Mφ. Antibodies used for this analysis were; N418/CD11c, GL-1/B7.2, RA3-6B2/CD45R/B220, 145-2C11/CD3ɛ, and MOMA-2/pan Mφ. With the exception of the pan anti-Mφ monoclonal antibody (mAb; clone Moma-2, Serotec, Oxford, UK), all antibodies were obtained from Pharmingen, San Diego, CA.
Assessment of antigen processing in vitro and in vivo
For assessment in vitro, APC were pulsed with VSV(G41) for 3 hr, washed and seeded into 96-well microtitre plates at the indicated concentrations. Each well contained VSV-immune T cells (5 × 105) depleted of accessory cells to ensure that APC function was not due to contaminating APC in the responding T-lymphocyte population.18 To evaluate the ability of different APC subsets to process and present VSV in situ, mice were infected i.p. with 2 × 108−5 × 108 PFU VSV and, at the indicated times, isolated APC populations were added directly to the microculture wells, as described above, to induce recall Th-cell and CTL responses in vitro. No exogenous VSV was added to these cultures.
Analysis of chemokine mRNA
Characterization and quantification of the primary VSV-induced mRNA response was achieved using the RiboQuant Multi-Probe RNase Protection Assay System (Pharmingen) using a mouse chemokine template set (mCK-5). Following RNase treatment, the protected probes were resolved by electrophoresis on a denaturing polyacrylamide gel and detected by autoradiography. Individual mRNA cytokine signals were compared to the signal generated by a house-keeping gene within the same sample to quantify individual chemokine mRNA signals.
Results
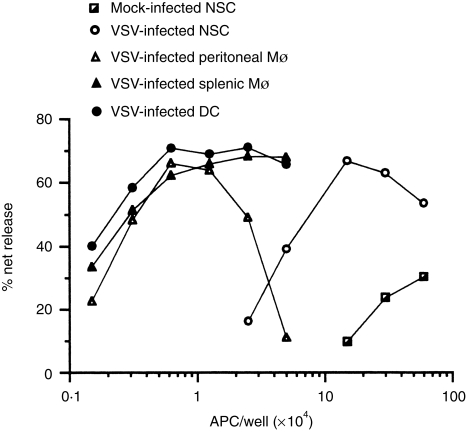
To assess the ability of distinct cell types to function as APC for a complex antigen, splenic Mφ, peritoneal Mφ and splenic DC were purified, pulsed with VSV and then tested for their ability to elicit recall CTL responses in vitro. This analysis clearly established that fractions enriched for splenic, as well as resident peritoneal Mφ, functioned as potent APC for this type of response (Fig. 1). On a per cell basis, both Mφ populations were comparable to splenic DC. Thus, both DC and Mφ generate and display VSV peptide/major histocompatibility complex (MHC) class I complexes following an antigen pulse in vitro and therefore represent potential candidate APC in vivo.
Figure 1.
Antigen-presenting activity of dendritic cells and macrophages assessed in vitro. Splenic Mφ and DC were isolated from normal spleen cells (NSC); whereas, resident peritoneal Mφ were obtained by flushing the peritoneal cavity of non-infected mice with PBS and then isolating the plastic-adherent population. The Mφ- and DC-enriched fractions contained ≥80% of the respective cell type as judged by immunofluorescence using mAb specific for either Mφ (MOMA-2) or DC (N418). Cells were either uninfected (mock) or pulsed with VSV for 3 hr, washed and the indicated number of APC co-cultured with accessory cell-depleted, VSV-immune T cells. On day 5, cultures were tested for CTL activity using radiolabelled,VSV-infected P815 target cells. The following APC were evaluated: mock-infected normal spleen cells; VSV-infected normal spleen cells; VSV-infected peritoneal Mφ; VSV-infected splenic Mφ; and VSV-infected DC. This experiment was repeated four times with similar results.
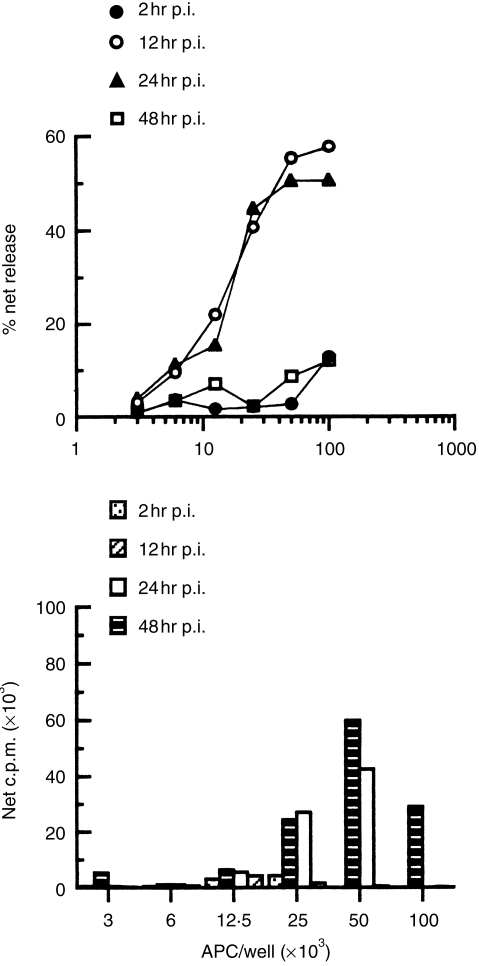
To determine if these cells functioned similarly in vivo, the kinetics of VSV epitope generation following infection with VSV was determined by titrating LDSC, obtained at the indicated times post-infection (p.i.), into cultures containing accessory cell-depleted, VSV-immune T cells. The ability to induce recall VSV proliferative and CTL responses was then assessed in the absence of exogenous virus. Preliminary studies indicated that APC activity was associated with the LDSC fraction but not with the HDSC fraction (see below). Figure 2 illustrates that VSV peptides displayed on class I (Fig. 2a) and II (Fig. 2b) molecules were first detected 12 hr p.i., became optimally expressed in the next 12 hr, and then had diminished by 48 hr. Thus, VSV T-cell epitopes are transiently displayed in a fraction enriched for DC (LDSC).
Figure 2.
Kinetics of in situ antigen processing. Mice were infected i.p. with VSV (2 × 108 PFU/mouse) and at the indicated times, LDSC were isolated by flotation on BSA columns. The indicated number of LDSC were then co-cultured with accessory cell-depleted, VSV-immune T cells. On days 3 and 5, cultures were tested for secondary, VSV-specific proliferative and CTL responses, respectively. (a) Recall CTL responses elicited by LDSC at 2, 12, 24 and 48 hr p.i. with VSV. (b) Recall proliferative responses induced by LDSC at 2, 12, 24 and 48 hr p.i. with VSV. No exogenous virus was added to any of the cultures. The failure to detect VSV-specific proliferation at high input cell numbers reflects, in this particular experiment, unusually high proliferation in cultures containing mock-infected LDSC. This background proliferation has been subjected from all values when the data are expressed as net counts per minute (c.p.m.) incorporated. Similar results were obtained in two additional independent experiments.
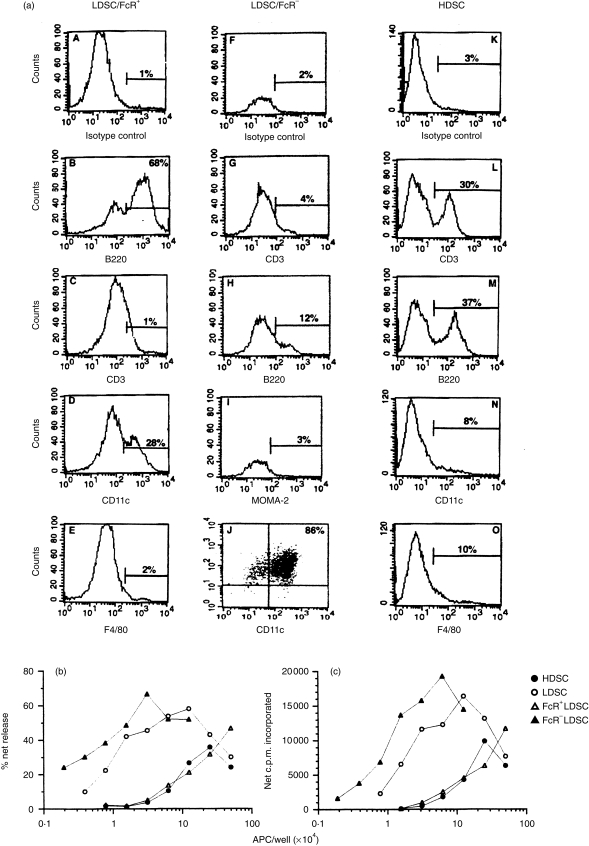
To identify those specific cell type(s) bearing VSV antigenic fragments in situ, LDSC were separated into FcR+ and FcR− populations before being tested in vitro. Most of the cells in the FcR− fraction were CD11c+ B7.2+, a phenotype characteristic of DC (Fig. 3a, panel J). Splenic DC (LDSC/FcR−) isolated from VSV-infected mice induced, in a dose-dependent fashion, potent recall CTL responses (Fig. 3b) and proliferative responses (Fig. 3c). Although the FcR+ fraction, composed primarily of B cells (Fig. 3a, panel B), contained APC activity it was not enriched above the HDSC fraction (Fig. 3b,c) or unfractionated spleen cells (data not presented). This fraction had approximately 10-fold less APC activity on a per cell basis relative to the DC-enriched fraction which probably reflected the presence of contaminating DC (Fig. 3a, panel D). This was confirmed by demonstrating the loss of APC activity following removal of DC from this fraction by plastic adherence for 2 hr at 37° (see Fig. 4). Thus, following infection with VSV, VSV epitopes were displayed on splenic DC in sufficient quantities to induce potent recall responses mediated by CD4+ and CD8+ T lymphocytes.
Figure 3.
Splenic DC are enriched for antigen-presenting activity after an antigen pulse in situ. Mice were injected i.p. with VSV (2 × 108 PFU) and 12–14 hr p.i. their spleens were digested with collagenase. The indicated cell populations were isolated as described in the Materials and methods and aliquots were removed, stained with the indicated mAb and then subjected to flow cytometric analysis to determine the phenotypic composition of each fraction (a). All panels indicate single colour analyses except panel J which represents a double stain (CD11c vs. CD86). The remaining cells were co-cultured with accessory cell-depleted, VSV-immune T cells to generate VSV CTL (b) and Th-cell (c) proliferative responses. The following fractions were evaluated: HDSC, LDSC, FcR+ LDSC and FcR− LDSC. No exogenous VSV was added to the cultures. This experiment was repeated a second time with similar results.
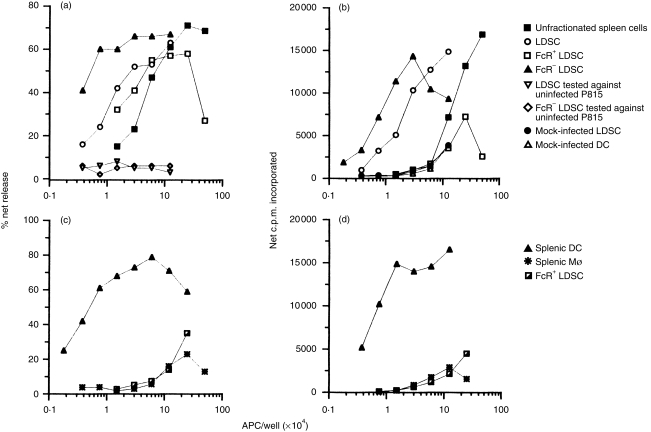
Figure 4.
Specificity of T cells elicited by antigen presenting cells pulsed in vivo. The experiment protocol was essentially identical to that described in Fig. 3. (a) Accessory cell-depleted, VSV-immune T cells were restimulated to elicit recall VSV CTL responses. Unless indicated otherwise, CTL activity was detected with VSV-infected P815 target cells. The following cell populations isolated from mice pulsed in vivo with VSV for 12 hr were evaluated: unfractionated normal spleen cells; LDSC; FcR+ LDSC; FcR− LDSC; LDSC tested against uninfected P815; and FcR− LDSC cells tested against uninfected P815 targets. (b) Recall proliferative response induced by the following cell fractions: mock-LDSC and mock-DC represent LDSC and DC (FcR− LDSC) isolated from uninfected mice, respectively. The other symbols represent the same fractions as in (a). In this experiment, 82% of the FcR− LDSC were N418+ as measured by immunofluorescence microscopy (data not shown). (c,d) Highly purified splenic DC and Mφ, isolated from VSV-infected mice, were obtained by overnight adherence and rosette formation (see the Materials and methods). FcR+ LDSC were depleted of contaminating DC by adherence to obtain purified B cells. This population was >95% B220+ and <2% CDllc+ as assessed by immunofluorescence microscopy. The purified cell populations were then co-cultured with accessory cell-depleted, VSV-immune T cells to elicit recall VSV CTL (c) and proliferative responses (d) in the absence of exogenous virus. Similar results were obtained in three independent experiments.
To assess the specificity and reproducibility of these responses, the experiment presented in Fig. 3 was repeated to include additional controls. The results, presented in Fig. 4(a,b), illustrate that unfractionated spleen cells induced recall CTL and Th-cell responses only at high input cell numbers. Again, FcR+ cells, isolated from the LDSC fraction, contained APC activity. This fraction contained primarily B cells and DC (see Fig. 3) and removal of the latter cell type dramatically reduced the APC activity of this fraction (see Fig. 4c,d). As expected, LDSC were enriched for APC activity and DC purified from this fraction were, on a per cell basis, the most potent APC. For example, substantial CTL and Th-cell responses were generated in cultures containing as few as 3 × 103 DC. These responses were specific for VSV because little cytolytic activity was detected against uninfected, syngeneic target cells (Fig. 4a) or VSV-infected, MHC incompatible EL-4 targets (data not shown). Thus, the effector cells appear to be virus-specific and MHC-restricted, characteristics of anti-viral CTL. A similar specificity pattern was noted for activation of class II-restricted Th cells (Fig. 4b). Mock-infected LDSC or DC failed to induce a significant CD4-mediated proliferative response, even at high input cell numbers, indicating that induction of these responses required prior exposure to VSV in vivo. Because the freshly isolated DC fraction contained a minor population of contaminating cells, we also obtained highly purified splenic DC and Mφ from the adherent LDSC and HDSC fractions, respectively (see the Materials and methods), and assessed their ability to induce VSV recall responses. Highly purifed DC, but not Mφ, induced potent CTL (Fig. 4c) and proliferative (Fig. 4d) responses which were still detectable at 900 input DC/well.
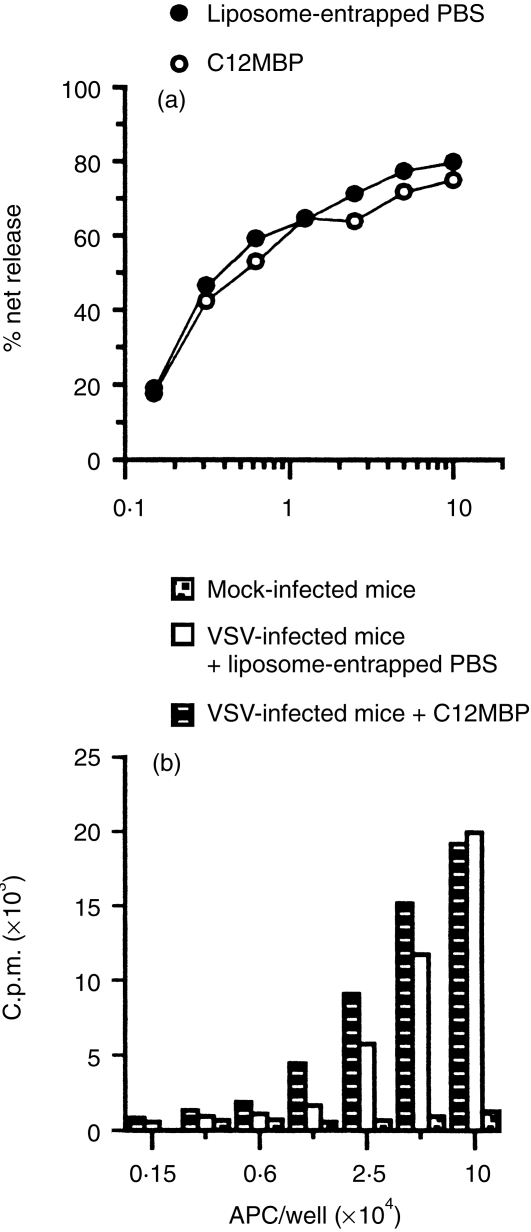
It is possible that following Cl2MBP-liposome treatment, VSV Th cells and CTL do not become primed because the remaining non-phagocytic IDC fail to capture VSV and present VSV peptides. MDC and/or RPM may be required to degrade virus particles and/or proteins (preprocess) prior to final presentation by IDC. To determine if IDC can present VSV peptides independent of MDC and Mφ, IDC were isolated from mice treated with Cl2MBP-liposomes prior to a 14-hr antigen pulse. Figure 5 demonstrates that IDC, isolated from VSV-infected, MDC/Mφ-deficient mice, still induced potent recall CTL (Fig. 5a) and Th-cell responses (Fig. 5b). Thus, these data suggest that IDC capture and process VSV antigens independent of phagocytic cells such as MDC and RPM.
Figure 5.
Interdigitating dendritic cells generate viral peptides in situ independent of marginal dendritic cells and macrophages. Mice were injected with either liposome-entrapped PBS or Cl2MBP and then infected i.p. with VSV (2 × 108 PFU) 24 hr later. Mock-infected animals were injected i.p. with PBS alone. (a) Splenic DC were isolated 14 hr p.i. from mice previously treated with either liposome-entrapped PBS or Cl2MBP and evaluated for their ability to induce recall CTL responses. (b) Recall proliferative responses to VSV induced by DC isolated from either mock-infected (dot-filled bars) mice or VSV-infected mice treated with either liposome-entrapped PBS (open bars) or Cl2MBP (filled bars). The results presented are representative of three experiments.
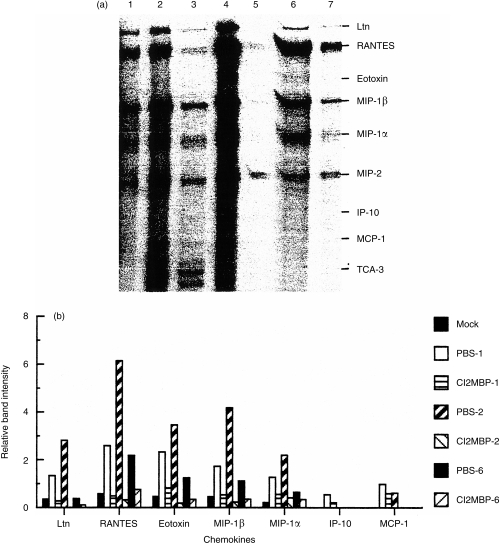
Because IDC can process and present VSV peptides in vivo in the absence of MDC and Mφ, we next considered the possibility that these cells fail to produce sufficient chemokines following infection with VSV to recruit T cells into the T-dependent areas of the spleen. To address this issue, mice were treated with Cl2MBP-liposomes, infected with VSV and, at the indicated times, VSV-induced chemokine mRNA levels were determined in the spleen. As can be seen from Fig. 6, VSV infection induced a potent chemokine response in control mice that quickly (<24 hr) exceeded the signal for a house-keeping gene. For example, 24 hr after infection, mRNA levels were six times greater for RANTES than L32. Acute depletion of phagocytic cells profoundly inhibited this response for all the indicated chemokines, essentially to control levels. Although this response had diminished by 6 days p.i., VSV-infected mice still had elevated chemokine mRNA levels relative to control mice.
Figure 6.
Acute depletion of phagocytic cells dramatically inhibits the primary VSV-induced chemokine response in vivo. Mice were treated with either PBS- or Cl2MBP-entrapped liposomes and then infected with VSV. At the indicated times, total RNA was isolated and the quantity of chemokine mRNA was determined by RNase protection assay. (a) Gel pattern of protected cytokine mRNA species. Lanes 2, 4 and 6 represent chemokine profiles of control (PBS-liposomes) mice obtained 1, 2 and 6 days, respectively, after infection with VSV. Lanes 3, 5 and 7 represent chemokine profiles of mice treated with Cl2MBP-liposomes obtained 1, 2 and 6 days, respectively, after infection with VSV. Lane 1 represents the constituitive chemokine mRNA levels detected in uninfected (mock) mice. (b) Quantification of chemokine response in control mice and mice treated with Cl2MBP-liposomes. Relative band intensity was determined by dividing chemokine band density by the house-keeping gene (L32) band intensity. The numbers after the treatment groups refer to the day total RNA was isolated following infection with VSV. Data are representative of two independent experiments.
Discussion
We previously reported that recall Th cells and CTL responses to VSV were markedly suppressed when mice were primed in the absence of Mφ.18 Normal T-cell priming returned at a time when a particular Mφ subset, namely RPM, reappeared in the spleen, suggesting that this Mφ subpopulation played a critical role in the priming event. Several mechanisms can be envisaged which may render priming Mφ-dependent. For example, Mφ may directly regulate T-cell responses because they function as the critical APC in vivo. If this view is correct, then Mφ should process and present VSV epitopes following infection with VSV. In the present study, we tested this hypothesis by evaluating the APC activity of splenic and peritoneal Mφ. Following an in vitro antigen pulse, both Mφ populations, as well as splenic DC, induced a potent recall CTL response to VSV. Thus Mφ satisfied this minimum criterion for APC function. In contrast, in situ antigen-processing studies indicated that APC activity fractionated with FcR− LDSC, a phenotype characteristic of DC. HDSC, the fraction where Mφ localized, contained relatively little APC activity. Indeed, Mφ purified from this fraction failed to elicit recall responses, indicating that these cells did not display VSV peptides/class I and II complexes in vivo. Although APC activity was detected in the FcR+ low-density fraction containing primarily B cells, the activity in this fraction was most probably mediated by DC contamination. This view is supported by studies demonstrating that removal of DC by adherence markedly reduced APC activity in this fraction. Furthermore, Brundler et al.28 demonstrated that T-cell priming to VSV, as well as vaccinia virus and lymphocytic choriomeningitis virus, was normal in B-cell-deficient mice, indicating that B cells were not critical for priming VSV T cells in vivo. Thus, splenic DC, but not Mφ (including RPM), display VSV peptide/class I and II complexes and therefore function as APC in situ.
T-cell priming may be still rendered Mφ-dependent by an indirect mechanism. Thus, epitopes buried within virions may require prior Mφ (pre)processing before regurgitated viral fragments can be presented by DC. This occurs during infection with Listeria monocytogenes, where interstitial lung Mφ augment T-cell activation by providing DC with bacterial-derived antigenic fragments.29 However, our results demonstrate that depletion of phagocytic cells does not prevent IDC-induced recall responses, indicating that MDC/RPM are not necessary for IDC to generate antigenic peptides. This result is not consistent with the view that antigen is captured only in the marginal sinus and transported to the white pulp by MDC for T-cell presentation. At least in the VSV model, viral antigens can be trapped, processed and displayed by IDC in the absence of phagocytic cells including MDC. Trapping of viral antigens in the white pulp following depletion of phagocytic cells with Cl2MBP-liposomes has also been recently reported for rabies virus, another rhabdovirus.30
The suicide technique used to deplete phagocytic cells eliminates several splenic Mφ subpopulations as well as MDC, and these cell types are known to be sources of a number of chemokines. IDC may be dependent on one of the depleted phagocytic populations to provide the necessary chemokines to recruit naive VSV T cells into the T-dependent areas of the spleen. Studies presented herein document that acute depletion of MDC/Mφ profoundly inhibits the VSV-induced chemokine response, suggesting that IDC do not contribute significantly to this response. Preliminary studies implicate a slowly repopulating cell population, such as MMM and MZM, as the source of VSV-induced chemokines because this response is still markedly reduced 14 days after liposome treatment (data not shown). However, it should be noted that we currently do not know which chemokine(s) is critical for recruitment of naive VSV T cells. Further studies will be required to establish which chemokines are biologically relevant for generating a primary anti-VSV CTL and Th-cell cytokine response in vivo.
In summary, we have demonstrated that following infection with VSV, viral peptide/class I and II complexes were detected within 12 hr on splenic DC; however, expression of VSV peptides recognized by CTL and Th cells was transient. These results are in sharp contrast with studies demonstrating that B-cell epitopes can still be detected weeks following infection with VSV.31 Although peritoneal and splenic Mφ presented VSV epitopes following an antigen pulse in vitro, splenic Mφ, isolated from VSV-infected mice, displayed little APC activity, suggesting that Mφ do not present VSV antigens to Th cells or CTL in situ. Furthermore, the failure of Cl2MBP-liposome treated mice to prime VSV T cells was not due to depletion of all splenic APC subsets since IDC displayed abundant viral antigens in vivo. The inability of IDC to prime VSV T cells may be related to insufficient chemokine production and the failure to recruit naive T cells into the T-dependent areas of the spleen. This indicates that VSV-induced chemokines are provided by one of the depleted cell populations. Future studies should assess the contribution of MDC and distinct Mφ subsets to the VSV-induced chemokine response.
Acknowledgments
These studies were supported by the Eastern Virginia Medical School Institutional Grant 86371 (to R.P.C.) and a Jeffress Memorial Trust Research Grant (to R.P.C.).
Abbreviations
- APC
antigen-presenting cell
- C12MBP
dichloromethylene bisphosphonate
- CTL
cytotoxic T lymphocyte
- FcR
Fc receptor
- HDSC
high-density spleen cells
- IDC
interdigitating dendritic cell
- LDSC
low-density spleen cells
- Mφ
macrophage
- MDC
marginal dendritic cell
- MMM
marginal metallophilic macrophage
- MZM
marginal zone macrophage
- PALS
periarteriolar lymphoid sheaths
- p.i.
post-infection
- RPM
red pulp macrophage
- SRBC
sheep red blood cells
- Th cell
helper T cell
- VSV
vesicular stomatitis virus
References
- 1.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 2.van Rooijen N, Sander. A. Liposome mediated depletion of macrophages: mechanism of action, preparation ofliposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 3.van Rooijen N, Van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of lipsomes-encapsulated dephosphonate. An enzyme-histochemical study. Cell Tiss Res. 1984;238:355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- 4.Claassen I, van Rooijen N, Claassen E. A new method for removal of mononuclear phagocytes from heterogeneous cell populations in vitro, using the liposome-mediated macrophage “suicide” technique. J Immunol Methods. 1990;134:153–61. doi: 10.1016/0022-1759(90)90376-7. [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Bunting AM, Rouse R, van Rooijen N, Huang L, Rouse BT. Role of macrophages and dendritic cells in primary cytotoxic T lymphocyte responses. Int Immunol. 1995;4:679–88. doi: 10.1093/intimm/7.4.679. [DOI] [PubMed] [Google Scholar]
- 6.Cua DJ, Hinton DR, Kirkman L, Stohlman SA. Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur J Immunol. 1995;25:2318–24. doi: 10.1002/eji.1830250830. [DOI] [PubMed] [Google Scholar]
- 7.Matsushima GK, Stohlman SA. Distinct subsets of accessory cells activate Thy-1+ triple negative (CD3–, CD4–, CD8–) cells and Th-1 delayed type hypersensitivity effector T cells. J Immunol. 1991;146:3322–31. [PubMed] [Google Scholar]
- 8.Delemarre FGA, Kors N, van Rooijen N. Elimination of spleen and of lymph node macrophages and its difference in the effect on the immune response to particulate antigens. Immunobiology. 1990;182:70–8. doi: 10.1016/S0171-2985(11)80584-X. [DOI] [PubMed] [Google Scholar]
- 9.Brewer JM, Richmond J, Alexander J. The demonstration of an essential role for macrophages in the in vivo generation of IgG2a antibodies. Clin Exp Immunol. 1994;97:164–71. doi: 10.1111/j.1365-2249.1994.tb06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buiting AMJ, DeRover Z, Kraal G, van Rooijen N. Humoral immune responses against particulate bacterial antigens are dependent on marginal metallophilic macrophages in the spleen. Scand J Immunol. 1996;43:398–405. doi: 10.1046/j.1365-3083.1996.d01-54.x. [DOI] [PubMed] [Google Scholar]
- 11.Karupiah GR, Buller RMML, Van Rooijen N, Duarte CJ, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–9. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leenen PJM, de Bruijin FTR, Voerman JSA, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 13.Leenen PJM, Radosevic K, Voerman JSA, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers and subpopulation turnover. J Immunol. 1998;160:2166–73. [PubMed] [Google Scholar]
- 14.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciavarra RP, Forman J. H-2Ld-restricted recognition of viral antigens. In the H-2d haplotype, anti-vesicular stomatitis virus cytotoxic T cells are restricted solely by H-2L. J Exp Med. 1982;156:778–90. doi: 10.1084/jem.156.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiss CS, Evans GA, Marguleis DH, Seidman JG, Burakoff SJ. Allospecific and virus-specific cytolytic T lymphocytes are restricted to the N or C1 domain of H-2 antigens expressed on L cells after DNA-mediated gene transfer. Proc Natl Acad Sci USA. 1983;80:2709–12. doi: 10.1073/pnas.80.9.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal KL, Oldstone BA, Hengartner J, Zinkernagel RM. Specificity of in vitro cytotoxic T cell clones directed against vesicular stomatitis virus. J Immunol. 1983;131:475–8. [PubMed] [Google Scholar]
- 18.Ciavarra RP, Buhrer K, van Rooijen Tedeschi B. T cell priming against vesicular stomatitis virus analyzed in situ. Red pulp macrophages, but neither marginal metallophilic nor marginal zone macrophages, are required for priming CD4+ and CD8+ T cells. J Immunol. 1997;158:1749. [PubMed] [Google Scholar]
- 19.Ciavarra RP, Kang CY, Forman J. Vesicular stomatitis antigens recognized by cytotoxic cells. analysis with defective interfering particles and reconstituted membrane vesicles. J Immunol. 1980;125:336–43. [PubMed] [Google Scholar]
- 20.Maravaldi JL, Lucas-Lenard J, Sekellick MJ, Marcus PI. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus requires the same gene functions. Virology. 1977;79:267–80. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- 21.Sekellick MJ, Marcus PI. Persistent infection. II. Interferon-inducing temperature sensitive mutants as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1979;95:36–47. doi: 10.1016/0042-6822(79)90399-4. [DOI] [PubMed] [Google Scholar]
- 22.Eikelenboom P. Characterization of non-lymphoid cells in the white pulp of the mouse spleen: an in vivo and in vitro study. Cell Tiss Res. 1978;195:445–60. doi: 10.1007/BF00233888. [DOI] [PubMed] [Google Scholar]
- 23.van Rooijen N, Kors M, van der Ende M, Dijkstra CD. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990;260:215–22. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- 24.Ciavarra RP, Tedeschi B. Priming antiviral cytotoxic T lymphocytes: Requirement for CD4+ cells is dependent on the antigen presenting cell in vivo. Cell Immunol. 1994;157:132–43. doi: 10.1006/cimm.1994.1211. 10.1006/cimm.1994.1211. [DOI] [PubMed] [Google Scholar]
- 25.Ciavarra RP, Silvester S, Brody S. Analysis of T-cell subset proliferation at afebrile and febrile temperatures: differential response of Lyt-1+23– lymphocytes to hypothermia following mitogen and antigen stimulation and its functional consequence on development of cytotoxic lymphocytes. Cell Immunol. 1987;107:293–306. doi: 10.1016/0008-8749(87)90238-3. [DOI] [PubMed] [Google Scholar]
- 26.Ciavarra RP, Burgess DH. Antigen-presenting B cells: efficient uptake and presentation by activated B cells for induction of cytotoxic T lymphocytes against vesicular stomatitis virus. Cell Immunol. 1988;114:27–40. doi: 10.1016/0008-8749(88)90252-3. [DOI] [PubMed] [Google Scholar]
- 27.Swiggard WJ, Nonacs RM, Witmer-Pack MD, Inaba K, Steinman RM. Isolation of dendritic cells. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: John Wiley and Sons; 1998. pp. 3.7.1–3.7.10. [Google Scholar]
- 28.Brundler MA, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel RM. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–62. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 29.Gong JL, McCarthy KM, Rogers RA, Schneeberger EE. Interstitial lung macrophages interact with DC to present antigenic peptides derived from particulate antigens to T cells. Immunology. 1994;81:343–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Claassen IJTM, Osterhaus ADME, Poelen M, van Rooijen N, Claassen E. Antigen detection in vivo after immunization with different presentation forms of rabies virus antigen, II. cellular, but not humoral, systemic immune responses against rabies virus immune-stimulating complexes are macrophage dependent. Immunology. 1998;94:455–60. doi: 10.1046/j.1365-2567.1998.00539.x. 10.1046/j.1365-2567.1998.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–69. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]