Abstract
Hapten immune pulmonary interstitial fibrosis (HIPIF) is induced by a recall cell-mediated immune response against the hapten 2,4,6-trinitrobenzene sulphonic acid (TNBS) in the lung. Studies here dissect the role of the cellular components of the bronchoalveolar lavage (BAL) cells (alveolar macrophages [AMs] versus monocytes and immature dendritic cells) in the fibrogenic inflammatory response. BAL cells from HIPIF mice were generally more activated and produced a greater amount of tumour necrosis factor-α (TNF-α) than controls. Liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP) that was inoculated intranasally (i.n.) into mice selectively depleted AMs. Following AM depletion, the number of TNF-α-containing cells was reduced, and both the number of immune inflammatory cells recruited into the alveolar space and the subsequent collagen deposition (hydroxyproline) were decreased in the sensitized and intratracheally (i.t.) challenged mice. In conclusion, AMs are required, in part, for the development of pulmonary fibrosis in HIPIF because AM-derived factors such as TNF-α are needed for initiation of chemokine and cytokine pathways and accumulation of immune inflammatory cells.
Introduction
Haptens are protein-reactive chemicals that are ubiquitous in both the industrial and domestic environment. Haptens (metals, salts and drugs) are considered major environmental noxae capable of modifying the immune system of vertebrates.1 The skin and the lung are major sites for individuals to come into contact with haptens. Also, depending on the route, dose and frequency, hapten exposure may lead to a resolving contact hypersensitivity (poison ivy),2,3 an allergic reaction (penicillin sensitivity),4–6 or as we show here, a non-resolving pulmonary interstitial fibrosis. Moreover, like viruses, haptens alter self-antigens and therefore contribute to an ensuing immune response that may include autoimmune mechanisms.1,7–9 The autoimmune aetiology of pulmonary interstitial fibrosis was a factor associated with the identification of activated T cells in the lung and autoantibodies in patients.10–12 Hapten immune pulmonary interstitial fibrosis (HIPIF) is a unique model for the study of mechanisms of autoimmune pulmonary interstitial fibrosis potentiated by environmental noxae. Pulmonary fibrosis is induced in mice by epicutaneous application of the hapten 2,4,6-trinitrobenzene sulfonic acid (TNBS), followed by intratracheal (i.t.) challenge with TNBS.13–17 HIPIF is antigen specific, requires both CD4+ and CD8+ T cells, and is dependent on the genetic susceptibility of test animals to express a contact hypersensitivity response to TNBS.13,14 Immune mechanisms are critically involved in HIPIF as mice that are made tolerant to the immunizing hapten do not develop fibrosis.16 Moreover, HIPIF can be adoptively transferred with sensitized cells to naive mice prior to i.t. challenge with TNBS.13–16
It is well established that resting alveolar macrophages (AMs) contribute to the creation of an immune-suppressive environment that limits the initiation of immune responses and activation of the antigen-specific T lymphocytes in the lung.18,19 AMs actually release factors that alter the antigen-presenting capacity of the pulmonary dendritic cells.20 Suppressed pulmonary immune responses, however, can be overcome by exposure to infectious agents or foreign antigen.21 During times of inflammation, there is prominent recruitment of cells from the periphery22 that changes the composition of the bronchoalveolar (BAL) cells and shifts immune responses towards inflammation rather than suppression.
While the role of AMs in regulating the initiation or afferent stage of an immune response has been studied in detail,20–24 there are only a few studies exploring how AMs might regulate immune responses in the lung during the efferent stage, i.e. when presensitized or when memory immune cells from other regions of the body arrive in the lung after local antigenic challenge.25 We reasoned that a HIPIF pulmonary environment was compatible with the development of a chronic immune inflammatory response and the eventual development of fibrosis, in part, because AMs were not immunosuppressive during elicitation of the immune response. Thus, the innate ability of AMs to down-regulate immune responses might be modified in the presensitized host, or overwhelmed by the influx of bone marrow-derived inflammatory prone cells recruited to the alveolar space by pulmonary antigenic challenge. Indeed, data reported here show that AMs from lungs of sensitized and lung-challenged mice expressed an altered functional phenotype (up-regulation of inflammatory cytokines) that promoted immune responses and recruitment of inflammatory cells. Moreover, selective removal of AMs prior to antigen challenge resulted in an absence of infiltrating cells in the lung after local antigen challenge. In addition, AMs were absolutely required for the collagen increases because selective depletion of AMs reduced hydroxyproline accumulation.
Materials and methods
Animals
Female BALB/c ByJ mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in Schepens Eye Research Institute Vivarium until they reached the desired weight (20–24 g) for the experiments. All animals were maintained and treated humanely in accordance with NIH guidelines and the approval of the Schepens’ Animal Care and Use Committee.
Reagents
Hapten TNBS, normal rat immunoglobulin G (IgG), normal rabbit IgG, normal hamster serum, ExtrAvidin® fluorescein isothiocyanate (FITC) conjugate, Brefeldin A and crystal violet were purchased from Sigma Chemical Co. (St. Louis, MO). The following reagents were purchased from PharMingen (San Diego, CA) and used according to the manufacturer’s instructions: FITC-conjugated anti-CD3ɛ (145-2C11); ‘Fc blocker’ (anti-CD16/32, 2.4G2); FITC-conjugated anti-tumour necrosis factor-α (TNF-α) (MP6-XT22); phycoerythrin (PE)-conjugated anti-interleukin (IL)-12 (C15.6); biotin-conjugated anti-I-Ad/I-Ed (2G9); biotin-conjugated Gr-1 (RB6–8C5); and Streptavidin Cy-Chrome® (Cy5). Anti-Mac-1 (M1/70-15) was purchased from Caltag Laboratories (Burlingame, CA). The PermeaFix reagent was purchased from Ortho Diagnostic Systems (Raritan, NJ). TNF-α-neutralizing antibody (AMC3012) was purchased from Biosource International (Camarillo, CA) and used at a dose of 50 000 units/treatment. Clodronate (dichloromethylene diphosphonate [Cl2MDP]) was a gift of Roche Diagnostics GmbH (Mannheim, Germany) and was encapsulated into liposomes, as previously described.26 Cl2MDP liposomes were inoculated either intranasally (i.n.) or i.t. at an effective dose of 100 µl, or intravenously (i.v.) (at 200 µl), as predetermined by preliminary studies.
Animal model
HIPIF
Mice were sensitized on the abdomens with a water-soluble form of the hapten TNBS (3% in phosphate-buffered saline [PBS], 100 µl/mouse) on day 0. Five days after sensitization, the mice were inoculated i.t. with 50 µl of 1% TNBS.13
Adoptive-transfer HIPIF (ADT-HIPIF)
Donor mice were sensitized on their abdomens with 100 µl of 3% TNBS. Five to 7 days after skin sensitization, spleen and draining lymph nodes (axillary, inguinal and brachial) were harvested and dissociated into a single cell suspension prior to determining cell viability by using the Trypan Blue exclusion method. Recipient mice were irradiated (200 rads) (Mark 1 irradiator; J. L. Shepherd and Associates, Glendale, CA) 24 hr before donor cells (3 × 107/mouse) were adoptively transferred via the tail vein. One day after the adoptive transfer, recipient mice were challenged (i.t.) with 50 µl of 1% TNBS in PBS. Control mice received naive cells (3 × 107) via their tail vein. Each experimental group contained five mice unless otherwise indicated.
Hydroxyproline assay
Changes in collagen deposition in the lung were measured by using a colorimetric hydroxyproline assay.27 In brief, lungs recovered from experimental mice 14 days post-i.t. challenge with antigen were minced and hydrolyzed in 6N HCl (2 ml/lung) for 16 hr at 110°. The samples were filtered through Whatman no.1 filter paper, diluted with H2O, neutralized with 10N NaOH, and assessed spectrophotometrically (at 557.5 nm) (Milton Roy Spectronic 1201; Milton Roy Company, Ivyland, PA). The amount of hydroxyproline in the lungs was calculated according to the standard curve generated using a serial dilution of trans-4-hydroxy-l-proline (Sigma Chemical Co.).
Flow cytometric analysis
BAL cells were collected by centrifugation (200 g) of ≈10 ml of wash recovered from the alveoli after i.t. inoculation of 1 ml of PBS 10–15 times. To assess the expression of IL-12 receptor (IL-12R) on T cells, BAL cells (106/tube) from the lungs of experimental mice were incubated with recombinant mouse IL-12 (100 ng/ml; 40 min at 4°) in staining buffer (PBS, 1% bovine serum albumin [BSA], 0·1% sodium azide) followed by blocking reagent (‘Fc blocker’ 2 µg/106 cells, rat IgG 20 µg/106 cells, hamster serum 5 µl/106 cells; 20 min at 4°) and then PE-conjugated anti-IL-12 (1 µg/106 cells) was added. Cells were co-stained with FITC-conjugated anti-CD3ɛ (1 µg/106 cells) to identify IL-12R+ T cells. To quench macrophage autofluorescence, cells were permeabilized and fixed with PermeaFix reagent (1 : 1 diluted with H2O; 0·5 ml/106 cells) to allow the intracellular access of crystal violet28 before the samples were analysed by using flow cytometry (EPICS XL; Beckman Coulter, Miami, FL). When cells were evaluated for intracellular protein, all media contained Brefeldin A (10 µg/ml) to block secretion. Intracellular TNF-α was identified by incubating permeabilized cells with FITC-conjugated anti-TNF-α (1 µg/106 cells) after the cells were stained with biotin-conjugated anti-I-Ad-I-Ed (anti-major histocompatibility complex [MHC] II, 1 µg/106 cells) and streptavidin Cy5 (2 µl/tube).
Peripheral blood monocytes were identified as Gr-1dim Mac-1+ cells in whole blood samples. The blood (100 µl/tube) was incubated for 20 min at 4° with blocking reagent (rat IgG, 20 µg/tube) followed by biotin-conjugated Gr-1 antibody (1 µg/tube). After washing twice with the staining buffer (1 ml/tube), the cells were incubated for 20 min on ice with PE-conjugated anti-Mac-1 (10 µl/tube) and ExtrAvidin® FITC conjugate (2 µl/tube). Red blood cells were lysed using the Easy-lyse Whole Blood Erythrocyte Lysing Kit (Leinco Technologies, Inc., Ballwin, MO) (1 ml/tube) before flow cytometry analysis.
Differential cell counts
Differential cells counts were performed on cytospins of BAL cells collected from the various experimental groups. Cells (5 × 104 cells/slide) were centrifuged onto coated slides (micro slides; VWR Scientific, West Chester, PA) in a Shandon cytocentrifuge (Shandon Southern Products, Ltd, Astmoor, UK). The cells were then stained vertically in HEMA 3® (Fisher Scientific, Pittsburgh, PA), according to the manufacturer’s instructions. Stained slides were evaluated using light microscopy (×100 under oil). A total of 1000 cells were counted in consecutive areas of an eye piece grid per slide and categorized according to the following morphology: AMs were identified by their large size and large cytoplasmic region and single, round nucleus; monocytes showed a kidney-shaped nucleus with light blue granules in the cytoplasm; lymphocytes had a spherical nucleus that was intensely stained, and a small cytoplasmic region; neutrophils had a ring-shaped nucleus or a nucleus consisting of two to five lobes linked by fine threads of chromatin; basophils had a nucleus with irregular lobes and purple granules in the cytoplasm; and eosinophils contained a bilobed nucleus and pink granules in the cytoplasm.
Reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from 3×106 BAL cells by using Trizol reagent (Gibco, Rockville, MD) and dissolved in 30 µl of diethylprocarbonate-treated H2O (DEPC; Sigma Chemical Co.). One microlitre of the RNA sample was used for the one-step RT–PCR amplification using the Access RT–PCR system (Promega, Madison, WI) and Gene Amp PCR System 9600 (Perkin Elmer, Norwalk, CT). The primer pairs29,30 for IL-12R β1 (sense: CCAGCACAGGAACCACACA; antisense: CAGAGACGCGAAAATGATG), IL-12R β2 (sense: AATTCAGTACCGACGCTCT CA; antisense: ATCAGGGGCTCAGGCTCTTCA) and β-actin (sense: GTGGGCCGCTCTAGGCACCAA; antisense: CTCTTTGATGTCACGCACGATTTC) were generated by Oligos Etc (Wilsonville, OR). The RT reaction was one cycle at 48° for 45 min followed by a 2-min incubation at 94°. The PCR amplification comprised 40 cycles of: 30 seconds at 94°, 1 min at 60° and 2 min at 68°; this was followed by one cycle at 68° for 7 min. The PCR products were separated on a 1% agarose gel and visualized using GelStar® nucleic acid gel stain (FMC BioProducts, Rockland, ME) and UV illumination. The density of the bands on the gel was measured by using Gel Doc 2000 (Bio-Rad, Hercules, CA).
Statistical analyses
The significant differences among data derived from the experimental groups were determined by analysis of variance (anova) and post-hoc tests.31 Differences were considered significant if P≤0·05.
Results
Cl2MDP liposome depletion of AMs prevents hydroxyproline accumulation in the ADT-HIPIF model
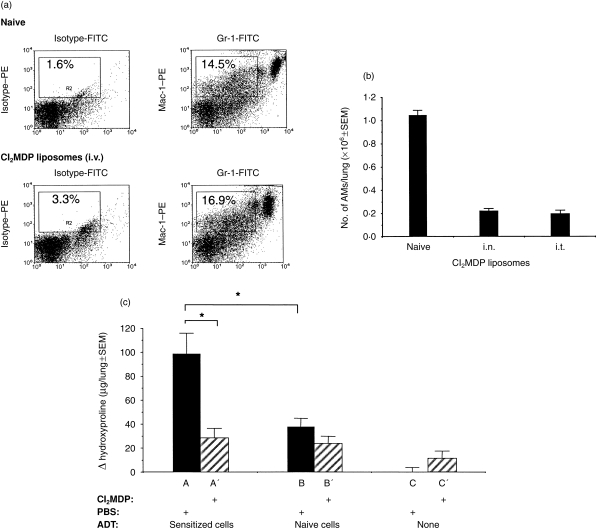
Immune cell infiltration is a prominent feature during the development of inflammation in the lung.22 The inflammatory cell recruitment changes the heterogeneity and phenotype of BAL cells from ≈90–95% to ≤60% AMs, depending on the type and time of inflammation, because of an influx of immune-inciting bone marrow-derived cells. As the cellular composition of BAL cells varies according to the immune status of the lung, we developed a strategy to differentiate the role of AMs versus monocytes in HIPIF by selectively eliminating AMs in vivo with Cl2MDP liposomes prior to i.t. challenge of hapten-sensitized mice. Cl2MDP liposomes induce apoptosis of cells that phagocytize them.26,32 In support of previous reports showing that only macrophages were depleted by Cl2MDP liposomes,26,32 we were unable to eliminate monocytes (poorly phagocytic) by direct i.v. inoculation of Cl2MDP liposomes (Fig. 1). The percentage of Gr-1dim Mac-1+ (marker for monocytes) cells in the peripheral blood of naïve mouse was no different from the percentage of peripheral blood monocytes from the mouse treated with Cl2MDP liposomes i.v. 2 days earlier.
Figure 1.
(a) Flow cytometric analysis of the Mac-1+/Gr-1dim population in peripheral blood after intravenous (i.v.) treatment with dichloromethylene diphosphonate (Cl2MDP) liposomes. The two-parameter histograms compare the percentage of Mac-1+/Gr-1dim cells in peripheral blood from naïve mice that were treated with or without Cl2MDP liposomes (i.v., 200 µl/mouse). The blood was collected 2 days after the treatment and stained with phycoerythrin (PE)-conjugated Mac-1 antibody and biotin-conjugated Gr-1 antibody plus fluorescein isothiocyanate (FITC)-conjugated ExtrAvidin®. The samples were analysed using flow cytometry. Peripheral blood monocytes were identified by their Mac-1+/Gr-1dim staining pattern and gated on the histograms. The percentage of Mac-1+/Gr-1dim cells is indicted in each gate by the rectangle. (b) Differential cell counts of alveolar macrophages (AM) in bronchoalveolar lavage (BAL) following intranasal (i.n.) or intratracheal (i.t.) Cl2MDP liposome treatment. The histogram shows the number of AMs in each lung (ordinate) after the naïve mice were treated or not treated with Cl2MDP liposomes (abscissa). One dose of 100 µl of Cl2MDP liposomes was given i.t. per naïve mouse. Three doses of 33 µl (total 99 µl) of Cl2MDP liposomes were given i.n. per naïve mouse during the course of 1 day. BAL was collected 2 days after i.t. or i.n. treatment. BAL cytospins were stained with Wright–Giemsa and evaluated by light microscopy (×40 Nikon). Three mice were used in each group. AMs were identified and counted by differential cell counts (see the Materials and Methods). The number of AMs in each lung was calculated by multiplying the total number of cells in the BAL preparation with the percentage of AMs from differential cell counts. The result is presented as mean±SEM. (c) Hydroxyproline deposition in the lungs of adoptive transfer-hapten immune pulmonary interstitial fibrosis (ADT-HIPIF) mice treated with Cl2MDP liposomes (i.n.). Recipient mice (groups A′, B′ and C′) were treated with Cl2MDP liposomes (33 µl/dose×three doses) and irradiated 1 day prior to receiving adoptive transfer of sensitized or non-sensitized cells. The mice in groups A, B and C were treated with phosphate-buffered saline (PBS) liposomes. One day before i.t. challenge, the mice in groups A and A′ received 3×107 2,4,6-trinitrobenzene sulphonic acid (TNBS)-sensitized spleen and draining lymph node (inguinal, brachial, axillary) cells; mice in groups B and B′ received 3×107 naïve spleen cells, and lymph node (inguinal, brachial, axillary) cells. Group C mice were untreated. Fourteen days after i.t. challenge, the lungs from experimental mice (five mice per group) were harvested and analysed for their hydroxyproline level. The histogram shows the change (Δ) in hydroxyproline level (experimental hydroxyproline – baseline hydroxyproline) in each experimental group (mean±SEM). The baseline hydroxyproline concentration was 20 µg/lung and was calculated as the mean hydroxyproline concentration from five naïve mice that were treated with PBS liposomes (i.n.). An asterisk (*) indicates a statistically significant difference (P=0·05) between indicated groups. The experiment was repeated twice.
Thepen and colleagues also showed that i.t. inoculation of Cl2MDP liposomes into mouse lungs eliminated AMs but not interstitial macrophages.26,32 To test our ability to selectively remove AMs, Cl2MDP liposomes were inoculated into naïve mice, either i.n. or i.t. Two days after the treatment, BAL cells were collected and stained with Wright–Giemsa prior to examination using light microscopy. AMs were identified as large granular cells containing single round nuclei and multiple vacuoles in the cytoplasm. By 48 hr, both i.n. and i.t. inoculations of Cl2MDP liposomes had depleted 80% of the AMs in the lungs of naïve mice (Fig. 1).
After establishing a route for Cl2MDP liposome delivery, we then evaluated the effect of AM depletion on the ability of treated mice to develop HIPIF. To define the role of AMs in the secondary immune response in the lungs (as opposed to the periphery) of hapten-sensitized and i.t.-challenged mice, we used the adoptive transfer modification of HIPIF, ADT-HIPIF. Trinitrophenyl (TNP)-sensitized spleen and draining lymph node (axillary, brachial, inguinal) cells were harvested from donor mice 5 days post skin sensitization and adoptively transferred i.v. to recipient mice treated with control or Cl2MDP liposomes 1 day before i.t. challenge with hapten. The depletion of AMs with Cl2MDP correlated with a significant reduction in hydroxyproline on day 14 in recipient mice (Fig. 1).
Effect of AM depletion on the BAL cellular profile in ADT-HIPIF
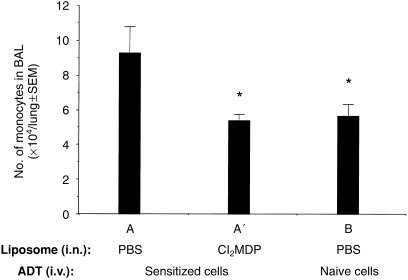
As the lungs of (adoptively) hapten-sensitized mice are associated with an influx of peripheral blood inflammatory cells13,17 after i.t. challenge, we analysed the role of AMs in recruitment of the adoptively transferred immune inflammatory cells to the lungs of ADT-HIPIF mice. Differential cell counts were performed on BAL cells from unmanipulated and AM-depleted ADT-HIPIF mice. In brief, recipient mice were treated with control or Cl2MDP liposomes 2 days prior to i.t. challenge and BAL was collected 2 days after i.t. challenge. The percentage and total number of the infiltrating monocytes/immature dendritic cells were calculated and compared among the ADT-HIPIF mouse groups treated with control or Cl2MDP liposomes. We observed a decrease (5·36±0·22×104 versus 9·27±0·87×104, P=0·007) of infiltrating monocytes/immature dendritic cells in BAL of ADT-HIPIF mice treated with Cl2MDP liposomes compared to the mice treated with control liposomes (Fig. 2). Because Cl2MDP liposomes did not decrease the number of monocytes when directly inoculated i.v., we reasoned that the lack of this cell population in the lung after i.t. challenge of treated mice was not a direct effect of the treatment.
Figure 2.
Infiltrating monocyte/immature dendritic cell counts in the bronchoalveolar lavage (BAL) of adoptive transfer-hapten immune pulmonary interstitial fibrosis (ADT-HIPIF) mice treated with dichloromethylene diphosphonate (Cl2MDP) liposomes intranasally (i.n.). Cl2MDP liposomes were given i.n. to recipient mice 2 days prior to intratracheal (i.t.) challenge (see the legend to Fig. 1c). BAL was collected from experimental mouse groups (A, A′ and B) 2 days after i.t. challenge with 2,4,6-trinitrobenzene sulphonic acid (TNBS). Differential cell counts were performed using light microscopy (×100 under oil) and an eye piece grid. One thousand cells were counted per slide per animal, and four slides per group (n = 4). Monocytes were distinguished from other cells as described in the Materials and Methods. The number of infiltrating monocytes/immature dendritic cells was calculated and is presented as mean ±SEM. An asterisk (*) indicates a statistically significant difference (P≤0·05) between the indicated group and group A. The experiment was repeated twice.
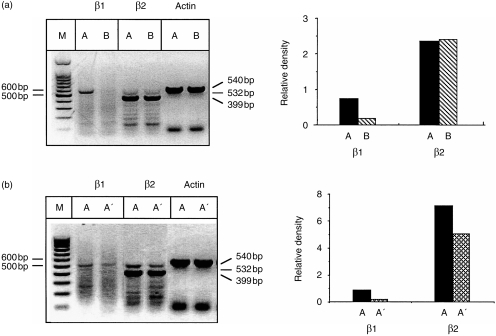
As HIPIF is a T-cell-mediated fibrotic response and requires presensitized T cells,13,14 we also measured the number of activated T cells in BAL from ADT-HIPIF mice and AM-depleted ADT-HIPIF mice. A functional IL-12R expresses both IL-12Rβ1 and β2 chains and is commonly expressed on a T helper 1 (Th1) cell.29,33,34 Th2 cells express IL-12Rβ1 chain and not the IL-12Rβ2 chain. As previous data showed that HIPIF is a result of a Th1 response,13,14,17 we reasoned that the effector lymphocytes in the lungs of mice developing fibrosis would express a functional IL-12R (i.e. IL-12Rβ1 and IL-12Rβ2). Because antibodies to the IL-12R chains are not available, mRNA of IL-12Rβ1 and -β2 chains in BAL cells was determined by using RT–PCR. Total RNA was purified from the BAL cells collected 1 and 2 days after i.t. challenge of recipient mice. The level of IL-12Rβ1 and -β2 mRNA was measured by RT–PCR using specific primers for each subunit. Electrophoresis (1% agarose gel) of the PCR product was followed by densitometric analyses. In contrast to conventional wisdom, cells from lungs of mice that were challenged i.t. expressed IL-12Rβ2 chain, regardless of their sensitization status. However, only presensitized mice that were challenged i.t. also expressed mRNA for IL-12Rβ1 and, presumably, a functional IL-12R (Fig. 3). AM depletion is correlated with a decrease in both IL-12Rβ1 and IL-12Rβ2 mRNA, implying a decrease in recruitment of Th1 cells expressing a functional IL-12R (Fig. 3).
Figure 3.
Reverse transcription–polymerase chain reaction (RT–PCR) analyses and densitometry measurements of the mRNA level of interleukin-12 (IL-12) β1 and β2 subunits in bronchoalveolar lavage (BAL) cells from adoptive transfer-hapten immune pulmonary interstitial fibrosis (ADT-HIPIF) mice treated with dichloromethylene diphosphonate (Cl2MDP) liposomes. (a) BAL was collected 2 days after intratracheal (i.t.) challenge from group A mice (skin sensitized and i.t. challenged) and group B mice (i.t. challenged only). (b) The recipient mice were treated with Cl2MDP liposomes (A′, 33 µl/dose×three doses/mouse) or control phosphate-buffered saline (PBS) liposomes (A) two days prior to i.t. challenge. BAL was collected 1 day after i.t. challenge. Total RNA was purified and RT–PCR was performed using IL-12Rβ1- and -β2-specific primer sets. The PCR product was separated on a 1% agarose gel and visualized by using GelStar® nucleic acid gel stain and UV illumination, as shown on the left side of each panel. The histograms on the right side of each panel show the density of the bands measured using Gel Doc 2000. The relative density of β1 (532 bp) and β2 (399 bp) bands was normalized to β-actin (540 bp). The experiment was repeated three times.
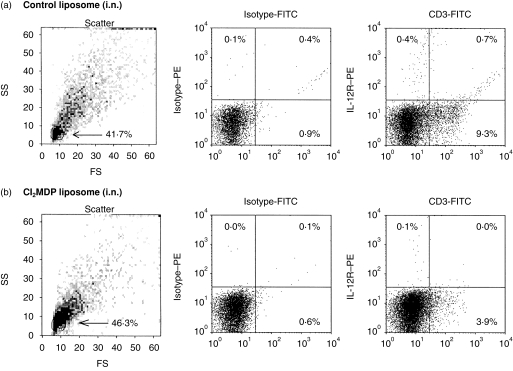
Because various cells (T cells, neutrophils and dendritic cells) may express a functional IL-12R,35 flow cytometric analysis was used to determine the relative number of CD3+ T cells that expressed the IL-12R. BAL cells were collected 1 day after i.t. challenge from mice receiving sensitized cells that were treated with control (A) or Cl2MDP liposomes (A′). Procedures to identify IL-12R+ cells were adapted from a report by Igarashi and colleagues.29 Samples were incubated with recombinant mouse IL-12 (rmIL-12) followed by PE-conjugated anti-IL-12 and counterstained with FITC-conjugated anti-CD3ɛ. The expression of CD3 and IL-12R on the cells that were gated for their small size and low granularity was plotted (Fig. 4). An equivalent number of BAL cells (3·41±0·4×105 versus 4±0·9×105, P=0·58) was found within these gates in samples from ADT-HIPIF mouse groups, with or without AMs. However, the number of both CD3+ IL-12R+, CD3+ IL-12R−, and CD3− IL-12R+ cells in ADT-HIPIF mice with AMs was significantly higher than the number of each subpopulation in the ADT-HIPIF mice without AMs. In fact, the recipient mice receiving sensitized cells and treated with Cl2MDP liposomes prior to i.t. challenge had 54% less total CD3+ cells (1·56±0·36×104 versus 3·4±0·39×104, P=0·026) in the lavage samples compared to the ADT-HIPIF mice treated with PBS liposomes. Also, without the AMs present in the lung, there was a significant difference (0·4±0·09×103 versus 3·41±0·39×103, P=0·01) in the number of CD3+ IL-12R+ and CD3− IL-12R+ cells in the BAL samples. T cells are not depleted by the Cl2MDP liposomes because they are not phagocytic. Thus, in the absence of AMs, adoptively sensitized mice were less able to recruit activated T cells (IL-12R+ CD3+) after hapten challenge.
Figure 4.
Flow cytometric dot-plot analyses of CD3+ and interleukin-12 receptor-positive (IL-12R+) lymphocytes in adoptive transfer-hapten immune pulmonary interstitial fibrosis (ADT-HIPIF) lungs treated with dichloromethylene diphosphonate (Cl2MDP) liposomes intranasally (i.n.). Recipient mice were treated i.n. with Cl2MDP liposomes (33 µl/dose×three doses) or control liposomes 2 days prior to i.t. challenge and received sensitized cells 1 day before i.t. challenge. One day after i.t. challenge, bronchoalveolar lavage (BAL) was collected, stained for surface expression of CD3 and IL-12R (see Materials and methods) and then analysed using flow cytometry. (a) Mice were treated with control liposomes. (b) Mice were treated with Cl2MDP liposomes. The percentage of the population that was gated (arrow) is indicated in the forward scatter (FS)/side scatter (SS) dot-plot in (a) and (b) of each panel. To the right of each panel are dot-plots showing CD3 and IL-12R expression on gated cells. The percentage of CD3+ and IL-12R+ cells is indicated in each quadrant. A minimum of three mice was used in each experimental group. The experiment was repeated twice. FITC, fluorescein isothiocyanate.
Effect of TNF-α depletion on the BAL cellular profile in ADT-HIPIF
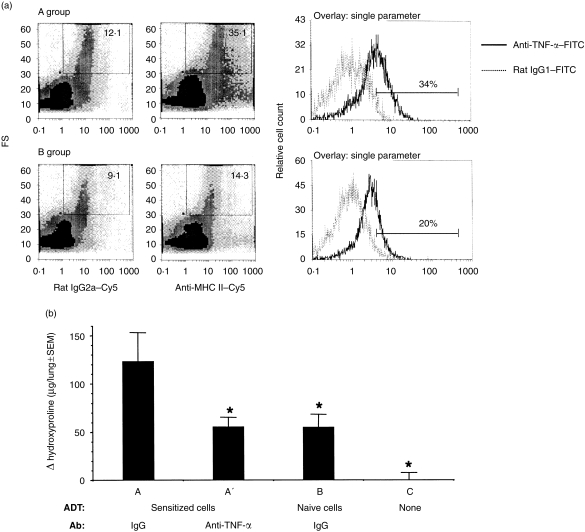
Activated macrophages produce inflammatory cytokines. In fact, AMs are known to produce large amounts of TNF-α during pulmonary inflammatory responses that lead to fibrosis.36,37 TNF-α is critical for the development of toxicity-induced fibrosis.36,38 In support of published results,36–38 AMs from the HIPIF mice produced an increased amount of TNF-α, as indicated by a shift in mean fluorescence intensity (MFI) and an increase in the percentage of AMs that were positive for intracellular TNF-α protein (Fig. 5, Table 1).
Figure 5.
(a) Flow cytometric analysis of intracellular tumour necrosis factor-α (TNF-α) in hapten immune pulmonary interstitial fibrosis (HIPIF) alveolar macrophages (AMs). Histograms show the intracellular staining of TNF-α in AMs from mice that were skin sensitized and challenged intratracheally (i.t.) with 2,4,6-trinitrobenzene sulphonic acid (TNBS) (HIPIF mice, group A) or from mice that were challenged i.t. only (group B). Three days after i.t. challenge, bronchoalveolar lavage (BAL) was collected. BAL cells were pooled from three experimental mice in each group and surface markers and intracellular proteins were stained with biotin-conjugated anti-major histocompatibility complex (MHC) II plus streptavidin Cy-Chrome® (Cy5) and fluorescein isothiocyanate (FITC)-conjugated anti-TNF-α antibodies, respectively. The group A and B dot-plots show gated cells (rectangle) that were selected as AMs because of their large size and expression of MHC IIdim. The mean fluorescence intensity of MHC II staining is indicated in each dot-plot. The expression of TNF-α in the gated cells was analysed and is presented as percentage (%) of positive cells. The experiment was repeated three times. FS, forward scatter. (b) Hydroxyproline deposition in the lungs of adoptive transfer (ADT)-HIPIF mice treated with TNF-α-neutralizing antibody. The level of hydroxyproline in the lung was compared among experimental groups (A, A′, B and C). The treatment of recipient mice is described under the abscissa: ADT, adoptive transfer of donor cells (TNBS-sensitized or naïve); Ab, antibody treatment. The donor cells (3×107/mouse) were transferred to the recipient mice 1 day before i.t. challenge. TNF-α-neutralizing antibody (50 000 units/mouse) or control immunoglobulin G (IgG) was given intraperitoneally (i.p.) 2 days before i.t. challenge and 2 hours after i.t. challenge (intranasally). Fourteen days after i.t. challenge, lungs were harvested and a hydroxyproline assay was performed. The histogram shows the change (Δ) in hydroxyproline level (experimental hydroxyproline – baseline hydroxyproline) in each experimental group (mean±SEM). The baseline hydroxyproline concentration was the mean hydroxyproline level of four naïve mice and was 180·3 µg per lung. An asterisk (*) indicates a significant difference between the indicated group and group A.
Table 1.
Expression of tumour necrosis factor-α (TNF-α) in hapten immune pulmonary interstitial fibrosis (HPFIF) alveolar macophages (AMs)
| Group A | Group B | |||||
|---|---|---|---|---|---|---|
| Exp. | Isotype MFI | Anti-TNF-α MFI | (%) | Isotype MFI | Anti-TNF-α MFI | (%) |
| Exp. 1* | 0·948 | 3·74 | (34·4%) | 0·857 | 2·69 | (20·0%) |
| Exp. 2* | 0·797 | 3·04 | (59·5%) | 0·891 | 2·21 | (46·7%) |
| Exp. 3** | 1·34 | 5·17 | (31·1%) | 1·47 | 4·18 | (17·0%) |
Flow cytometric analysis of TNF-α expression in AMs from three independent experiments. Group A mice were skin sensitized and challenged intratracheally (i.t.) with 2,4,6-trinitrobenzene sulphonic acid (TNBS) (HIPIF mice). Group B mice were challenged i.t. with TNBS only (control mice). Bronchoalveolar lavage (BAL) cells were collected 3 days after i.t. challenge. BAL cells from naïve mice do not produce a measurable amount of TNF-α.MFI, mean fluoresence intensity of AMs.(%), percentage of AMs that were positive for TNF-α staining conpared with the isotype control.
BAL cells were stained for surface major histocompatibility complex II and intracellular TNF-α expression. AMs were selected by their large size and MHC IIdim expression, as shown in Fig. 5(a).
BAL cells were stained for intracellular TNF-α expression. AMs were selected by their large size.
To test if the small but consistent increase of TNF-α in BAL cells was of biological significance, experimental animals in the ADT-HIPIF model were treated or not treated with TNF-α-neutralizing antibody prior to i.t. challenge with TNBS. Mice that received TNP-sensitized cells prior to i.t. challenge showed a 1·5–2-fold increase in hydroxyproline deposition compared to mice that received naive cells and control IgG. The group of mice that received sensitized cells, but were also treated with TNF-α-neutralizing antibody prior to the i.t. challenge, showed no increases in hydroxyproline deposition compared with the toxicity control (challenged only, IgG control group B). Thus, the relative increases of TNF-α in adoptively sensitized and challenged mice compared with control groups is of biological significance in this immune-mediated model of pulmonary fibrosis (Fig. 5).
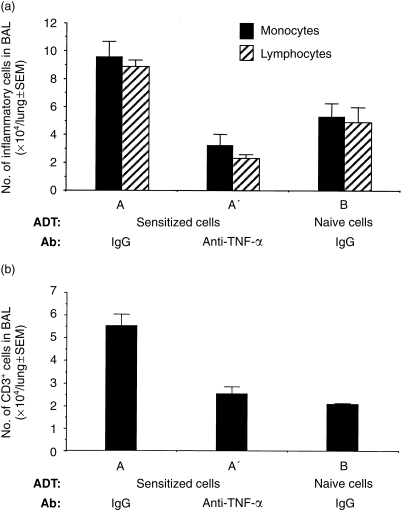
In addition to being fibrogenic and directly influencing the production of collagen, TNF-α is critical for recruitment of inflammatory cells during the development of fibrotic lesions.36,37 Therefore, we reasoned that AM-depleted mice may lack sufficient amounts of AM-derived TNF-α to recruit the inflammatory cells required for the immune-mediated fibrogenic response in ADT-HIPIF. To confirm that TNF-α is indeed required for the recruitment of cells, TNF-α-neutralizing antibody was given to recipient mice in the ADT-HIPIF model, i.p., 1 day before transfer of sensitized cells and again, 2 hr after i.t. challenge. The BAL cells were collected 3 days after i.t. challenge. Wright–Giemsa-stained cytospin preparations were blinded and differential cell counts performed. BAL cells from ADT-HIPIF mice treated with TNF-α-neutralizing antibody (the A′ group) contained fewer lymphocytes (2·33±0·27×104 versus 8·9±0·45×104, P=0·0028) and monocytes/immature dendritic cells (3·23±0·81×104 versus 9·58±1·1×104, P=0·0031) than BAL cells from control antibody-treated HIPIF mice (group A) (Fig. 6). CD3+ T cells in the BAL were further identified by flow cytometric analyses. The number of CD3+ T cells was also significantly decreased (2·54±0·31×104 versus 5·54±0·52×104, P=0·0046) in the ADT-HIPIF mice treated with TNF-α-neutralizing antibody (group A′) compared with the untreated ADT-HIPIF mice (group A) (Fig. 6). As the TNF-α-neutralizing antibody was given to ADT-HIPIF mice prior to i.t. hapten challenge, we reasoned that this antibody neutralized AM-derived TNF-α which was necessary for initiating cascades of cytokines required for extravasation of the adoptively transferred inflammatory cells. Thus, we concluded that removal of AMs from lungs prior to antigen challenge in sensitized mice reduced a cellular source of cytokines (one of which might be TNF-α) needed for recruitment of inflammatory cells.
Figure 6.
Cell counts of infiltrating inflammatory cells in the bronchoalveolar lavage (BAL) of adoptive transfer-hapten immune pulmonary interstitial fibrosis (ADT-HIPIF) mice treated with tumour necrosis factor-α (TNF-α)-neutralizing antibody. Blinded differential counts were performed on Wright–Giemsa-stained BAL cytospins. (a) The bar graph shows the mean number (±SEM) of infiltrating lymphocytes and monocytes/immature dendritic cells in the BAL (ordinate) of experimental mice (groups A, A′ and B [abscissa]) 3 days after intratracheal (i.t.) challenge with 2,4,6-trinitrobenzene sulphonic acid (TNBS). The T cells in the BAL samples were identified by flow cytometry of fluorescein isothiocyanate (FITC)-labelled CD3+ BAL cells. (b) The bar graph shows the mean number of CD3+ T cells in the BAL of experimental mice (A, A′ and B) 3 days after i.t. challenge. Five mice were used in each experimental group. An asterisk (*) indicates a statistically significant difference (P≤0·05) between groups A and A′ or groups A and B.
Discussion
The primary function of the lung is gas exchange. It appears that regional specialization of the macrophage allows AMs to be critical negative regulators of immune responses in the lung, primarily by releasing nitric oxide to limit the antigen-presenting capacity of the pulmonary dendritic cells and thus blocking T-cell activation.18,20,39 As in other specialized regions of the body (gut, eye, testis),40–43 the lung has a transforming growth factor-β (TGF-β)-rich environment that may contribute to local down-regulation of immune cells and their responses.44,45 However, during a chronic immune response induced by a virus or a hapten, this mechanism is broken, perhaps because the agents alter pulmonary proteins and remain in the lung as an antigen, thereby contributing to chronic immune responses. Similarly to pulmonary virus infections, hapten exposure leads to a chronic autoimmune response in the lung that progresses into pulmonary interstitial fibrosis. Here we explored the mechanisms involved in eliciting a secondary immune response to hapten in the lung, which lead to the loss of pulmonary immune-suppressive qualities.
This report demonstrates that AMs are absolutely required for eliciting a Th1 secondary immune response to hapten, in part because they are a cellular source of cytokines, such as TNF-α, needed for recruitment of Th1-related inflammatory cells. These observations differ from studies examining the role of AMs in T helper 2 (Th2)-mediated inflammation, where removal of AMs correlated with increased inflammatory cell responses,25,46 potentially owing to increases in the concentration of IL-4.47 Also, the reduction in the number of immune inflammatory cells recruited to the lungs associated with AM depletion ultimately reduced the hydroxyproline deposition in the pulmonary interstitium.
TNF-α is a known initiator of cell recruitment, in part, by promoting up-regulation of genes encoding adhesion molecules, E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells, thus leading to increased cellular traffic.37,48,49 However, TNF-α can also synergize with IL-1β (another potential macrophage-derived factor in ADT-HIPIF) and induce the synthesis of inflammatory chemokines through the nuclear factor κB (NFκB) pathway in epithelial cells (and perhaps other cells), thus promoting the recruitment of immune inflammatory cells.50,51 It is probable that AM-derived TNF-α initiates the inflammatory chemokine pathway as well as the expression of adhesion molecules during the development of ADT-HIPIF in the lung.
The role of TNF-α in fibrosis has been studied in detail and it is common knowledge that TNF-α is a critical cytokine in the development of toxicity-induced fibrosis.36,38 Although less physiological in nature, over-expression of TNF-α correlates with lymphocytic and fibrosing alveolitis.48 TNF-α is a fibrogenic cytokine, in part because it directly activates fibroblasts to produce collagen.48 Our data show that during elicitation of immune inflammation, AM-derived TNF-α contributes to recruitment of immune inflammatory cells that respond to the local antigen and initiate the fibrogenic process.
The fact that AMs are crucial for the development of ADT-HIPIF was directly supported by results from AM-depletion studies with Cl2MDP liposomes. In fact, AM-derived recruitment was necessary for the development of fibrosis, in part because activated T cells were not present and the local Th1 response was not stimulated and therefore resulted in reduced fibrosis.
The data in this report showed that the respiratory routes of inoculation of the toxic liposomes depleted AMs. We, similarly to others, observed a slight inflammatory response (neutrophil infiltration) in Cl2MDP liposome-treated lungs after inoculation via the i.t. and i.n. routes. The neutrophil-mediated inflammation however, did not lead to an immune inflammatory infiltrate or development of fibrosis when unsensitized mice were challenged with hapten. Furthermore, the number of neutrophils did not increase in the ADT-HIPIF mice treated with Cl2MDP liposomes compared to the ADT-HIPIF mice treated with control liposomes.
The following observations suggest that the liposomes given into the lung remained locally and had minimal or no effect on the cells in the periphery:
Liposomes are not able to pass vascular barriers, such as capillary vessel walls.26,32
When Cl2MDP liposomes were inoculated directly into the blood, the number of monocytes and immature dendritic cells (Mac-1+ Gr-ldim) in the peripheral blood remained unchanged on flow cytometric analyses.
Previously, Thepen and colleagues established that the inoculation of Cl2MDP liposomes i.t. had no effect on interstitial macrophages in the lung.32
i.t. instillation of Cl2MDP liposomes into the lungs of naïve mice does not deplete lung dendritic cells or block their function.32
Hence, there are both published and new experimental data to support the conclusion that the Cl2MDP liposome treatment in the lung (i.n. or i.t.) of mice selectively removed AMs.
Although the hapten (TNBS) used in these studies may not be a hapten that is encountered in the environment, many small reactive chemicals with haptenic properties are ubiquitous in both the domestic and the industrial environment. The following environmental haptens could potentially cause lung disease: formaldehyde is used to treat clothing, rugs and fabrics before marketing; cigarette smoke, which contains haptens; and aerosolized urushiol (hapten) from burning poison ivy. Thus, as with viruses, humans may be constantly exposed to small reactive chemicals, both on the skin and in the respiratory tract.
Pulmonary fibrosis induced by an immune response to haptens provides a paradigm for studying mechanisms that might be important in some forms of idiopathic pulmonary fibrotic conditions seen in humans. While much of the fibrogenic immune inflammatory process that occurs in lungs of ADT-HIPIF mice is understandably caused by the infiltration of immune inflammatory cells, the infiltration of these cells is, nevertheless, dependent on an alteration of the suppressive phenotype of the resident AMs. Understanding mechanisms that might restore the immunosuppressive phenotype of AMs in the lung may lead to novel therapies in conditions of autoimmune pulmonary fibrosis.
Acknowledgments
We appreciate the critical and helpful discussions of our data with Drs Patricia Finn, David Perkins (Brigham and Women’s Hospital) and J. Wayne Streilein (Schepens Eye Research Institute). We thank Drs Jeffrey Drazen and Patricia Finn (Brigham and Women’s Hospital) for their critical reading of this manuscript and Dr Ann Elsner (Schepens Eye Research Institute) for her advice on appropriate statistical approaches for our data. We appreciate the administrative support of Ms Gayle Barry and her preparation of this manuscript.
Abbreviations
- ADT-HIPIF
adoptive transfer-hapten immune pulmonary interstitial fibrosis
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- Cl2MDP
dichloromethylene diphosphonate
- DEPC
diethlyprocarbonate
- HIPIF
hapten immune pulmonary interstitial fibrosis
- i.n.
intranasal
- i.p.
intraperitoneal
- i.t.
intratracheal
- i.v.
intravenous
- MFI
mean fluorescence intensity
- RT–PCR
reverse transcription–polymerase chain reaction
- TNBS
2,4,6-trinitrobenzene sulphonic acid
References
- 1.Weltzien HU, Moulon C, Marin C, Padovan S, Harmann U, Kohler J. T cell immune response to haptens. Structural models for allergic and autoimmune reactions. Toxicology. 1996;107:141–51. doi: 10.1016/0300-483x(95)03253-c. 10.1016/0300-483x(95)03253-c. [DOI] [PubMed] [Google Scholar]
- 2.Kalish RS, Wood JA. Induction of hapten-specific tolerance of human CD8+ urushiol (poison ivy)-reactive T lymphocytes. J Invest Dermatol. 1997;108:253–7. doi: 10.1111/1523-1747.ep12286447. [DOI] [PubMed] [Google Scholar]
- 3.Gelber C, Gemmell L, McAteer D, et al. Down-regulation of poison ivy/oak-induced contact sensitivity by treatment with a class II MHC binding peptide–hapten conjugate. J Immunol. 1997;158:2425–35. [PubMed] [Google Scholar]
- 4.Padovan E, von Greyerz S, Pichler WJ, Weltzien HU. Antigen-dependent and -independent IFN-gamma modulation by penicillins. J Immunol. 1999;162:1171–7. [PubMed] [Google Scholar]
- 5.Coleman JW. Protein haptenation by drugs. Clin Exp Allergy. 1998;28:79–82. [PubMed] [Google Scholar]
- 6.Horton H, Weston SD, Hewitt CR. Allergy to antibiotics: T-cell recognition of amoxicillin is HLA-DR restricted and does not require antigen processing. Allergy. 1998;53:83–8. doi: 10.1111/j.1398-9995.1998.tb03778.x. [DOI] [PubMed] [Google Scholar]
- 7.Grabbe S, Steinert M, Mahnke K, Schwarz A, Luger TA, Schwartz T. Dissection of antigenic and irritative effects of epicutaneously applied haptens in mice. Evidence that not the antigenic component but nonspecific proinflammatory effects of haptens determine the concentration-dependent elicitation of allergic contact dermatitis. J Clin Invest. 1996;98:1158–64. doi: 10.1172/JCI118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 9.De Weck AL. Drug allergy, immunotherapy, immune complexes and anaphylaxis. Curr Opin Immunol. 1990;2:548–57. doi: 10.1016/0952-7915(90)90009-6. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard R, Lewis S, Richards K, Johnson I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1999;347:284–9. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 11.Sauty A, Rochat T, Schoch OD, Hamacher J, Kurt AM, Dayer JM, Nicod LP. Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-Jo-1 antibodies. Eur Respir J. 1997;10:2907–12. doi: 10.1183/09031936.97.10122907. [DOI] [PubMed] [Google Scholar]
- 12.Grigolo B, Mazzetti I, Borzi RM, et al. Mapping of topoisomerase II alpha epitopes recognized by autoantibodies in idiopathic pulmonary fibrosis. Clin Exp Immunol. 1999;114:339–46. doi: 10.1046/j.1365-2249.1998.00747.x. 10.1046/j.1365-2249.1998.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Stein-Streilein J. Hapten-immune pulmonary interstitial fibrosis (HIPIF) in mice requires both CD4+ and CD8+ T lymphocytes. J Leukoc Biol. 1993;54:414–22. doi: 10.1002/jlb.54.5.414. [DOI] [PubMed] [Google Scholar]
- 14.Kimura RH, Hu H, Stein-Streilein J. Delayed type hypersensitivity (DTH) responses regulate collagen deposition in the lung. Immunology. 1992;77:550–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Stein-Streilein J, Lipscomb MF, Fisch H, Whitney PL. Pulmonary interstitial fibrosis induced in hapten immune hamsters. Am Rev Respir Dis. 1987;136:119–23. doi: 10.1164/ajrccm/136.1.119. [DOI] [PubMed] [Google Scholar]
- 16.Kimura R, Hu H, Stein-Streilein J. Immunological tolerance to hapten prevents subsequent induction of hapten-immune pulmonary interstitial fibrosis (HIPIF) Cell Immunol. 1992;145:351–8. doi: 10.1016/0008-8749(92)90337-o. [DOI] [PubMed] [Google Scholar]
- 17.Garcia H, Salter-Cid L, Stein-Streilein J. Persistent interleukin-2 activity and molecular evidence for expression of lymphotoxin in the hapten-immune model for pulmonary interstitial fibrosis. Am J Respir Cell Mol Biol. 1992;6:22–8. doi: 10.1165/ajrcmb/6.1.22. [DOI] [PubMed] [Google Scholar]
- 18.Strickland DH, Thepen T, Kees UR, Kraal G, Holt PG. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology. 1993;80:266–72. [PMC free article] [PubMed] [Google Scholar]
- 19.Steele MG, Herscowitz HB. Suppression of murine IgM, IgG, IgA and IgE antibody responses by alveolar macrophages. Immunology. 1993;80:62–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Holt PG, Oliver J, Bilyk N, Mcmenamin C, McMenamin PG, Kraal IG, Thepen T. Downregulation of the antigen-presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilyk N, Holt PG. Cytokine modulation of the immunosuppressive phenotype of pulmonary alveolar macrophage populations. Immunology. 1995;86:231–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrecht BN, Carro-Muino I, Vermaelen K, Pauwels RA. Allergen-induced changes in bone-marrow progenitor and airway dendritic cells in sensitized rats. Am J Respir Cell Mol Biol. 1999;20:1165–74. doi: 10.1165/ajrcmb.20.6.3484. [DOI] [PubMed] [Google Scholar]
- 23.Toews GB, Vial WC, Dunn MM, Guzzetta P, Nunez G, Stastny P, Lipscomb MF. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol. 1984;132:181–6. [PubMed] [Google Scholar]
- 24.Lipscomb MF, Toews GB, Lyons CR, Uhr JW. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981;126:286–91. [PubMed] [Google Scholar]
- 25.Thepen T, McMenamin C, Girn B, Kraal G, Holt PG. Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin Exp Allergy. 1992;22:1107–14. doi: 10.1111/j.1365-2222.1992.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 26.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–85. doi: 10.1016/s0167-7799(97)01019-6. 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 27.Woessner Jf. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 28.Hallden G, Skold CM, Eklund A, Forslid J, Hed J. Quenching of intracellular autofluorescence in alveolar macrophages permits analysis of fluorochrome labelled surface antigens by flow cytofluorometry. J Immunol Methods. 1991;142:207–14. doi: 10.1016/0022-1759(91)90108-r. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi O, Yamane H, Imajoh-Ohmi S, Nariuchi H. IL-12 receptor (IL-12R) expression and accumulation of IL-12Rβ1 and IL-12Rβ2 mRNAs in CD4+ T cells by costimulation with B7-2 molecules. J Immunol. 1998;160:1638–46. [PubMed] [Google Scholar]
- 30.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 31.Spatz C. Basic Statistics, Tables of Distributions. 5. Pacific Grove, CA: Brooks/Cole Publishing Co.; 1993. [Google Scholar]
- 32.Thepen T, van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 34.Presky DH, Yang H, Minetti LJ, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–7. doi: 10.1073/pnas.93.24.14002. 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity. 1998;9:315–23. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 36.Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH. TNF-α-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–9. [PubMed] [Google Scholar]
- 38.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–21. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- 39.Strickland D, Kees UR, Holt PG. Regulation of T-cell activation in the lung: isolated lung T cells exhibit surface phenotypic characteristics of recent activation including down-modulated T-cell receptors, but are locked into the G0/G1 phase of the cell cycle. Immunology. 1996;87:242–9. doi: 10.1046/j.1365-2567.1996.460541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Ren J, Dhabuwala CB, Shichi H. Immunotolerance induced by intratesticular antigen priming: expression of TGF-beta, Fas and Fas ligand. Ocular Immunol Inflamm. 1997;5:75–84. doi: 10.3109/09273949709085055. [DOI] [PubMed] [Google Scholar]
- 41.Gonnella PA, Chen Y, Inobe J, Komagata Y, Quartulli M, Weiner HL. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160:4708–18. [PubMed] [Google Scholar]
- 42.Streilein JW. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilige in the eye. Eye. 1995;9:236–40. doi: 10.1038/eye.1995.46. [DOI] [PubMed] [Google Scholar]
- 43.Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD. Immunolocalization of TGF-beta 1, TGF-beta 2, and TGF-beta 3 in the anterior segment of the human eye. Invest Ophthalmol Vis Sci. 1993;34:23–30. [PubMed] [Google Scholar]
- 44.Khalil NK, O’Connor RN, Flanders KC, Unruh H. TGF-β1, but not TGF-β2 or TGF-β3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14:131–8. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 45.Sacco O, Romberger D, Rizzino A, Beckmann JD, Rennard SI, Spurzem JR. Spontaneous production of transforming growth factor-β2 by primary cultures of bronchial epithelial cells. J Clin Invest. 1992;90:1379–85. doi: 10.1172/JCI116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thepen T, McMenamin C, Oliver J, Kraal G, Holt PG. Regulation of immune responses to inhaled antigen by alveolar macrophages: differential effects of in vivo elimination on the induction of tolerance vs. immunity. Eur J Immunol. 1991;21:2845–50. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- 47.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 1 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–47. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki Y, Araki K, Vesin C, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–9. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Pober JS. TNF initiates E-selectin transcription in human endothelial cells through parallel TRAF-NF-κB and TRAF-RAC/CDC42-JNK-c-Jun/ATF2 pathways. J Immunol. 1997;159:3508–18. [PubMed] [Google Scholar]
- 50.Dekaris I, Su-Ning Z, Dana MR. TNFα regulates corneal Langerhans’ cell migration. J Immunol. 1999;162:4235–9. [PubMed] [Google Scholar]
- 51.Avane M, Andres PG, Li DJ, Reinecker H-C. NF-KappaB-inducing kinase is a common mediator of IL-17-, TNF-Alpha-, and IL-1Beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–44. [PubMed] [Google Scholar]