Abstract
When the developing embryo implants into the uterine wall, resident maternal immune cells may encounter antigens present on the fetal tissues. The nature and constituents of the ensuing maternal immune response, and its regulation, are of considerable interest in understanding normal and abnormal pregnancy. Here, we report the presence of natural killer (NK)1.1+ αβ T cells in the murine periimplantation uterus. These cells account for a large portion of both the T-cell and natural killer cell populations in early pregnancy, while their numbers in the non-pregnant uterus and later in pregnancy are greatly reduced. Phenotypically, these NK1.1+ αβ T cells belong to a previously described subset of cells that bear a Vα14-Jα281-encoded T-cell receptor. Unlike other organs, where both CD4+ and CD4−/CD8− NK1.1+ αβ T cells are found, the placental/decidual population appears to be entirely CD4−/CD8−. The Vβ repertoire of the placental/decidual population is also altered from that of other organs, with a majority of cells expressing Vβ3. Together, these features suggest the possibility of local development. NK1.1+ αβ T cells are known to recognize the class I-like CD1 molecule. Consistent with this association, we demonstrate CD1 expression by tissues within the pregnant uterus. Our findings define an additional organ-specific immune environment where NK1.1+ αβ T cells may play a role, and continue to demonstrate the specialized nature of the maternal intrauterine immune system during pregnancy.
Introduction
At implantation, fetally derived cells invade the uterine tissues, and ultimately form the placenta. The uterine stroma, known as decidua in humans, and which forms a more anatomically specialized structure in rodents known as the metrial gland,1,2 is known to contain numerous maternal immune cells.3 Despite the immunologically foreign nature of the fetal tissues, invasion and proliferation normally proceed successfully.
Recent studies indicate several interesting aspects of maternal–fetal immunology that may be important in resolving this apparent paradox. For example, major histocompatibility complex (MHC) class I molecule expression by the trophoblasts, the fetal cells most likely to be encountered by maternal immune cells, is highly regulated, with reduced or absent expression of polymorphic MHC class I and class II molecules.4–6 Whereas expression of the oligomorphic class-I-like human leucocyte antigen (HLA)-G molecule by human trophoblasts is well characterized,7 elucidation of other MHC/MHC-like molecules that may be expressed by murine trophoblasts is incomplete.5,6
Secondly, the cytokine milieu of pregnancy in both mice and humans appears to be skewed towards a T helper 2 (Th2) type environment, with high levels of interleukin-4 (IL-4) and reduced levels of interferon-γ (IFN-γ), and studies suggest this skewing is important for pregnancy maintenance.8–11
Studies by ourselves and others have also indicated the presence of specialized immune cell populations within the pregnant uterus, including γδ T cells and natural killer (NK) cells,12–15 in addition to αβ T cells and macrophages.3
Here we demonstrate the presence of another specialized immune cell population within the pregnant uterus, NK1.1+ αβ T (NKT) cells, a novel T-cell subset previously reported in the thymus, liver, bone marrow, and other organs.16–18 NKT cells populate the periimplantation uterus in a highly temporally regulated fashion, and are distinct from previously described uterine NK (uNK) cells, which are CD3 – by definition.12,13 The uterine NKT cells described here are CD3+ and bear a Vα14-Jα281-encoded T-cell receptor (TCR),16 known from previous studies to recognize the MHC class I-like CD1 molecule.19 In this regard, we also report here expression of CD1 within the pregnant uterus. Interestingly, the uterine population of NKT cells is entirely CD4−/CD8− (double negative, DN), whereas other NKT cell populations contain both CD4+ and DN cells.16–18 These uterine NKT cells also appear to express different Vβ phenotypes from other described NKT cell populations. Together, these differences suggest the possibility of in-situ development of a unique NKT cell lineage. In addition to other possible functions,20 NKT cells are know to secrete large amounts of IL-4 upon engagement of the TCR,21 a function that may be important in inducing the Th2 environment critical for pregnancy success. Our findings provide an additional example of the specialized nature of maternal intrauterine immune system, and suggest possible functions for NKT cells in pregnancy.
Materials and methods
Mice
Six to 8-week-old, pathogen-free C57Bl/10, and BALB/c mice were originally obtained commercially from Jackson Laboratories (Bar Harbor, ME) and Harlan Spraguef–Dawley (Indianapolis, IN). CD1 knockout (CD1−, generated as described22) males and females, non-pregnant virgin C57Bl/10 and timed pregnant C57Bl/10 females bred with BALB/c males (referred to as timed pregnant [C57Bl/10 × BALB/c] F1 females) mice were raised and cared for in the animal care facility at Swedish Medical Center, Denver, CO. All experiments were approved by the HealthONE Institutional Animal Care and Utilization Committee, which acts for Swedish Medical Center.
Cell preparation
Maternal and fetal tissues from the pregnant uterus were obtained at three time points of pregnancy: early (days 6–8 [day of plug detection = day 0]), middle (days 12–13) and late (days 16–18) pregnancy, with implantation beginning around day 4·5. Mice were killed using rapid cervical dislocation or CO2 inhalation and placental/decidual tissues were collected, placed in a Cellector tissue sieve (VMR Scientific Productions, Willard, OH), and mechanically dispersed into balanced salt solution (BSS) medium. In the early pregnancy experiments, the embryo cannot easily be separated from the other tissues, and so was included in the tissue preparations. Because the embryo does not contain lymphoid cells at this time, its inclusion will not alter the results. The fetus was removed prior to tissue processing in middle and late pregnancy experiments. Because of the intimate apposition of the fetal trophoblasts and maternal tissues, they cannot be easily separated, and circulating cells, especially within the highly perfused middle and late pregnancy placenta, may contaminate the tissue preparations. In preliminary middle and late pregnancy experiments, we carefully dissected away as much of the placenta as possible, and found that absolute and relative numbers of T and NK cells thus obtained did not significantly change from those obtained when the entire placenta was included, indicating that contamination by circulating cells is not a significant problem. For simplicity, at all gestations, we refer to the fetal and maternal tissues thus obtained as ‘placenta/decidua’.
Spleens and thymi were obtained from the same pregnant mice and similarly prepared. Placental/decidual cells, splenocytes, or thymocytes were centrifuged at 250 g for 5 min, cell pellets were resuspended in 1 ml of BSS and 3 ml of Gey's solution (0·155 m NH4CL and 0·01 m KHCO3) for 5 min at room temperature to lyse red blood cells (RBC), washed with BSS, resuspended in 2 ml of BSS/5% fetal calf serum (FCS; Sigma, St. Louis, MO), and lymphocytes enriched over nylon wool as previously described.23 For CD1 staining by flow cytometry, cells were prepared in a similar fashion, but the nylon wool purification step was omitted. Instead, cells were passed rapidly over a smaller nylon wool column simply to remove tissue fragments.
Monoclonal antibodies and cytofluorographic analysis
Monoclonal antibodies (mAb) were generated as cell culture supernatants or ascites from existing cell lines, purified by affinity chromatography on protein A or G-Sephadex G25 columns (Sigma, St. Louis, MO), concentrated, dialysed, and conjugated with N-hydroxysuccinoimidobiotin (Sigma) or fluoroscein isothiocyanate (FITC) (Sigma). Antibodies used include 145-2C11 (anti-CD3ɛ chain),24 H57.597 (anti-pan TCR αβ),25 GK1.5 (anti-CD4),26 53.6.7 (anti-CD8),27 and PK136 (anti-NK1.1).28 CMS-5 (anti-Vα14, biotin- and phycoerythrin (PE)-conjugated) was a generous gift from Dr M. Taniguchi and Dr M. Amano, Chiba University, Japan.29 Anti-CD1 mAb 5C64 and 3H3, developed in Dr C.-R. Wang's laboratory at University of Chicago (22 and M. Mandal and C.-R. Wang, unpublished data) were obtained from culture supernatants and used unconjugated. Commercially available antibodies used include 1B1 (anti-CD1.1), 145.2C11 (anti-CD3ɛ chain, PE-conjugated), GK1.5 (anti-CD4, PE-conjugated), PK-136 (anti-NK1.1, PE conjugated) and Ly-2 (anti-CD8α, PE-conjugated; PharMingen, San Diego, CA), and goat anti-hamster immunoglobulin G (IgG; PE-conjugated; Caltag, San Francisco, CA). A panel of FITC-conjugated anti-Vβ mAb including B20.6 (anti-Vβ2), KJ25 (anti-Vβ3), KT4 (anti-Vβ4), MR9-4 (anti-Vβ5.1/2), RR47 (anti-Vβ6), TR310 (anti-Vβ7), MR5-2 (anti-Vβ8.1/2), 1B3.3 (anti-Vβ8.3), MR10-2 (anti-Vβ9), B21.5 (anti-Vβ10), RR3-15 (anti-Vβ11), MR11-1 (antiVβ12), MR12-3 (anti-Vβ13), 14-2 (anti-Vβ14) and KJ23 (anti-Vβ17) were also obtained commercially (PharMingen). For cytofluorographic analysis, cells (0.1–1 × 106/well in 96-well plates) were preincubated with rat anti-mouse FcγR antibody (24G2, 1 µg/ml)30 to block non-specific binding, one-, two-, or three-colour staining was performed, and cells were analysed. In experiments using unconjugated primary antibodies, neat normal mouse serum was used to block non-specific binding instead of anti-FcγR. In two- and three-colour experiments, biotinylated antibodies were developed with streptavidin–PE (Caltag) and streptavidin–spectral red (Southern Biotechnology, Birmingham, AL), respectively.
Cells were analysed by flow cytometry using a Becton-Dickinson FACSort cytometer (San Jose, CA), and data plots were generated using the CELLQuest version 1.2 software package supplied by the manufacturer. In all experiments, set up and calibration were performed with nylon-wool prepared splenocytes, and these cells were also used to set gates for thymocytes and decidual lymphocytes. In all cases, appropriate negative and isotype control experiments were performed to verify staining specificity.
Cell sorting and hybridoma production
Placental/decidual cells were obtained from (C57Bl/10 × BALB/c)F1 gestations at early gestations, as described, NK1.1+ cells were sorted by flow cytometry after single-colour staining, and plated in 100 µl of culture medium with 10% FCS in anti-CD3 mAb (10 µg/ml) precoated 96-well plates. IL-2 (10 units/ml) was added into the culture medium the following day. After 4–6 days with media changes as appropriate, the cells were fused with the α-β-variant of the AKR BW5147 cell line,31 as previously described.32 The fusion products were plated in 96-well plates, and selected in hypoxanthine/aminopterin/thymidine (HAT) medium. The generated hybridomas were screened for expression of αβ TCR by flow-cytometry, and the αβ-TCR+ hybridomas were further analysed with anti-Vα14. Four Vα14+ hybridomas were identified.
Reverse transcription–polymerase chain reaction (RT–PCR) and sequence analysis
Placental/decidual tissues obtained from early pregnant mice were mechanically dispersed into BSS medium, centrifuged, and total cellular RNA extracted from the cell pellet using RNA STAT-60 (TEL-TEST, Friendswood, TX) according to the manufacturer's direction. Total cellular RNA was also extracted from the four Vα14+ hybridomas with RNA STAT-60. cDNA was synthesized from total cellular RNA using 3′Cα ext primer and superscript RNase H-reverse transcriptase (Life Technology, Grand Island, NY), and first PCR was carried out using the synthesized cDNA as template and the 3′Cα int and 5′Vα14 primers. The PCR products were electrophoresed in 2% agarose gel (FMC, Rockland, ME) to confirm the existence of the expected size DNA band, and used as template for secondary PCR, performed using 3′Jα281 and 5′Vα14 as primers. The primer sequence was as follows. 3′Cα ext–AGATTCCATGGTTTTCGGCAC; 3′Cα int–ACTGAATTCGGTACACAGCAGCAGGTTCTG; 5′Vα14–TAAGCACAGCACGCTGCA CAT; and 3′Jα281–CAGGTATGACAATCAGCTGAGTCC. All primers were produced and purified by gel filtration by the Midland Certified Reagent Company (Midland, TX).
The final PCR products were cloned into a plasmid vector (pCR 2.1) using the TA-cloning kit (Invitrogen, Carlsbad, CA). Nucleotide sequences were determined by using AmpliTaq FS cycle sequencing with two ABI automated fluorescent sequencing instruments. M13/pUC forward was employed as the primer.
Results
High relative frequencies of NKT lymphocytes in early pregnancy
In preliminary experiments, we analysed the T lymphocyte population in placental/decidual tissues of early (days 6–8), middle (days 12–13) and late (days 16–18) (C57Bl/10 × BALB/c)F1 gestations. As previously reported by ourselves and others, αβ T cells comprise a significant cellular population in the placenta/decidua throughout murine pregnancy.6,14
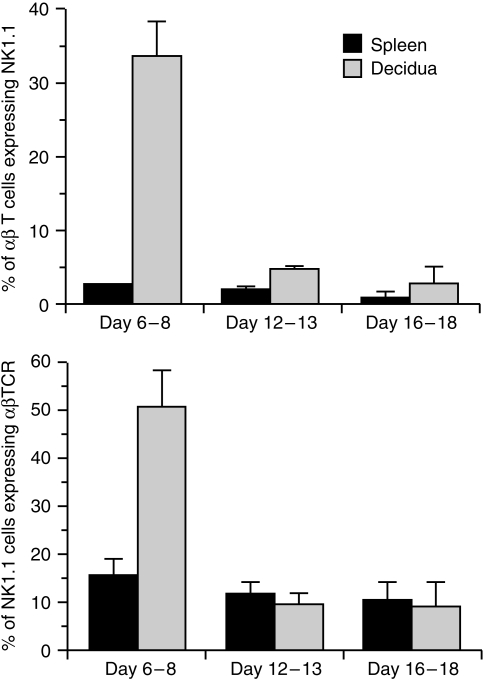
Upon further study of the αβ-TCR+ lymphocyte population, we found that about one third of these cells express the cell marker NK1.1 early in pregnancy (Fig. 1, top), a percentage 13-fold higher than that present in spleen, and similar to that noted in other organs rich in NKT cells, such as thymus, bone marrow, and liver.16–18,20 As pregnancy progresses, the proportion of αβ-TCR+ cells co-expressing NK1.1 in placenta/decidua decreases dramatically, so that by middle and late pregnancy it has fallen to that of spleen.
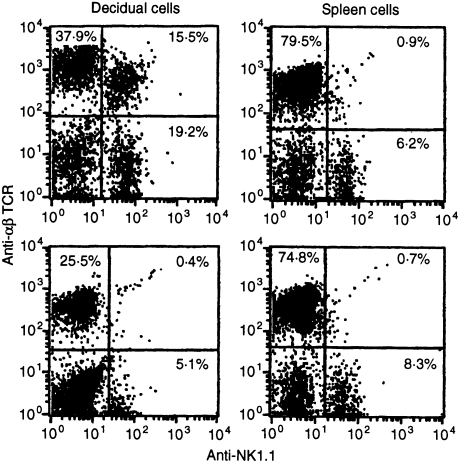
Figure 1.
Results of cytofluorographic analysis of NKT cells in pregnant mice at days 6–8, days 12–14, and days 16–18. FITC-conjugated PK-136 (anti-NK1.1) and biotin-conjugated H57.597 (anti-αβ TCR) were used to measure the percentage of αβ TCR+ cells co-expressing NK1.1 (top), and the percentage of NK1.1+ cells co-expressing αβ TCR (bottom). The mean ± standard deviation was obtained from four mice. Representative staining data are depicted in Fig. 2.
We also analysed the placental/decidual NK1.1+ cell population to see what proportion was accounted for by cells co-expressing the αβ-TCR. We found that approximately half of NK1.1+ cells express αβ-TCR in early pregnant placenta/decidua, a threefold increase over that found in spleen (Fig. 1, bottom). In mid and late gestation, the proportion of NK1.1+ cells expressing the αβ-TCR decreases to that of the spleen. Representative cytofluorographic data from early and late pregnancy are shown in Fig. 2.
Figure 2.
Representative staining date of NKT cells at early and late gestation. FITC-conjugated PK136 (anti-NK1.1) and biotin-conjugated H57.597 (anti-αβ-TCR) were used to analyse placental/decidual cells (left) or splenocytes (right) at early (top) or late (bottom) gestation. Percentages indicated are relative to the total number of cells in all four quadrants. The mean anti-αβ-TCR staining log fluorescence of NKT cells is 39% ( ± sd 2%, data obtained from four mice, absolute data not shown) that of conventional αβ T cells, indicating these cells are TCRint.
For comparison, we also studied proportions of NKT cells in non-pregnant uteri. Small cell numbers precluded precise cytofluorographic analysis. Twenty to 25% of NK1.1+ cells appear to be αβ-TCR+, and 17–23% of αβ TCR+ cells co-express NK1.1 (data not shown). The absolute number of T cells present in the early pregnant uterus is increased about 20-fold over that in the non-pregnant uterus,14 indicating that the total NKT cell population expands at least 40-fold from non-pregnant to early pregnant uterus.
Together, these results demonstrate a dramatic, temporally regulated increase in the proportion of NK1.1+ αβ T cells at periimplantation.
Characterization of NK1.1+ αβ T cells in early pregnancy
Since several NKT cell populations have been described,33 the next set of experiments was designed to further categorize placental/decidual NKT cells.
We noted a threefold reduction in surface density of αβ-TCR by NKT cells as compared with conventional placental/decidual αβ T cells (Fig. 2), a result similar to that reported with NK1.1+ αβ-TCR+ thymocytes and referred to as ‘TCRint’.
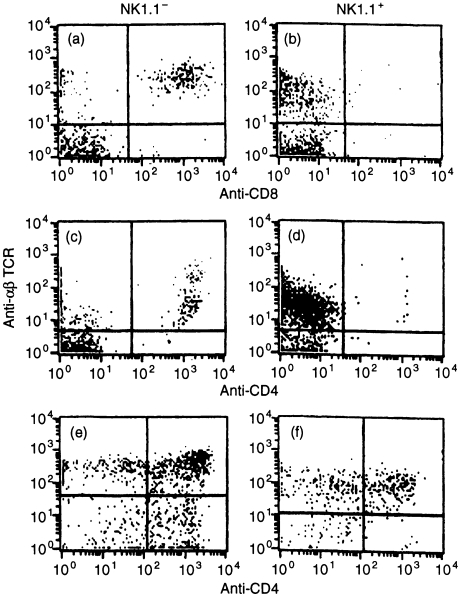
NK1.1+ αβ-TCR+ cells found in the thymus, liver, and bone marrow are either CD4+ or DN,16–18,20 although one report indicates predominantly DN cells in bone marrow.29 By contrast, NK1.1+ αβ-TCR+ placental/decidual cells are uniformly CD4−, whereas NK1.1+ αβ-TCR+ thymocytes contain a substantial CD4+ population, and NK1.1-αβ TCR+ cells in the placenta show typical CD4 and CD8 expression (Fig. 3). Possible explanations for this altered accessory molecule expression are discussed below.
Figure 3.
Expression of CD4 and CD8 molecules by NK1.1+ and NK1.1− uterine and thymic T cells. Placental/decidual cells from a day 7 pregnancy (a–d) or from thymus (e–f) were prepared as described and analysed in a three-colour experiment, using FITC-conjugated PK136 (anti-NK1.1), PE-conjugated GK1.5 and Ly-2 (anti-CD4 and anti CD8α), and biotin-conjugated H57.597 (antiαβ-TCR). NK1.1 negative (left) and positive (right) cells were gated and further analysed for expression of αβ-TCR and CD8 (a, b) and CD4 (c–f). Five mice were studied in each experiment; representative data are shown.
The majority of NK1.1+ αβ-TCR+ thymocytes manifest a single Vα gene rearrangement (Vα14-Jα281) rarely found in conventional αβ T cells.16,34 To further characterize the TCR repertoire of NKT cells in early pregnancy, we studied their Vα14 expression.
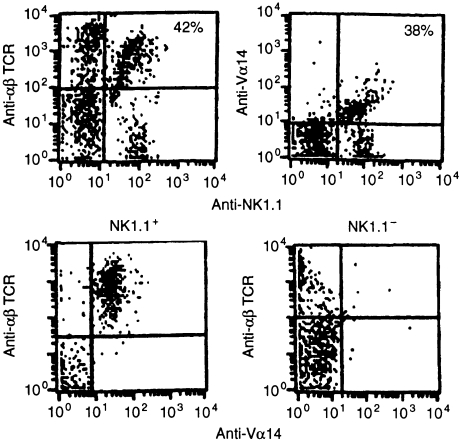
In two-colour staining experiments of early pregnancy tissues, we found percentages of NK1.1+ cells co-expressing αβ-TCR and percentages of cells co-expressing Vα14 to be similar, suggesting uniform expression of Vα14 by NKT cells (Fig. 4, top). To directly confirm this, we performed three-colour experiments gated on NK1.1+ and NK1.1− cells. Amongst NK1.1+ cells, all αβ-TCR+ cells are Vα14+, while amongst NK1.1− αβ-TCR+ cells; few Vα14+ cells are found (Fig. 4, bottom).
Figure 4.
Two- and three-colour analysis of Vα14 expression by NKT cells. Cells from early placenta/decidua were stained with FITC-conjugated PK136 (anti-NK1.1), PE-conjugated CMS-5 (anti-Vα14), or biotin-conjugated H57.597 (anti-αβ-TCR), as indicated, in two- and three-colour experiments. Percentages are the percent of NK1.1+ cells staining positive for αβ-TCR, or Vα14, as indicated. Top: Similar percentages of αβ-TCR+ (left, 42%) and Vα14+ (right, 38%) cells were found among nylon-wool purified NK1.1+ placental/decidual cells, indicating essentially uniform expression of Vα14 by NK1.1+ αβ-TCR+ cells. Bottom: NK1.1 positive (left) or negative (right) cells were gated and further analysed as indicated. Amongst NK1.1+ cells, all αβ-TCR+ cells were also Vα14+. Amongst NK1.1− cells, there were very few Vα14+ αβ-TCR+ cells noted. Three mice were studied in each experiment; representative data are shown.
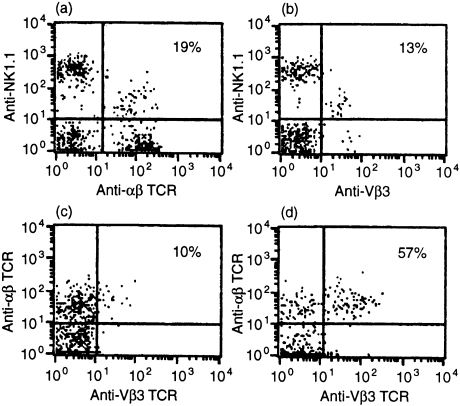
Cytofluorographic analysis of the four Vα14+ hybridomas reveals one each as expressing Vβ3, Vβ6, Vβ8 (but not Vβ8.3) and Vβ13 (data not shown). Cytofluorographic analysis of uterine NKT cells indicates that approximately 57–68% express Vβ3, a Vβ chain present on one of the four Vα14+ hybridomas (Fig. 5). There appear to be very small numbers of uterine NKT cells expressing Vβ7 and Vβ8, two of the Vβ chains commonly found in NKT cells in other organs,20 and also very small numbers of Vβ6+and Vβ13+NKT cells (data not shown). These data are summarized in Table 1. Apparent differences in Vβ expression as compared with other NKT cell populations may, like the altered CD4 expression by these cells noted above, suggest independent development, as discussed below.
Figure 5.
Two- and three-colour analysis of Vβ3 expression by NKT cells. Cells from early placenta/decidua were stained with PE-conjugated PK136 (anti-NK1.1), biotin-conjugated H57.597 (anti-αβ-TCR) and FITC-conjugated KJ25 (anti-Vβ3). In this experiment, 19% of NK1.1+ cells express αβ-TCR (a), and 13% express Vβ3 (b), indicating that approximately 68% (calculated, 13/19) of NKT cells express Vβ3. Amongst NK1.1− cells (c), 10% co-express αβ-TCR and Vβ3, while amongst NK1.1+ cells, 57% co-express αβ-TCR and Vβ3. Three mice were studied in each experiment; representative data are shown.
Table 1.
Summary of Vβ expression by uterine NKT cell hybridomas and lymphocytes
| Cell | Vβ3 | Vβ6 | Vβ7 | Vβ8 | Vβ13 | Vβ2,4,5,9–12,14,17 |
|---|---|---|---|---|---|---|
| Uterine NKT cell hybridomas | 1 of 4 | 1 of 4 | None | 1 of 4 | 1 of 4 | None |
| Uterine NKT lymphocytes | 57–68% | + | + | + | + | None |
+The presence of a small number of cells, with small cell numbers precluding the precise quantitation of percentages.
Verification of Vα14-Jα281 encoded TCR expression by NKT cells in early pregnancy
The above experiments indicate that the NK1.1+ αβ T cells found in early pregnancy are phenotypically similar to TCRint, DN, Vα14+ thymocytes. Another important feature of these NK thymocytes is their invariant usage of Vα14 rearranged to Jα281.16 In the next set of experiments, we verified this feature in our placental/decidual cells.
To further analyse the Vα gene rearrangement found in uterine NKT cells, we sequenced the Vα rearrangement from the four Vα14+ hybridomas. RT–PCR yielded a signal of the expected length for all four hybridomas. The integrity of the amplified product was verified by sequence analysis of the Jα281Cα join, with the expected sequence16 obtained in all cases (Table 2). In addition, 14 Vα14 clones obtained from RNA prepared from early pregnant tissues were sequenced, and the Vα14–Jα281 joins are shown in Table 2. Six of the 14 joins demonstrate the canonical sequence previously reported by Lantz and Bendelac.16 Six others demonstrate conservative amino acid substitutions, and two demonstrate non-conservative substitutions (G→A at amino acid 93) as shown in Table 2.
Table 2.
Vα14-Jα281 nucleotide and amino acid junctional sequences from NKT-cell derived hybridomas and clones from early pregnancy decidua
| Germline Vα14 | TGT | GTG | GTG Ctg | GGC tgTA | Gcac GAT | AGA AGA | GGT GGT | TCA TCA | Germline Jα281 |
|---|---|---|---|---|---|---|---|---|---|
| Vα sequences | 93 | 94 | |||||||
| 6/14 | TGT | GTG | GTG | GGG | GAT | AGA | GGT | TCA | |
| C | V | V | G | D | R | G | S | ||
| 3/14 | ... | ... | ... | GGC G | ... | ... | ... | ... | |
| 2/14 | ... | ... | ... | GGA G | ... | ... | ... | ... | |
| 1/14 | ... | ... | ... | GGT G | ... | ... | ... | ... | |
| 2/14 | ... | ... | ... | GCC A | ... | ... | ... | ... | |
| 4/4 hybridoma | ... | ... | ... | GGC | ... | ... | ... | ... |
Thus, we conclude that the NKT cells present in periimplantation placenta/decidua belong to the previously described subtype of cells that are TCRint, DN, Vα14-Jα281+.
CD1 expression in early pregnancy
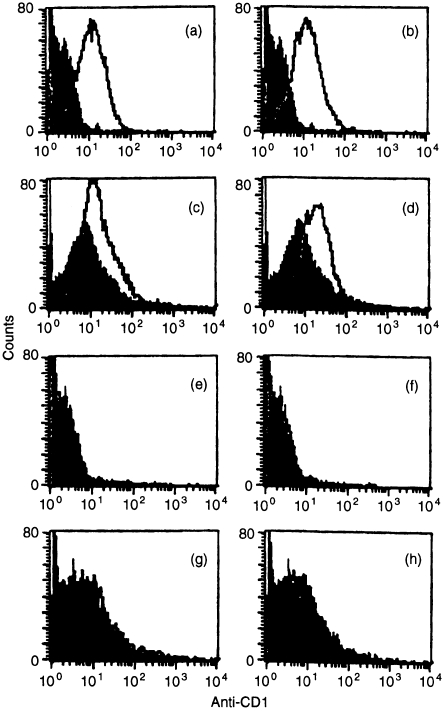
The presence of CD1 on early pregnancy tissues was assessed cytofluorographically by staining early pregnancy tissues with two anti-CD1 mAb. Both antibodies stained these cell preparations with an intensity similar to that seen with thymocytes (Fig. 6). Staining with isotype controls at identical concentrations, and staining of tissues from CD1− × CD1− mice were used to verify the specificity of the staining.
Figure 6.
Cytofluorographic analysis of CD1 expression by early pregnant placental/decidual cells. Thymocytes (a–b, e–f) or placental/decidual cells (c–d, g–h) without nylon wool purification from day 7 C57Bl/10 × BALB/c (a–d) or CD1− × CD1− (e–h) gestations were stained with undiluted anti-CD1 mAb 5C64 (left) and 3H3 (right), and binding detected with PE-conjugated anti-hamster IgG (1: 15). Control staining with isotype control is shown by the shaded curves. Six mice were studied in each experiment; representative data are shown.
Discussion
Previous studies characterizing cellular populations within the pregnant murine uterus have documented significant populations of both NK cells and T cells, 6,12–6,14 but NKT cells have not been previously reported. That this numerically significant population has been overlooked in the past may be due to several factors. For example, many studies have employed primarily immunohistological techniques, with staining for only one antigen at a time, and such studies would likely overlook the simultaneous expression of NK1.1 and TCR. Other studies have not focused on the narrow window of periimplantation when this population is prevalent. Indeed, the small size of the decidua and the relatively small number of cells present therein at day 6–8 makes these studies somewhat tedious. The recent interest in NKT cells provided the impetus to investigate their presence during pregnancy.
Typical NK cells resident in the uterus, which have been referred to in the past in mice as ‘granulated metrial gland (GMG) cells’, and more recently as uterine natural killer (uNK) cells, appear to be the most prevalent immune cell population within the pregnant uterus.12,13 In addition to previously hypothesized functions regarding cytokine secretion and control of placentation,12,13 recent studies with genetically altered mice lacking uNK cells (Tgɛ26) suggest an important role for uNK cells in the development of the metrial gland and the subsequent success of pregnancy.35 Mechanisms involved in these experiments are unknown, but because abrogation of the deleterious effects associated with uNK cell absence can be achieved in bone marrow reconstitution experiments using severe combined immunodeficient (SCID) mice, a role for NKT cells in these experiments seems unlikely.36 In any case, it must be stressed that the NKT cells described here are distinct from uNK cells, which are CD3 – by definition.
Functions of uterine T cells are largely unknown. Reduced expression of polymorphic MHC molecules within the pregnant uterus may limit their function, although some antigen-presenting cells (APC, e.g. macrophages) are present.37 Presumably some T cells serve conventional roles, such as combating infection. Pregnancy-specific functions, such as recognition of fetal tissues, have remained hypothetical, although we have previously reported recognition of a conserved trophoblast antigen by a γδ T cells expressing Vδ6 in conjunction with a variety of Vγ chains.38
The presence of maternal NKT cells in close proximity to fetal tissues expressing CD1 provides another scenario for maternal immune recognition of fetal cells, since NKT cells expressing the Vα14-Jα281-encoded TCR are known to bind CD1.19 Demonstration of CD1 expression by the fetal trophoblasts also adds an interesting aspect to the issue of class I molecule expression within the pregnant uterus, an area of significant interest. In the human, placental expression of the oligomorphic class I molecule HLA-G is well established.7 Class I/I-like molecule expression within the pregnant murine uterus is less well understood.
The presence within the pregnant uterus of NKT cells is also intriguing because of their ability to elaborate large amounts of IL-4 rapidly upon engagement of the TCR.21 This characteristic is consistent with the known Th2 environment known to be important to pregnancy maintenance. An excess of Th2-type cytokines has been shown to exist in both murine and human pregnancy,10,11 and a shift in this milieu towards a Th1-type environment is associated with reproductive failure.8,9 The placental expression of CD1 suggest the possibility of immune signalling between the fetus and mother, with fetal expression of CD1 inducing, by way of IL-4 production by NKT cells, a favourable immune environment within the local environment of the pregnant uterus. The periimplantation window may be an especially important time for such regulation to occur, as the developing embryo is attempting to gain a foothold. Future experiments will directly investigate the production of IL-4 by uterine NKT cells.
The relevance of our findings to human pregnancy is speculative at present. Similar NKT cell populations exist in the human, with homologous TCR expression and CD1 restriction.39–42 CD1 expression has been noted in the uterus in humans,43,44 as well as on choriocarcinoma cell lines and trophoblasts.45
The source of uterine NKT cells is not addressed by this study. Details of the development of NKT cells in general remain under investigation, with current data supporting both thymic and extrathymic development (reviewed in 20). Development does appear to be class-I dependent, based on findings in β2-microglobulin deficient mice,17,20,46–48 and a major role for CD1 seems likely.22,49 Whether uterine NKT cells develop in situ or migrate from other sites is not known. Mechanisms allowing class-I mediated selection of CD4 expressing NKT cells remain unknown, although other NKT-cell populations contain significant CD4+ components.17–20 The uterine population appears to differ from populations in other organs in terms of absence of CD4 expression and a different Vβ repertoire. These features would seem to favor in situ development of uterine NKT cells, in turn suggesting involvement of trophoblast CD1 expression as a possibility. Why uterine NKT cells would manifest altered CD4 and Vβ expression as compared to other NKT cells is not known. Possibilities include altered level of CD1 expression or the local cytokine milieu.
Finally, our findings continue to reinforce the intriguing and highly specialized nature of the maternal immune system within the pregnant uterus. It seems likely that further understanding of these features will allow a better comprehension of normal and abnormal pregnancy.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HD34079-03) to K. H. We thank Drs M. Tanguchi and M. Amano for kindly providing anti-Vα14 mAb.
References
- 1.Benirscke K. The placenta: normal development. In: Creasy R, Resnick R, editors. Maternal–Fetal Medicine: Principles and Practice. Philadelphia: W.B. Saunders; 1989. p. 123. [Google Scholar]
- 2.Enders A. A comparative study of the fine structure of the trophoblast in several hemochorial placentas. Am J Anat. 1965;116:29. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- 3.Heyborne K, Silver R. Immunology of postimplantation pregnancy. In: Bronson R, Alexander N, Anderson D, Branch D, Kutteh W, editors. Reproductive Immunology. Cambridge, MA: Blackwell Science; 1996. p. 383. [Google Scholar]
- 4.Hunt JS, Fishback JL, Andrews GK, Wood GW. Expression of class I HLA genes by trophoblast cells. Analysis by in situ hybridization. J Immunol. 1988;140:1293. [PubMed] [Google Scholar]
- 5.Hedley ML, Drake BL, Head JR, Tucker PW, Forman J. Differential expression of the class I MHC genes in the embryo and placenta during midgestational development in the mouse. J Immunol. 1989;142:4046. [PubMed] [Google Scholar]
- 6.Redline RW, Lu CY. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal–fetal immunological relationship. Lab Invest. 1989;61:27. [PubMed] [Google Scholar]
- 7.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion [see comments] JAMA. 1995;273:1933. [PubMed] [Google Scholar]
- 9.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653. [PubMed] [Google Scholar]
- 10.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562. [PubMed] [Google Scholar]
- 11.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy [see comments] Immunol Today. 1997;18:478. doi: 10.1016/s0167-5699(97)01127-4. 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 12.Croy BA, Luross JA, Guimond MJ, Hunt JS. Uterine natural killer cells: insights into lineage relationships and functions from studies of pregnancies in mutant and transgenic mice. Nat Immun. 1996;15:22. [PubMed] [Google Scholar]
- 13.Head JR. Uterine natural killer cells during pregnancy in rodents. Nat Immun. 1996;15:7. [PubMed] [Google Scholar]
- 14.Heyborne KD, Cranfill RL, Carding SR, Born WK, O’Brien RL. Characterization of gamma delta T lymphocytes at the maternal–fetal interface. J Immunol. 1992;149:2872. [PubMed] [Google Scholar]
- 15.Mincheva-Nilsson L, Baranov V, Yeung MM, Hammarstrom S, Hammarstrom ML. Immunomorphologic studies of human decidua-associated lymphoid cells in normal early pregnancy. J Immunol. 1994;152:2020. [PubMed] [Google Scholar]
- 16.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4− 8 − T cells in mice and humans. J Exp Med. 1994;180:1097. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+ 8− and CD4− 8 − subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994;180:699. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+ CD4− CD8− and alpha beta TCR+ NK1.1+ cells. J Immunol. 1990;145:3209. [PubMed] [Google Scholar]
- 19.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 20.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1− deficient mice. Immunity. 1997;6:459. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 23.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 24.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989;142:2736. [PubMed] [Google Scholar]
- 26.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445. [PubMed] [Google Scholar]
- 27.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 28.Koo GC, Peppard JR. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984;3:301. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- 29.Masuda K, Makino Y, Cui J, et al. Phenotypes and invariant alpha beta TCR expression of peripheral V alpha 14+ NK T cells. J Immunol. 1997;158:2076. [PubMed] [Google Scholar]
- 30.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White J, Blackman M, Bill J, Kappler J, Marrack P, Gold DP, Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989;143:1822. [PubMed] [Google Scholar]
- 32.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald HR. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arase H, Arase N, Ogasawara K, Good RA, Onoe K. An NK1.1+ CD4+ 8 − single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc Natl Acad Sci USA. 1992;89:6506. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 36.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt JS, Manning LS, Mitchell D, Selanders JR, Wood GW. Localization and characterization of macrophages in murine uterus. J Leukocyte Biol. 1985;38:255. doi: 10.1002/jlb.38.2.255. [DOI] [PubMed] [Google Scholar]
- 38.Heyborne K, Fu YX, Nelson A, Farr A, O’Brien R, Born W. Recognition of trophoblasts by gamma delta T cells. J Immunol. 1994;153:2918. [PubMed] [Google Scholar]
- 39.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells [see comments] Nature. 1994;372:691. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 40.Davodeau F, Peyrat MA, Necker A, et al. Close phenotypic and functional similarities between human and murine alphabeta T cells expressing invariant TCR alpha-chains. J Immunol. 1997;158:5603. [PubMed] [Google Scholar]
- 41.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4− CD8− cytolytic T lymphocytes. Nature. 1989;341:447. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 42.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4− 8 − T lymphocytes to a microbial antigen. Nature. 1992;360:593. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 43.Blumberg RS, Terhorst C, Bleicher P, McDermott FV, Allan CH, Landau SB, Trier JS, Balk SP. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518. [PubMed] [Google Scholar]
- 44.Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561. [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkinson HJ, Wainwright SD, Simpson KL, Perry AC, Fotiadou P, Holmes CH. Expression of CD1D mRNA transcripts in human choriocarcinoma cell lines and placentally derived trophoblast cells. Immunology. 1999;96:649. doi: 10.1046/j.1365-2567.1999.00726.x. 10.1046/j.1365-2567.1999.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 47.Bix M, Coles M, Raulet D. Positive selection of V beta 8+ CD4− 8 − thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993;178:901. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coles MC, Raulet DH. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J Exp Med. 1994;180:395. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]