Abstract
A panel of four mimotopes of the epitope recognized by the highly protective monoclonal antibody against Schistosoma mansoni (152-66-9B) was obtained by screening a solid-phase 8mer random peptide library. Three of the four mimotopes (p28, p29 and p30) were efficiently recognized in an in vitro radioimmunoassay by the monoclonal antibody and by sera from infected mice and one (p30) induced in vitro proliferation of primed lymphocytes. When the mimotopes were conjugated to bovine serum albumin (BSA) and the conjugates used to immunize C57BL/6J mice, only the p30–BSA-induced antibodies which were effective at complement-mediated killing of schistosomula. The level of complement-mediated killing obtained with the anti-p30 antibodies was comparable to that seen with serum from mice immunized with the protective 9B-antigen. Furthermore, following challenge infection of mimotope–BSA-immunized mice, a greater than 40% reduction in worm burden was observed in p30–BSA-immunized mice, a level comparable to that seen following immunization with the intact 9B-antigen. These results show that a simple synthetic peptide immunogen comprising an eight-amino acid mimotope of a conformational epitope on the 9B-antigen can induce protective immune responses against S. mansoni that are comparable to those obtained following immunization with the far more complex intact antigen. This mimotope may well represent a potential component of a synthetic peptide vaccine against S. mansoni. The inclusion of other B-cell- and T-cell-stimulating synthetic epitopes in such a vaccine, together with a more appropriate carrier, adjuvant and delivery systems may well result in a level of protection even greater than that seen with the single mimotope.
Introduction
Schistosomiasis is a serious parasitic disease with world-wide distribution, causing an estimated 200 000 deaths per year. Despite the fact that the global distribution of schistosomiasis has changed significantly in the past 50 years, particularly in regions where control strategies have been successfully employed, the disease remains endemic in over 70 developing countries and more than 200 million people are estimated to be harbouring the disease.1 Chemotherapy, does not provide a satisfactory solution since, although effective, it does not prevent re-infection, and in addition, partial drug resistance to the most commonly used chemotherapeutic agent against schistosomiasis, Praziquantel, has been reported.2,3 Hence, immunological intervention in the form of a vaccine would contribute to the success of the present efforts if added to existing control strategies.1 Furthermore, as has been amply discussed,4 even partial protection, i.e. reduction of worm burden, could lead to significant alleviation of the morbidity of this parasitic disease.
To date there is no available vaccine against schistosomiasis. Furthermore, due to the complex life cycle of the schistosomes and the inability to culture them in vitro, it is obvious that any practical vaccine against it must be based on either recombinant DNA technology – cloning and expression of relevant protective antigen(s) – or on a chemically synthesized compound – such as a peptide. Synthetic peptide vaccines based on relevant epitopes of protective antigens, besides being inexpensive to prepare, can be used to direct the immune response to cellular or humoral immunity by selection of specific T- and B-cell epitopes. Coupling of such peptides to carriers and/or the employment of appropriate adjuvants may help enhance the specific immune response. Synthetic peptide vaccines are also attractive because they may avoid toxic effects associated with the pathogen and could be designed to emphasize the immunogenic elements that may be hidden in the native antigen.5,6 Peptides for use as potential vaccines could be comprised of linear amino acid sequences representing B-cell epitopes derived from protective antigens of the pathogen.7,8
Although many antibodies are directed to linear epitopes, it is likely that antibodies to conformational epitopes on infectious agents are of particular importance in protective immunity. The identification and synthesis of peptides representing conformational epitopes is not easy, since the epitopes are made up of amino acids brought together by folding of the protein chains. One approach which has been successfully employed in a number of systems is the production of mimotopes. Combinatorial peptide libraries, in which vast numbers of different peptides are expressed either on filamentous phage or solid-phase resin beads,9,10 have been used to identify mimics of epitopes recognized by virus-neutralizing and protective antibodies. It should be remembered that mimotopes of conformational epitopes will not necessarily bear sequence identity or homology with the primary amino acid sequence of the epitope in the protein. It might, however, assume a conformation that mimics that of the epitope.
In the realm of parasite vaccines, research towards the development of synthetic peptide vaccines has been focused mainly on malaria.11,12 In the case of schistosomiasis, efforts have been made to direct specific B-cell and T-helper cell responses to the parasite by identifying different epitopes in protective antigens such as Sm23 and Triose-Phosphate Isomerase (TPI).13,14 A cytotoxic T-cell C-terminal lipopeptide has also been derived from the vaccine candidate Sm28 GST.15
In our laboratory we have studied a highly protective monoclonal antibody against Schistosoma mansoni, denoted 152-66-9B, which leads to efficient killing of the parasite in vitro (67%) and to 82% inhibition of its infectivity.16 Affinity chromatography of the parasite extract on this antibody led to the isolation of an antigen denoted 9B-antigen which has molecular weight (MW) 450 000 in its native form, but migrates as a 200 000 MW band in the presence of sodium dodecyl sulphate, and under reducing conditions exhibits two main subunits of 45 000 and 30 000, respectively.16 This 9B-antigen is a highly protective antigen inducing immune responses leading to 45% protection following administration in complete Freund's adjuvant (CFA),16–18 and up to 65% protection when delivered with proteosomes.19 It is recognized by antibodies in sera from mice vaccinated with irradiated cercaria as well as by antibodies in sera from individual patients infected with Schistosoma mansoni or S. haematobium,19 indicating that it could be a promising vaccine candidate.
The work described in this paper was performed to determine whether mimotopes selected from a synthetic combinatorial peptide library by the protective monoclonal antibody 152-66-9B could serve as the basis for a peptide vaccine. The results show that an eight-residue peptide selected from the library in this way, when conjugated to a carrier, induced an antibody response which conferred a significant level of protection in mice (up to 42%) against challenge infection.
Materials and methods
Reagents
All materials and chemicals used in this study were of analytical grade or the best grade available.
Parasite
A Puerto Rican strain of S. mansoni was maintained in outbred CD1 mice and Biomphalaria glabrata snails. Schistosoma mansoni cercariae were artificially transformed into schistosomula and the bodies were separated from the tails in a 63% Percoll gradient.20 These schistosomula were incubated for 3 hr at 37° in defined synthetic medium (DSM) in an atmosphere of 5% CO2. Adult worms were obtained by liver perfusion from chronically infected mice 6–7 weeks postinfection as described.21
Sera
Normal mouse serum (NMS) and sera from acutely infected mice taken 9 weeks after exposure to 150 cercariae17 were obtained from CD1 and C57BL/6J mice.
Monoclonal antibody 152-66-9B and 9B-antigen
The monoclonal antibody 152-66-9B and the immunoaffinity-purified protective antigen, 9B-antigen, were prepared as described previously.16
Synthesis of peptide library and selection of mimotopes
A solid-phase peptide library was synthesized on NovasynTG, a polystyrene–polyoxyethylene resin functionalized with amino groups in such a way that each resin bears a different 8mer amino acid peptide.10 The resin allows the deprotection of the peptide without its cleavage from the resin and there are 2·86 × 106 beads per g of resin with a capacity of 0·24 mol/g. The synthesis of the library was performed with 10 g of resin. Nineteen reaction vessels containing the resin were used for synthesis by fluorenyl-methoxy-carbonyl (Fmoc) chemistry with all amino acids with the exception of cysteine.
The beads were blocked with 10% bovine serum albumin (BSA) −1% Tween-20 in phosphate-buffered saline (PBS) at room temperature for 2 hr. Then, 500 µl of a 1 : 50 dilution of ascitic fluid containing monoclonal antibody 152-66-9B was added to 50 mg of the resin library and incubated for 2 hr at room temperature and for 18 hr at 4°. Beads bound by the antibody were identified following the addition of peroxidase-conjugated rabbit anti-mouse immunoglobulin antibody (Nordic, Tilburg, the Netherlands) at a final dilution of 1 : 1000 followed by the addition of diaminobenzidine chloronaphthol substrate. Darkly stained beads were manually selected,10 the bound antibody was dissociated by washing with trifluoroacetic acid and the peptides on the individual beads were microsequenced.
Peptide synthesis and preparation of conjugates
The eight-residue peptides were synthesized by Prof. M. Fridkin of the Department of Organic Chemistry at The Weizmann Institute by the solid-phase method using a multi-peptide synthesizer (Abimed AMS 422, Langfield, Germany) and were purified by high-performance liquid chromatography (HPLC). The peptides were coupled to BSA by using the water-soluble carbodiimide reagent 1-ethyl-3(3-dimethyl-amino propyl)carbodimide (EDCI) for protein conjugation.22 A 1 : 38 molar ration of carrier to peptide was used.
Radioimmunoassay (RIA)
Solid-phase RIA was performed essentially as described by Pierce and Klinman.23 Briefly, 96-well microtest flexible assay plates (Falcon, Becton-Dickinson Labware, Oxnard, CA) were coated with 10 µg cercariae sonicate or 1 µg purified 9B-antigen in 100 µl PBS/well, or 5 µg of 9B-peptide p28 to p31 in 2% gluteraldehyde PBS solution at room temperature for 2 hr. After washing and blocking with 1% casein in PBS, with various dilutions of mouse antisera were added (10−1−10−4) and 2–4 hr later, were washed and incubated for 2 hr at room temperature or overnight at 4° with affinity-purified 125I-labelled anti-mouse F(ab′)2 (105 c.p.m./well). The washed wells were dried, cut out and counted in an auto gamma-counter.
Enzyme-linked immunosorbent assay (ELISA)
Whole parasite sonicate (10 µg/ml), or 9B-peptides p28 to p31 in PBS solution were allowed to dry in the wells of ELISA microtitre plates (Immunoplate, Nunc, Denmark) overnight at room temperature, or alternatively peptide–protein conjugates (5 µg/ml) in sodium carbonate buffer, pH 9·6, were allowed to adsorb to the plates for 2 hr at 25° or 18 hr at 4°. The plates were washed three times with PBS containing 0·1% Tween-20 (PBS-Tween). The wells were then blocked for 90 min at 25° with PBS containing 1% casein. After three washes with PBS-Tween, serial dilutions of the different antibodies as specified were added and incubated for 2 hr at 37° or for 18 hr at 4°. Unbound antibody was washed off with PBS-Tween and goat anti-mouse or goat anti-human immunoglobulin conjugated to peroxidase was added and incubated for 2 hr at 37°. Finally, 2,2-azino-bis(3-ethylbenz-thiazoline-6-sulphonic acid) diammonium salt (ABTS) was added as substrate. The colour produced by the hydrolysis of the substrate was determined at 405 nm by an ELISA reader (Multiskan MCC/340 MK II, Lab Systems, Helsinki, Finland).
Immunization and protection studies
Groups of 8–10 mice (C57BL/6J) were immunized intradermally and in the footpads with the BSA–peptide conjugates (50 µg/100 µl in CFA) as follows. The first injection was administered in the footpads and into the base of the tail and after 3-week intervals, two booster injection with the same conjugate in incomplete Freund's adjuvant (IFA), were given intraperitoneally or subcutaneously. Sera were collected 3 weeks after the second and third immunizations and analysed by ELISA. Four weeks after the last booster, infection with 70–90 cercaria was performed by the ring method.21 Control groups in the various experiments included untreated mice and mice injected as described above with either the carrier (BSA) alone in adjuvant, or adjuvant alone.
Complement-dependent cytotoxicity assay
The susceptibility of schistosomula to antibodies and complement was determined as reported previously.17 Briefly, 200 schistosomula in 65 µl DSM medium were incubated for 20 min with an equal volume of specific antiserum or control serum diluted 1 : 2 at 37° in 5% CO2 atmosphere. As a source of complement fresh guinea-pig serum was added at 1 : 9 final dilution, with heat-inactivated complement serving as a control. The cultures were incubated for an additional 18 hr following which live and dead parasites were counted under an inverted microscope.
Lymphocyte proliferation assay
C57BL/6J mice were immunized with 50 µg/100 µl peptide conjugated with BSA emulsified with an equal volume of CFA (H37 Ra). The preparation was injected into the footpads and into the base of the tail. Draining lymph nodes were dissected 10 days after immunization and lymphocyte proliferative responses to 9B-peptide mimotopes were tested in vitro in 96-well flat-bottomed plates using triplicates of 0·2 ml cultures containing 5 × 105 cells/well in RPMI-HEPES.17 Lymphocytes were stimulated with different concentrations of the various peptides and after 72 hr in culture, the cells were pulsed with 1 mCi (37 Bq) of [3H]thymidine ([3H]TdR) overnight and then harvested. The thymidine incorporation was determined in a Packard β-counter.
Statistical analysis
Statistical analysis was performed using the stat view ii program (Abacus Concepts Inc., Berkeley, CA) on a Macintosh II Ci. F-test was utilized to calculate probability (P) values. Result are presented as means±standard errors.
Results
Amino acid sequences of mimotopes
Following screening of resin beads from the peptide library with the monoclonal antibody 152-66-9B, the amino acid sequences of the peptides on six darkly stained beads were determined by microsequencing and four were chosen for detailed study. The sequences of these four peptides are shown in Table 1. The four peptides representing 9B-mimotopes were synthesized in preparative quantities. All the peptides were > 95% pure by HPLC and their amino acid analyses were in agreement with the expected values. Peptide p30 appeared to be the most hydrophobic of the four peptides (retention time in HPLC of 32·8 min) and p31 was the most hydrophilic (retention time of 27·52 min). Interestingly, none of the mimotope peptides showed any sequence homology with schistosome protein data bases.
Table 1.
Amino acid sequences of mimotopes of the monoclonal antibody 152-66-9B
| Mimotope | Sequence |
|---|---|
| p28 | VLLRRIGG |
| p29 | HLLRLSEI |
| p30 | SLLTYMKM |
| p31 | YLLQKLRN |
Recognition of the 9B-peptides by the 155-66-9B monoclonal antibody
The four synthetic peptides were evaluated for their recognition by the original protective monoclonal antibody. Each peptide in the free form was bound to the RIA plates (5 µg/well) and incubated with different concentrations of 152-66-9B over a range of dilutions from 1 : 10 to 1 : 1280. The amount of bound monoclonal antibody was measured with 125I-labelled goat anti-mouse immunoglobulin. The results show that three of the four tested peptides were recognized by the monoclonal antibody, with peptide p28 demonstrating the highest level of binding (Fig. 1). The only peptide not recognized was the more hydrophilic p31.
Figure 1.
Recognition of mimotopes by the monoclonal antibody 152-66-9B in a radioimmunoassay. Recognition was measured by monitoring antibody binding to unconjugated peptides p28–p31: p28 (▪); p29 (♦); p30 (•); p31 (▴); and binding of p28 to a non-related monoclonal antibody (□).
Humoral immune responses
Groups of mice were immunized with conjugates of the peptides p28, p29, p30 and p31 coupled to BSA. Anti-peptide antisera were tested in ELISA for their reactivity with the respective homologous peptide. Serum from mice infected with 150 cercaria was used as a positive control. As depicted in Fig. 2(a–d) each of the peptides was recognized by the respective homologous antiserum as well as by sera from infected mice, with comparable titres (Fig. 2a–d). A negative control, namely the interaction with an irrelevant peptide (influenza haemagglutinin 307–319), gave no response with any of the anti-mimotope sera. The isotype profile of the anti-peptide antibodies was analysed and clear-cut results were obtained only in the case of anti-p30 antibodies, which were of the immunoglobulin G1 subclass (results not shown).
Figure 2.
Recognition of the four mimotopes by their respective homologous anti-peptide antibodies and by serum from acutely infected mice. Recognition was quantified in ELISA by monitoring antibody binding to free peptides adsorbed on the plates: p28 (a); p29 (b); p30 (c); p31 (d). Homologous antipeptide serum (○); serum from infected mouse (▵) and NMS (□).
The anti-peptide antisera were also evaluated for their capacity to recognize the native schistosome antigens. As shown in Fig. 3(a–c), all four anti-peptide antisera bound to ELISA plates coated with the schistosomula extract, at levels comparable to those obtained with antibodies against the intact 9B-antigen and with antibodies from infected mice used as positive controls.
Figure 3.
Recognition of the schistosomula extract by anti-mimotope antisera. (a) anti-p28; (b) anti-p29; (c) anti-p30. Binding by the homologous anti-mimotope antisera (○); anti-9B-antigen antibodies (◊); serum from infected mouse (▵); and NMS (□).
Complement-dependent cytotoxicity of anti-9B-peptides
The 9B-antigen is a surface antigen,16 therefore, antisera produced by immunization with the mimotope peptides p28 to p31, were evaluated for their capacity to mediate complement-dependent killing of schistosomula. Sera from mice immunized with CFA or normal untreated mice were used as a negative control and sera from mice immunized with 9B-antigen were used as a positive control. Experiments were performed with fresh guinea-pig serum as a source of complement and used heat-inactivated complement as an internal control. The efficiency of killing was assessed by the difference between the percentage of dead schistosomula in the presence of active and inactivated complement. The most effective killing (43%) was observed with anti-mimotope peptide p30 (Fig. 4), indicating significant cytotoxicity (P = 0·05). This anti-p30 antibody caused also tegumental blebbing and granulation of the parasite in the presence of fresh guinea-pig complement during an 18-hr incubation. The complement-dependent killing observed was similar to the level of cytotoxicity induced by the positive control, anti-9B-antigen mouse serum (Fig. 4), which induced an average 51% killing. Anti-p28 and anti-p29 antisera induced a lower level of cytotoxicity (27–29% killing), and anti-p31 did not lead to any specific killing. In the presence of control sera, obtained from mice immunized with either BSA or adjuvant alone, non-specific killing of 15–20% was observed. Similar low levels of non-specific killing was observed in the presence of heat-inactivated complement.
Figure 4.
Complement-mediated cytotoxicity of anti-mimotope antibodies. Sera from mice immunized with the various peptide conjugates were added to 200 schistosomula (3 hr post-transformation) and incubated for 30 min. The susceptibility to complement-mediated lysis was determined in the presence of fresh guinea-pig complement (C). Results are expressed as percentage killing of the schistosomula as compared with the killing in the presence of heat-inactivated complement (HIC).
Induction of proliferative lymphocyte response by the mimotope–BSA conjugates
The ability of mimotope peptide–BSA conjugates to prime and restimulate lymphocytes was investigated in vitro using lymph node cells from mice immunized with peptides p28 to p31 conjugated to BSA and with BSA alone. Following culture in vitro with free peptides, significant positive proliferative responses were obtained only with lymphocytes from mice immunized with the mimotope p30–BSA conjugate, which, following in vitro restimulation with the homologous p30, showed a stimulation index of 14·5. In addition, lymphocytes from p30–BSA-immunized mice were also stimulated to proliferate following incubation with the mimotope p28 at a concentration of 20 µg/well with a stimulation index of 7·5 (Fig. 5). Low proliferative responses were observed towards p29 and p31 and no proliferation was demonstrated in the presence of the carrier protein BSA. Lymphocytes from mice immunized with the other three mimotopes (p28, p29 and p31) conjugated to BSA, were induced to only very low cellular proliferation (data not shown).
Figure 5.
Proliferative responses of lymph node cells from mice immunized with mimotope p30–BSA conjugate. Lymph node cells were stimulated with various concentrations of unconjugated mimotopes or with BSA and the proliferative response was assayed as the [3H]TdR uptake measured following a pulse label for the last 12 hr of incubation.
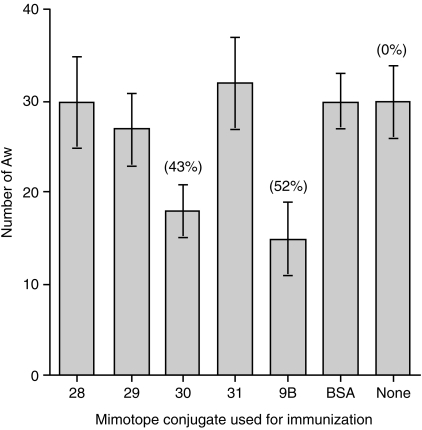
Protection conferred by the 9B-peptides
The most important issue is whether or not these mimotope peptides can induce a protective response against challenge infection with the parasite. To analyse the protective properties of the mimotopes, mice were immunized with 50 µg of the mimotope–BSA conjugates, twice or three times at 3-week intervals. Mice were challenged with 70–90 cercariae (ring assay) 1 month later. As shown in Fig. 6, worm burden was reduced by an average of 42% ± 3 as a result of immunization with the p30–BSA conjugate (P = 0·0001–0·004) and by 6% ± 3 after immunization with the p29–BSA conjugate (P = 0·5). The other two peptides did not induce a protective immune response (3% and 1% reduction of worm burden, respectively) compared with control mice that were injected with carrier (BSA), with adjuvant alone, or were not immunized. In the positive control group, consisting of mice immunized with the intact 9B-antigen, 52% protection was observed.
Figure 6.
Protection of mice immunized with mimotope–BSA conjugates against challenge infection. Mice were immunized with 50 µg of the respective peptide conjugate in CFA. One month after the last injection, mice were infected percutaneously with 70–90 cercariae. Six or seven weeks after infection, their livers were perfused and the numbers of adult worms (Aw) were determined.
Discussion
Synthetic peptides have several advantages as vaccine candidates: they are relatively easy and inexpensive to produce and therefore high quantities of a homogeneous product may be obtained without the necessity for complicated purification procedures.5,6 Furthermore, peptide vaccines have the advantage that they can be designed to include peptides that stimulate specific T-cell and B-cell responses. Strategies for the use of peptide epitopes as vaccines against S. mansoni have been developed in several laboratories.13–15,24 Work described in this paper was designed to explore this approach, as related to a protective antigen (9B-antigen) which has been extensively investigated in this laboratory.16–19 This antigen was purified using the protective monoclonal antibody 152-66-9B. The epitope reactive with the antibody is not characterized as yet and could be either conformational or linear. The fact that the antibody reacts with the antigen in Western blotting may serve as an indication that a linear sequence may be involved. However, it is possible that the epitope recognized by the antibody assumes a particular unique conformation.
A combinatorial peptide library was screened with this protective monoclonal antibody assuming that it is directed towards conformational epitopes on the 9B-antigen. In this way, it was hoped to determine whether or not mimotopes of the conformational epitope/s recognized by this antibody could be identified and used to induce protective antibody responses. It should be noted that B-cell responses are particularly important in the course of schistosomiasis and have already been used to identify potential vaccine candidates. Thus, passive transfer of serum from mice vaccinated with irradiated cercariae can confer partial resistance to naive recipients and such serum has been used to identify several potential vaccine candidates.25,26 Similarly, the surface antigen, glyceraldehyde 3-phosphate dehydrogenase, which is preferentially recognized by the serum of resistant patients, as well as peptides derived from it, have also been suggested as a vaccine candidate.27
In the present study, four mimotopes selected from a combinatorial peptide library using the protective monoclonal antibody 152-66-9B were synthesized and were shown to be bound by the monoclonal antibody in ELISA (Fig. 1), indicating that these 9B-related peptides are mimotopes of B-cell epitopes in the native antigen. Of the four mimotopes studied, p28 was bound by the monoclonal antibody at a higher level than the others, but as indicated by its lower effectiveness in mediating complement-dependent cytotoxicity and protection, this in vitro antibody-binding assay does not seem to be an effective correlate with potential vaccine efficacy.
Serum from acutely infected CD1 mice also contained antibodies that recognized the mimotopes (Fig. 2), with the highest binding against mimotope p30. The presence of anti-mimotope binding in sera following natural infection indicates that in the course of the disease, antibodies of this specificity are indeed generated and thus immunization with these peptides could result in the induction of protective responses. To investigate the potential protective capacity of the 9B-derived peptides, these four peptides were coupled to BSA and were used to immunize mice. In vitro, antibodies from mice immunized with the mimotope–BSA conjugate induced specific killing of schistosomula in the presence of complement, with anti-p30 demonstrating the most significant effect. Even though the antibodies were specific for a single epitope of a surface component of the parasite, the extent of killing by the anti-p30 sera was relatively high and was comparable to that seen with serum of mice vaccinated with the intact protective 9B-antigen (Fig. 4). Antibodies and complement interact with the surface membrane causing damage28,29 and the damage to the tegument (blebbing) that was observed with anti-p30 was consistent with these findings. The biological activity of anti-mimotope p30 antibodies indicates the important role of the humoral response in protection against challenge infection and underlies the notion that even a single antigen, if relevant, could serve as a suitable target for effective, immunologically mediated damage to the parasite.
Following challenge infection of the mice immunized with the four mimotope–BSA conjugates, significant protection (over 40%) was observed only following immunization with the p30–BSA conjugate (Fig. 6). This level of protection is similar to that achieved following immunization with the intact 9B-antigen (i.e. approximately 50%). It is of interest that this p30 mimotope was also the one which was best recognized by serum from infected mice (Fig. 2) and was the only one of the four peptides which induced lymphocyte proliferation (Fig. 5). Furthermore, it was also the most effective in inducing complement-dependent cytotoxicity (Fig. 4). Immunization with the other three mimotopes led to less than < 10% protection following challenge infection.
The data presented in this study, demonstrate that protective immune responses can be induced following immunization with an 8mer synthetic peptide conjugated to BSA. These results are encouraging in terms of vaccine development because of the simplicity of peptide synthesis and the short length of the peptide. The efficacy of the approach may be enhanced by replacement analysis, with PEPSCAN or SPOTs system, to replace each amino acid of the original mimotope sequence with all other amino acids to identify substitution/s resulting in improved binding with the antibody. This procedure could produce sequences which manifest higher protective capacity. Potentiation of immune responses to the mimotope by employing different carriers and the use of appropriate adjuvants could also augment the protective effect observed and should be further investigated. Furthermore, other synthetic peptide epitopes or mimotopes could be used in conjunction with mimotope p30 to increase the level of protection. However, even the presently observed reduction in worm burden could lead to reduced morbidity and suggests that this mimotope may well represent a potential vaccine candidate.
Acknowledgments
This work was supported in part by European Commission Contract BIO4-CT98-0294.
REFERENCES
- 1.Savioli L, Renganathan E, Montresor A, Davis A, Behbehani K. Control of Schistosomiasis – A global picture. Parasitol Today. 1997;13:555–562. doi: 10.1016/s0169-4758(97)01141-1. [DOI] [PubMed] [Google Scholar]
- 2.Fallon PG, Sturrock RF, Niang AC, Doenhoff MJ. Diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:61–2. [PubMed] [Google Scholar]
- 3.Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennnett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villager that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–8. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist NR. Controlling Schistosomiasis by vaccination: a realistic option? Parasitol Today. 1995;11:191–4. [Google Scholar]
- 5.Arnon R. Synthetic peptides as the basis for vaccine design. Mol Immunol. 1991;28:209–15. doi: 10.1016/0161-5890(91)90063-p. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Yedidia T, Arnon R. Design of peptide and polypeptide vaccines. Curr Opin Biotechnol. 1997;8:442. doi: 10.1016/s0958-1669(97)80066-3. [DOI] [PubMed] [Google Scholar]
- 7.Arnon R. Immuno-parasitological parameters in schistosomiasis – a perspective view of a vaccine-oriented immunochemist. Vaccine. 1991;9:379–94. doi: 10.1016/0264-410x(91)90123-n. [DOI] [PubMed] [Google Scholar]
- 8.Arnon R, Levi R. Synthetic recombinant vaccines induce anti-influenza long-term immunity and cross-strain protection. In: Cohen S, Shafferman A, editors. Novel Strategies in Design, Production of Vaccines. New York: Plenum Press; 1996. pp. 23–9. [Google Scholar]
- 9.Devlin JJ, Panganiban LC, Devlin PE. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404–9. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- 10.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski W, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–4. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira G, Clavijo P, Nussenzweig RS, Nardin EH. Immunogenicity of an alum-adsorbed synthetic multiple-antigen peptide based on B- and T-cell epitopes of the Plasmodium falciparum CS protein: possible vaccine application. Vaccine. 1994;12:1012–7. doi: 10.1016/0264-410x(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 12.Patarroyo ME, Romero P, Torres ML, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabazas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–32. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds SR, Schoemaker CB, Harn DA. T and B cell epitope mapping of SM23 an integral membrane protein of Schistosoma mansoni. J Immunol. 1992;149:3995–4001. [PubMed] [Google Scholar]
- 14.Reynolds SR, Dahl CE, Harn. DA. T and B epitopes determination and analysis of multiple antigenic peptides for Schistosoma mansoni. Experimental vaccine Triose-Phosphate Isomerase. J Immunol. 1994;152:193–200. [PubMed] [Google Scholar]
- 15.Pancre V, Gras-Masse H, Delanoye A, Herno J, Capron A, Auriault C. Induction of cytotoxic T-cell activity by the protective antigen of Schistosoma mansoni Sm28GST or its derived C-terminal lipopeptide. Scand J Immunol. 1996;44:485–92. doi: 10.1046/j.1365-3083.1996.d01-340.x. [DOI] [PubMed] [Google Scholar]
- 16.Tarrab-Hazdai R, Levi-Schaffer F, Brenner V, Horowitz S, Eshhar Z, Arnon R. Protective monoclonal antibodies against Schistosoma mansoni antigen isolation, and suitability for active immunization. J Immunol. 1985;135:2772–9. [PubMed] [Google Scholar]
- 17.Mendlovic F, Tarrab-Hazdai R, Puri J, Arnon R. Genetic control of immune response to a purified Schistosoma mansoni antigen. I. Effect of MHC class II antigens on the cellular, humoral and protective responses. Parasite Immunol. 1989;11:667–82. doi: 10.1111/j.1365-3024.1989.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 18.Mendlovic F, Arnon R, Tarrab-Hazdai R, Puri J. Genetic control of immune response to a purified Schistosoma mansoni antigen. II. Establishment and characterization of specific I-A and I-E restricted T-cell clones. Parasite Immunol. 1989;11:683–94. doi: 10.1111/j.1365-3024.1989.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 19.Tarrab-Hazdai R, Schechtman D, Lowell G, Pirak E, Arnon R. Proteosome delivery of protective 9B-antigen against Schistosoma mansoni. Intern J Immunopharm. 1999;21:205–18. doi: 10.1016/s0192-0561(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 20.Ramalho-Pinto FJ, de Rossi R, Smithers SR. Murine Schistosomiasis mansoni: anti-schistosomula antibody and the IgG sub-classes involved in the complement- and eosinophil-mediated killing of the schistosomula. In-Vitro Parasite Immnunol. 1979;1:2295–308. doi: 10.1111/j.1365-3024.1979.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 21.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of S. mansoni and the recovery of adults worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 22.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–2. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 23.Pierce SK, Klinman NR. Allogenic carrier specific enhancement of hapten-specific secondary B-cell responses. J Exp Med. 1967;114:1254–61. doi: 10.1084/jem.144.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankovic D, Aslund L, . Oswald IP, et al. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996;157:806–14. [PubMed] [Google Scholar]
- 25.Richter D, Harn DA, Matuschka FR. The irradiated cercariae vaccine model: looking on the bright side of radiation. Parasitoltoday. 1995;8:288–93. doi: 10.1016/0169-4758(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 26.Soisson LM, Masterson CP, Tom TD, McNally MT, Lowell GH, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–20. [PubMed] [Google Scholar]
- 27.Goudot CV, Caillol D, Jabali MD, Dessein AJ. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989;170:2065–80. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishelson Z. Complement and parasitic trematodes. Parasitol Today. 1989;5:19–25. doi: 10.1016/0169-4758(89)90218-4. [DOI] [PubMed] [Google Scholar]
- 29.Fishelson Z, Parizade M, Ghendler Y, Arnon R. Complement and Schistosoma mansoni. Complem Inflamm. 1990;7:23–123. [Google Scholar]