Abstract
We have previously presented evidence of a highly organized and compartmentalized structure of the small intestinal lamina propria of the pig. In this work, we have identified at least two major populations of cells in this site expressing high levels of major histocompatibility complex (MHC) class II antigens. One is CD45 positive and is a potent initiator of a primary immune response, this is a function usually associated with dendritic cells. These cells have characteristic dendritic morphology, but show evidence of phagocytosis as well as other phenotypic markers of immature dendritic cells. Some cells show evidence of ongoing immune maturation. We have also isolated CD45 negative endothelial cells bearing significant amounts of MHC class II, which do not trigger a mixed lymphocyte reaction. These findings have implications for the functional role of healthy gut lamina propria and clearly implicate this site as capable of differential antigen presentation by a heterogeneous population of antigen-presenting cells.
Introduction
The immunological role of the intestinal lamina propria (LP), populated predominantly by T cells of memory phenotype, is generally seen as a site of secondary responses, primary antigen presentation is usually associated with the organized lymphoid tissue of the Peyer's patches. However, the presence of macrophages1–3 as well as of cells with dendritic morphology, suggestive of primary presentation,4,5 has been described in several species. Cells with dendritic morphology and functional characteristics have been isolated from lymphatics draining the rat intestine,6 however, the origin of these cells in either LP or Peyer's patches is not clear. Functional studies of a relatively impure cell population isolated from mouse LP in a primary antigen-specific system in vitro, has suggested that LP antigen-presenting cells (APC) are capable of initiating a primary response, but cause T cells to respond with a different cytokine profile compared to the one elicited by APC isolated from other sites.6,7 However, the APC reported by most workers in this site appear to be macrophages, for example, cells isolated by centrifugal elutriation from the human small intestine have been classified phenotypically as CD14– macrophages.1 Surprisingly, an increase in the numbers of dendritic cells (DC) has been associated with increased immunological tolerance,8 whereas an influx of CD14+ macrophages has been observed in inflamed or transplanted tissue.9–13 Therefore, we know relatively little about the antigen-presenting role of the gut LP; but relative numbers of macrophages, DCs and other types of APC in the healthy and diseased LP appear to have clear implications for the immunological role of this site. An additional complication are the functional and phenotypic similarities between immature DCs and monocytes/macrophages which are increasingly reported: This has decreased the usefulness of the conventional categories of APC and may well have led to overlapping classification of the same cells.
Additionally, major histocompatibility complex class II (MHC II) has been found in healthy individuals on stromal cells such as epithelial cells in rodents14 and humans15 and on endothelial cells in pigs.16 The relevance of these stromal cells as APC also needs to be assessed. Functional studies of the role of endothelial cells as APC in humans, however, have been carried out only after MHC II induction on cultured or interferon-γ (IFN-γ)-treated endothelial cells.17–19
In summary, there is evidence for at least the potential for primary presentation, possibly in an unusual way, as well as for secondary presentation by more than one type of intestinal presenting cell, but we lack a detailed characterization of the cells involved. In order to assess an antigen-presenting system, functional as well as detailed phenotypic studies of purified cell populations are required. To date, functional studies of freshly isolated, purified cell populations from this site are relatively rare. The large numbers of pure cells required for such studies are difficult to isolate in sufficient numbers from rodents; studies of normal human tissue and comparisons with cells from other tissue sites are also difficult.
We have therefore chosen the pig for these studies, as a model for antigen presentation in the healthy omnivorous mammalian gut. Additionally, a better understanding of the porcine immune system is useful not only because it is an important farm species, but also in view of the increasing interest in this species as a donor for xenotransplantation.
We have previously shown the presence of large numbers of cells expressing high levels of MHC II in the pig jejunal LP.16,20 We have also shown that it is possible to isolate large numbers of LP cells from normal animals without contaminating cells from either the Peyer's patches or from epithelium.21 In this work, we have studied two highly purified MHC II-high cell populations, freshly isolated by flow cytometric cell sorting from this site.
Materials and methods
Animals
Large White/Landrace hybrid pigs aged 4–5 months and raised under conventional husbandry conditions were used for histology, isolation and characterization of presenting cell populations. Pigs were killed by exsanguination following electrical stunning. Responder lymphocytes for mixed lymphocyte reactions (MLR) were isolated from venous blood taken from MHC-inbred Minnesota minipigs, cc strain aged 1–9 months.22
Two-colour flow cytometry was carried out on five different animals, with quantification of the distribution of MHC II for three animals; three-colour flow cytometry was carried out for four animals. Flow cytometric cell sorting for the preparation of cytospins as well as for functional studies (MLR) was carried out on six animals. Immunostaining for extra- and intracellular determinants was carried out on three animals. Representative results are shown in each case.
Immunohistology
Three-colour fluorescent immunohistology was carried out on cryosections, taken from mid-jejunum of slaughterweight Large White/Landrace pigs as previously described,16 as previous studies had shown the presence of large numbers of MHC II-positive cells in this site.16,20 Briefly, 4 µm thick cryosections were cut from OCT (Tissue Tek, BDH, Lutterworth, UK) embedded and snap-frozen tissue. Sections were air dried and fixed for 10 min in ice-cold acetone. They were used either fresh or following storage at −20° for a maximum period of 2 weeks. Fc receptors were blocked for 30 min with phosphate-buffered saline (PBS) containing 5% pig serum and 5% goat serum. Combinations of pretitrated monoclonal antibodies were applied in PBS for a minimum of 2 hr. Slides were washed thoroughly in three changes of PBS for 5 min each. Secondary reagents were isotype specific goat anti-mouse anti-sera (Southern Biotechnology, Birmingham, AL) conjugated to biotin, fluoroscein isothiocyanate (FITC) or Texas Red, applied in combination at optimal dilutions. For antibody cocktails containing the monoclonal antibody MIL11 of immunoglobulin E (IgE) isotype, the secondary reagent to mouse IgE was a biotinylated rat anti-mouse IgE monoclonal antibody (Southern Biotechnology). Goat anti-mouse IgG1 antisera also bound to this rat monoclonal antibody. Therefore, the goat anti-mouse IgG1 conjugate was preabsorbed with 10% rat serum for 1 hr before use. Conjugates were incubated for 1 hr. Following three more thorough washing steps, the biotinylated reagents were detected with 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-Avidin D (Vector Laboratories, Burlingame, CA). After a final wash, the sections were mounted in Fluoromount (Vector Laboratories) and sealed with nail varnish. Stained slides were examined using a Leica epifluorescence microscope fitted with a combined excitation and emission filter block specific for FITC, Texas Red (TXRD) and AMCA. Images were recorded on a digital camera (Photonic Science, Milham Mountfield, UK). The coexpression of MHC II and other surface molecules was quantified by counting five representative tissue sections each.
Monoclonal antibodies
Antibodies used for this study are listed in Table 1.
Table 1.
Specificity and source of antibodies
| Clone | Specificity | Isotype | Source |
|---|---|---|---|
| MSA3 | MHC II DR | IgG2a | Dr J. Lunney39 |
| K274.3G8 | MHC II DQ | IgG1 | Authors40 |
| 76-7-4 | CD1 | IgG2a | Dr J. Lunney41 |
| TMG.6-5 | CD11b (human) | IgG1 | Dr I. Ando |
| MIL2 | CD14 | IgG2b | Authors42 |
| G7 | CD16 | IgG1 | Dr B.Y. Kim43 |
| CC51 | CD21 | IgG2b | Dr C. Howard44 |
| LC1-4 | CD31* | IgG1 | Dr P. Kilshaw45 |
| K252.1E4 | CD45 | IgG1 | Authors46 |
| Bb-1 | CD80 (CD74) | IgM | Pharmingen |
| 74-22-15 | SwC3 | IgG2b | Dr J. Lunney23 |
| C4 | SwC9 | IgG1 | Dr T. Whittall24 |
| MIL11 | Capillary endothelium* | IgE | Authors16 |
The cell surface expression of CD31 was used to identify endothelial cells in flow cytometry; MIL11 was used to identify endothelium in immunohistology.
Cell isolation
Leucocytes were isolated from jejunal LP by collagenase digestion as described previously.21 Briefly, an inverted inflated gut sac was incubated for 2–3 hr in several changes of calcium and magnesium-free Hank's balanced salt sloution (HBSS), containing 1 mm of ethylenediamine tetra-acetic acid (EDTA), at 37° on a shaking platform. This procedure largely removed the epithelial layer. The remaining tissue was then digested in RPMI-1640 (Dutch Modification, Life Technologies Ltd, Paisley, UK. Cat. no. 22409-015) containing 100 units/ml of collagenase (Collagenase Grade V, Sigma, St Louis, MO; Cat. No. C9263) at 37° for 2 × 45 min. Similarly, cells were isolated from spleen tissue fragments by collagenase digestion using identical conditions. Cells from both tissues were suspended in a 30% Percoll in HBSS solution and underlaid with a 50% Percoll solution. Low-density cells were recovered from the 30%/50% gradient interface.
Flow cytometry, cell sorting and cytospin preparations of sorted cells
Leucocytes (1 × 106) were suspended in 50 µl of PBS containing 5% pig serum plus 0·2% sodium azide (PBSA) and incubated with 50 µl each of optimized concentrations of monoclonal antibodies for 1 hr at 4°. Cells were washed in PBSA, and incubated with 50 µl each of pretitrated isotype-specific affinity-purified goat anti-mouse anti-sera, biotinylated or conjugated to FITC and phycoerythrin (PE), respectively (Southern Biotechnology). For two-colour flow cytometry, only PE and FITC conjugates were used, for three-colour flow cytometry, the third colour was visualized using the appropriate isotype-specific biotinylated antiserum followed by a third step consisting of the addition of 1 µl of streptavidin-Tricolor (Caltag Laboratories, Burlingame, CA). Cells were washed in PBSA, and fluorescence was quantified using an EPICS CS or XL Flow Cytometer (Coulter Electronics, Luton, UK). Appropriate isotype-matched controls were used. Cells were electronically gated on forward light scatter (FLS) to exclude small contaminating debris. For flow cytometric cell sorting and subsequent cell culture, PBS at 4° without azide was used for all washing and incubation steps.
Sorted cells were also used to make cytospin preparations using a Shandon Southern cytospin centrifuge (Shandon Scientific, Runcorn, UK). These preparations from sorted cells were viewed stained with Leishmann's stain using standard methods.
Mixed lymphocyte reaction (MLR)
Peripheral blood lymphocytes (PBL) isolated from Minnesota minipigs were cultured at 106 cells/ml in triplicate 200 µl wells in RPMI-1640, Dutch modification, supplemented with 1 mm mercaptoethanol, 1 mm glutamine, 10 IU/ml penicillin/streptomycin and 2% normal pig serum. To these were added the stimulator cell populations from Large White/Landrace pigs in ratios indicated on Figs 6 and 7. Preliminary experiments had indicated an optimum ratio of APC : responders of 1 : 100. Unfractionated stimulator cells were used with and without staining with primary and secondary reagents, as well as before and after enrichment by flow cytometric cell sorting. Cells were cultured for five days, pulsed for 6 hr with 1 µCi 3H-thymidine (Amersham International), and harvested onto glass fibre mats. The incorporated 3H-thymidine was quantified with a Rackbeta 1219 liquid scintillation counter (LKB).
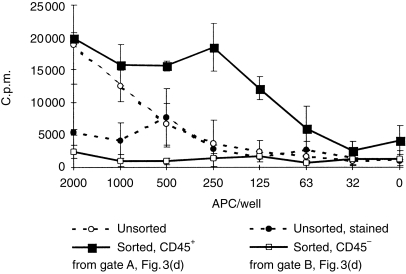
Figure 6.
Proliferative response of peripheral blood mononuclear cells (PBMC) isolated from cc Minnesota minipigs, 200 000 cells/well. Means of triplicate wells ± sd. x-axis shows numbers of stimulator APC isolated from Large White/Landrace LP.
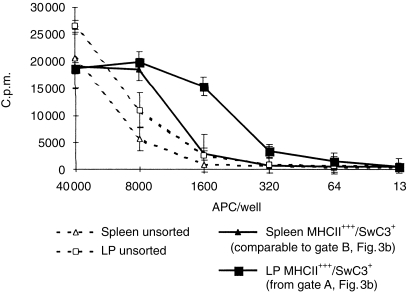
Figure 7.
Proliferative response of PBMC isolated from cc Minnesota minipigs, 200 000 cells/well. Means of triplicate wells ± sd. x-axis shows numbers of stimulator APC isolated from Large White/Landrace LP and spleen.
Three-colour staining for extra- and intracellular determinants
To investigate the distribution of extra- and intracellular CD3, MHC class II or other determinants, the following procedure was carried out. Unfractionated cell preparations, obtained after a brief collagenase digestion of 15 min in order to minimize the risk of in vitro-induced phagocytosis of lymphocytes, were stained in solution, as described for flow cytometric staining, with the first antibody and labelled with FITC (extracellular determinant, green). Cytospin preparations were then made of stained cells, re-stained with the first antibody and labelled with AMCA (blue, intra-and extracellular staining).
Results
Immunohistology
The presence of many cells bearing MHC II on a section from mid-jejunum is shown in Fig. 1. High levels of MHC II were found on many cells in the LP, predominantly on and immediately below the capillary plexus. Occasionally, we observed MHC II+ cells in the epithelium itself or protruding through the capillary network into the epithelial layer beyond (arrow head). Most of these MHC II+ cells coexpressed CD45, as shown by their yellow to orange (green + red) colouring. The presence of MHC II on capillary and lacteal endothelium is indicated by the purple to mauve coloration. However, we found consistently that some MHC II inside the LP was not coexpressed with either CD45 or endothelium (red coloration only).
Figure 1.
Section from mid jejunum stained with (a) anti-CD45 (FITC, green) (b) anti-MHC II (TXRD, red) and (c) the endothelial marker MIL11 (AMCA, blue). In the composite image (d), orange to yellow colouring shows cells coexpressing MHC II and CD45, mauve colouring indicates MHC II on capillary endothelium. Arrow head shows MHC II expressing cell penetrating the capillary network, (E) marks endothelium, (L) a lacteal and the dot shows an area which is pure red and therefore indicative of MHC II on other (non-endothelial) stromal cells. Magnification ×500.
We have quantified the coexpression of MHC II with CD45 as well with CD16 (not shown). Flow cytometry suggested that CD16 was the most characteristic cell surface marker of the population under study, see Fig. 3) by counting single- and dual-expressing cells on tissue other than the capillary endothelium. Table 2 shows that approximately 50% of CD45+ cells expressed MHC II, MHC II+ cells represented a very numerous cell population. Conversely, approximately 80% of total MHC II in LP was accounted for by CD45 (or CD16) positive cells, suggesting that high levels of MHC II together with CD45 or CD16 identified the same or largely overlapping cell populations. The remaining 20% of MHC II appeared to be in stromal tissue deep inside the LP. In contrast to findings in other species but consistent with previous reports in the pig, intestinal epithelium did not express MHC II.
Figure 3.
Two-colour flow cytometry on lamina propria cells, isolated by collagenase digestion and density gradient isolation. Coexpression of cell surface MHC II dR (y-axis) with (a) MHC II dQ (x-axis) (b) SwC3 (x-axis) (c) CD16 (x-axis) and (d) CD45 (x-axis). Gates A, B and C were used for flow cytometric cell sorting.
Table 2.
MHC class II co-expression with other cell-surface molecules by immunohistology
| Surface antigen | % coexpressing MHC II (sd) | % of total MHC II (sd) |
|---|---|---|
| CD45 | 52·0 (14·2) | 83·8 (3·9) |
| CD16 | 77·5 (10·5) | 78·4 (6·1) |
Figure 2 shows the coexpression of CD80 with MHC II, together with labelling of the endothelial network. CD80 (green) is present on a subset of MHC II (red) expressing cells as shown by the yellow (green+red) colouring, mainly close to the capillary plexus.
Figure 2.
Section from mid jejunum stained with (a) anti CD80 (FITC, green) (b) anti-MHC II (TXRD, red) and (c) the endothelial marker MIL11 (AMCA, blue). In the composite image (d), orange to yellow colouring shows cells coexpressing MHC II and CD80, mauve colouring indicates MHC II on capillary endothelium. Arrow head shows MHC II expressing cell penetrating the capillary network. Magnification ×250.
Immunohistology therefore suggested a minimum of three different cell populations bearing high levels of MHC II and therefore presumably capable of antigen presentation in normal pig jejunum: a population expressing CD45 as well as CD16, capillary endothelium and stromal tissue in the core of the LP.
Phenotypic studies of isolated cells
Following cell isolation by tissue digestion and density gradient centrifugation, the resultant low density cell population was analysed by two- and three-colour flow cytometry. Additionally, cells isolated by flow cytometric cell sorting were used for cytospin preparations and stained with Leishmann's.
Figure 3 shows evidence for two major MHC II-high cell populations: population A expressed CD45 (Fig. 3d, gate A) and CD16 (Fig. 3c) as well as SwC3 (Fig. 3b, gate A), the myeloid/monocytic cell surface cluster determinant in pigs.23 Note also that the expression of MHC II dR was synchronous with MHC II dQ on all cells. Similar gates were also used for three-colour flow cytometry and cell sorting. Co-expression of high levels of surface MHC II and CD16 appeared the most characteristic markers for population A. Gate B showed a population of cells, expressing MHC II, but none of the other cell surface molecules in this study. Leishmann's-stained cytospin preparations of cells sorted from gate A are shown in Fig. 4(a). These cells showed dendritic morphology. They were also strongly adherent, both with each other as well as with small lymphocytes, which were frequently found adhering to these large DC. Occasionally, the nucleus of a lymphocyte appeared within the DC, suggesting phagocytosis (arrow). In contrast, although expressing comparably high levels of MHC II, population B showed a different morphology (Fig. 4b), with very pale cytoplasmic staining and dense oval nuclei. These cells tended to associate in an end-to-end arrangement, had no adhering lymphocytes and were negative for all leucocytic cell surface molecules. The CD45+ cells expressing low amounts of MHC II were shown to consist predominantly of lymphocytes by flow cytometric cell sorting (Fig. 3d, gate C, results not shown).
Figure 4.
Cytospin preparations from cells sorted by flow cytometry, stained with Leishmann's. (a) Morphology of population A isolated from lamina propria, sorted from gate A in Fig. 3(d). Arrow shows DC containing a small lymphocyte. (b) Morphology of population B isolated from lamina propria, sorted from gate B in Fig. 3(d). Morphology is compatible with endothelial cells. Magnification ×750.
The identity of population A as CD45+/CD16+/SwC3+ and of population B as CD45–/CD16–/SwC3– suggested by two-colour flow cytometry was established in three-colour flow cytometry, with the addition of several other cell surface molecules for more detailed phenotyping (Fig. 5a–j). Gating based on the coexpression of high levels of MHC II as well as SwC3 showed that the majority of MHC II+++/CD45+ cells also expressed SwC3 (approximately 90%, not shown). Conversely, all MHC II+++/SwC3+ cells (Fig. 5e) were CD45+, and MHC II++/SwC3– cells were CD45– (Fig. 5f). We could thus identify population A as MHC II+++/SwC3+/CD16+/CD45+/CD11b+/CD31–(Fig. 5a,c,e,g,i). Population B was MHC II++/SwC3–/CD16–/CD45–/CD11b–/CD31+ (Fig. 5b, d, f, h, j). CD31 had been confirmed by immunohistology to be expressed on capillary endothelium (not shown), as reported for other species.
Figure 5.
Three-colour flow cytometry. Cells gated as MHC II+++/SwC3+ cells (a, c, e, g, i) and MHC II++/SwC3– cells (b, d, f, h, j), as shown in Fig. 3(b). Traces show staining with third reagent: control mouse serum, diluted 1 : 1000 (a, b), CD16 (c, d), CD45 (e, f), CD11b (g, h) and CD31 (i, j). The dotted lines show the cut-off values for both populations with control mouse serum.
Other cell surface molecules studied by flow cytometry were SwC9, a marker for pig tissue macrophages,24 CD14, CD1 and CD21. None of these surface molecules were found on cells isolated from LP, but were present on cells isolated in parallel studies from spleen (not shown). The lack of these cell surface markers was also confirmed by immunohistology (not shown), preliminary immunohistochemistry studies had shown no CD1 or CD21+ cells and only very low numbers of CD14+ cells in this tissue.
The existence of a third population (MHC II+/CD45–) in the LP, suggested by immunohistology (Table 2) could not be confirmed, as we could not positively identify this cell type on isolated cells with any cell-surface marker.
Functional studies of isolated cells, MLR
We also assessed the ability of both cell types to trigger unprimed T cells, using purified cell preparations obtained by flow cytometric cell sorting (gated as shown in Fig. 3). Figure 6 is a typical trace of one of five repeat experiments, and shows that the CD45+ subset isolated from LP is a potent initiator of a mixed lymphocyte reaction. The magnitude of the stimulatory effect of the sorted population A is particularly significant in view of a clear inhibitory effect of staining with primary and secondary reagents. The CD45– population does not have a stimulatory capacity, in spite of considerable MHC II expression. In one experiment, we compared cells isolated from LP with those isolated similarly from spleen. In this case, sorting was based on the coexpression of high levels of MHC II and SwC3 for both tissues, to avoid the numerous CD21+ B cells in spleen. Figure 7 shows that cells isolated from LP were more effective as stimulators of an MLR.
Evidence for phagocytosis and intracellular MHC II
The granularity and size – forward light scatter/side scatter (FLS/SS) – characteristics of the MHC II-high population A from LP were found to be relatively high in comparison to similar cells isolated from spleen (not shown). Additionally, Fig. 4(a) indicated the intracellular presence of a lymphocyte (arrow) in a DC. This was suggestive of ongoing phagocytosis.
Multicolour staining for extra- and intracellular MHC II showed the presence of intracellular MHC class II as well as extracellular MHC II in many cells (Fig. 8). This, as well as the evidence for phagocytosis, confirmed the immature status of some of these DC.
Figure 8.
Composite picture of representative cells, selected from cytospin preparations from unfractionated cellular digests, stained for extra- and intracellular MHC II. Extracellular staining in solution for MHC II (FITC, green), followed by cytospin preparation and staining in situ with MHC II (AMCA, blue). Magnification ×1000.
Discussion
In this study of the healthy gut of young adult pigs, we have found evidence of an antigen-presenting environment that is both complex as well as capable of providing strong stimuli to local T cells in the jejunal LP. However, we have found little evidence for the presence of classic macrophages, a cell type linked predominantly to inflamed gut tissue.9–13
We have found a large population of APC, potent stimulators in an MLR and therefore capable of primary presentation and by definition functional DC. This classification was supported by characteristic DC morphology of sorted cells. However, other functional and phenotypic characteristics, such as the ability to phagocytose and the expression of CD11b and CD16, would conventionally classify these cells as macrophages. Thus, macrophages have been reported in this site in humans1,2 and the pig.3 However, these cells were classified as ‘CD14–’ macrophages in humans.1 Our studies showed little evidence of monocytic APC other than the DC population A, CD14+ cells were present but exceedingly rare. However, recent evidence shows that the ability to phagocytose, the presence of large amounts of intracellular MHC II and the expression of the cell surface molecules CD16 and CD11b are compatible with the phenotype of immature DC.25,26 This would suggest that the major professional APC population in the small intestine of the pig is a population of immature DC, capable of phagocytosis, yet potent stimulators of a primary response. Some of these cells appear to be undergoing functional maturation and express the costimulatory molecule CD80. Most evidence to date suggests that costimulation by CD80 is not essential for antigen-experienced cells. Therefore, the role of CD80 in the LP, dominated by T cells of this phenotype, is not entirely clear. DCs are highly migratory. It is possible to speculate that mature CD80-expressing DC are destined for emigration, and may prime antigen-naive cells in other lymphoid sites such as mesenteric lymph nodes, inducing a productive proliferative primary response there. These T cells in turn may return as mature memory cells to the LP.
Recent work shows many parallels between macrophages and immature DC, and monocytes can be converted to DC in vitro by a suitable cytokine environment.27,28 Many cell-surface molecules are shared by both cell types.29 Therefore, the classification of cells to one or other of these APC types can be ambiguous, and our evidence is compatible with all available evidence in the pig as well as in other species, although the presence of some classic macrophages cannot be excluded in other species or other intestinal sites.
Many of the factors triggering the maturation of immature DC such as the ingestion of apoptotic30,31 or virally infected cells,26 bacterial products of commensal as well as pathogenic organisms and cytokines such as interleukin-4 (IL-4)32,33 are likely to be present in this site. Apoptotic endothelial fragments have been reported in DC cells emigrating via the thoracic duct,34 other authors have reported epithelial-derived apoptotic fragments in macrophages in the human intestine.35 The transport of bacteria from the intestinal lumen to blood via CD18+ cells in the LP has also been shown.35,36
There appears to be a contradiction between the presence of potent primary presenting cells and T cells of predominantly memory type in the same environment. However, memory T cells such as those present in the LP37 show an increased propensity to activation-induced apoptosis.38 One of the roles of the DC may therefore be the provision of a strong signal to memory T cells, specific for environmental antigens and prone to apoptosis, together with the capacity for phagocytosing the resulting apoptosing T cells. Increased numbers of DC have indeed been linked to the induction of oral tolerance.8 Additionally, there is clearly the possibility of primary presentation to the less numerous T cells without antigen experience in this site.
Additionally, we have confirmed that pig capillary endothelium expresses high levels of cell-surface MHC II, in agreement with previous findings of MHC II expression on porcine endothelial cells in many but not all tissues.16 These freshly isolated endothelial cells appear functionally incapable of primary presentation, but may be capable of stimulating secondary responses. The third population of CD45–/MHC II+ stromal cells indicated by immunohistology in the LP itself could not be identified by flow cytometric studies and requires further study.
Acknowledgments
This work was supported by a grant from the BBSRC, UK. We also thank all donors for the use of their antibodies.
Abbreviations
- APC
antigen-presenting cells
- LP
lamina propria
- MHC class II (MHC II)
major histocompatibility complex II
References
- 1.Smith PD, Janoff EN, Mosteller-Barnum M, Merger M, Orenstein JM, Kearney JF, Graham MF. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 2.Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer's-patches compared with normal ileal lamina propria and mesenteric lymph-nodes. Gut. 1993;34:1357–63. doi: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kambarage DM, Bland PW, Stokes CR, Brown P, Skuse AM. Ultrastructural, histochemical and immunohistochemical features of porcine intestinal lamina propria macrophages, peripheral-blood monocytes and splenic adherent cells. J Comp Pathol. 1995;112:63–77. doi: 10.1016/s0021-9975(05)80090-8. [DOI] [PubMed] [Google Scholar]
- 4.Pavli P, Hume DA, Vandepol E, Doe WF. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology. 1993;78:132–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Maric I, Holt PG, Perdue MH, Bienenstock J. Class-II MHC antigen (Ia) -bearing dendritic cells in the epithelium of the rat intestine. J Immunol. 1996;156:1408–14. [PubMed] [Google Scholar]
- 6.Liu LM, MacPherson GG. Rat intestinal dendritic cells – immunostimulatory potency and phenotypic characterization. Immunology. 1995;85:88–93. [PMC free article] [PubMed] [Google Scholar]
- 7.Harper HM, Cochrane L, Williams NA. The role of small-intestinal antigen-presenting cells in the induction of T-cell reactivity to soluble-protein antigens – association between aberrant presentation in the lamina propria and oral tolerance. Immunology. 1996;89:449–56. doi: 10.1046/j.1365-2567.1996.d01-760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viney JL, Mowat AMO, Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 9.Forbes GM, Horne R, Erber WN, Collins BJ, Papadimitriou JM. Ultrastructural evidence of intestinal mucosal macrophage activation after bone marrow transplantation. Pathology. 1996;28:251–4. doi: 10.1080/00313029600169094. [DOI] [PubMed] [Google Scholar]
- 10.Waraich T, Sarsfield P, Wright DH. The accessory cell populations in ulcerative colitis: a comparison between the colon and appendix in colitis and acute appendicitis. Human Pathol. 1997;28:297–303. doi: 10.1016/s0046-8177(97)90127-1. [DOI] [PubMed] [Google Scholar]
- 11.Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel-disease mucosa. J Gastroenterol Hepatol. 1995;10:387–95. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 12.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel-disease – apparent recruitment from peripheral-blood monocytes. Gut. 1994;35:669–74. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Brandtzaeg P, Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493–505. doi: 10.1016/s0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- 14.Bland PW, Warren LG. Antigen presentation by epithelial cells of the rat small intestine. Immunology. 1986;58:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Mayrhofer G, Spargo LD. Subcellular distribution of class II major histocompatibility antigens in enterocytes of the human and rat small intestine. Immunol Cell Biol. 1989;67:25–60. doi: 10.1038/icb.1989.38. [DOI] [PubMed] [Google Scholar]
- 16.Wilson AD, Haverson K, Southgate K, Bland PW, Stokes CR, Bailey M. Expression of major histocompatibility complex class-II antigens on normal porcine intestinal endothelium. Immunology. 1996;88:98–103. doi: 10.1046/j.1365-2567.1996.d01-640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pober JS, Collins T, Gimbrone MA. Lymphocytes recognise human vascular endothelium and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983;305:726. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- 18.McCarron RM, Racke M, Spatz M. Class II MHC antigen expression by cultures of human vascular endothelial cells. Brain Res. 1991;566:325–7. doi: 10.1016/0006-8993(91)91718-g. [DOI] [PubMed] [Google Scholar]
- 19.Haraldsen G, Sollid LM, Bakke O, et al. Major histocompatibility complex class II-dependent antigen presentation by human intestinal endothelial cells. Gastroenterology. 1998;114:649–56. doi: 10.1016/s0016-5085(98)70578-5. [DOI] [PubMed] [Google Scholar]
- 20.Vega-Lopez MA, Telemo E, Bailey M, Stevens K, Stokes CR. Immune cell distribution in the small-intestine of the pig – immunohistological evidence for an organized compartmentalization in the lamina propria. Vet Immunol Immunopathol. 1993;37:49–60. doi: 10.1016/0165-2427(93)90015-v. [DOI] [PubMed] [Google Scholar]
- 21.Bailey M, Hall L, Bland PW, Stokes CR. Production of cytokines by lymphocytes from spleen, mesenteric lymph-node and intestinal lamina propria of pigs. Immunology. 1994;82:577–83. [PMC free article] [PubMed] [Google Scholar]
- 22.Sachs DH, Leight G, Cone J, Schwarz S, Stuart I, Rosenberg S. Transplantation in miniature swine. I. fixation of the major histocompatibility complex. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Blecha F, Kielian T, McVey DS, et al. Workshop studies on monoclonal-antibodies reactive against porcine myeloid cells. Vet Immunol Immunopathol. 1994;43:269–72. doi: 10.1016/0165-2427(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez J, Ezquerra A, Alonso F, et al. Workshop studies with monoclonal antibodies identifying a novel porcine differentiation antigen, SWC9. Vet Immunol Immunopathol. 1998;60:343–9. doi: 10.1016/s0165-2427(97)00110-4. 10.1016/s0165-2427(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 25.Luft T, Pang KC, Thomas E, Hertzog P, Hart DNJ, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. Jimmunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 26.Regnault A, Lankar D, Lacabanne V, et al. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L-J, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triozzi P, Aldrich W. Phenotypic and functional differences between human dendritic cells derived in vitro from haemapoietic progenitors and from monocytes/macrophages. J Leukocyte Biol. 1997;61:600–8. doi: 10.1002/jlb.61.5.600. [DOI] [PubMed] [Google Scholar]
- 29.Woodhead VE, Binks MH, Chain BM, Katz DR. From sentinel to messenger: an extended phenotypic analysis of the monocyte to dendritic cell transition. Immunology. 1998;94:552–9. doi: 10.1046/j.1365-2567.1998.00547.x. 10.1046/j.1365-2567.1998.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M, Ricciardi Castagnoli P, Rugarli C, Manfredi AA. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–71. [PubMed] [Google Scholar]
- 31.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class-II compartment – down-regulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A. Efficient presentation of soluble-antigen by cultured human dendritic cells is maintained by granulocyte–macrophage colony-stimulating factor plus interleukin-4 and down-regulated by tumor-necrosis-factor-alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacPherson GG, Huang FP, Platt N, Liu LM, Jenkins CD, Major J, Powell T, Wykes M. A discrete sub-population of dendritic cells constitutively transports apoptotic epithelial cells from the intestine to T cell areas of mesenteric nodes. J Leukocyte Biol. 1998;S2:D4. [Google Scholar]
- 35.Nagashima R, Maeda K, Imai Y, Takahashi T. Lamina propria macrophages in the human gastrointestinal mucosa – their distribution, immunohistological phenotype, and function. J Histochem Cytochem. 1996;44:721–31. doi: 10.1177/44.7.8675993. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Torres A, Jones-Carson J, Baeumler A, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:727–8. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 37.Haverson K, Stokes CR, Bailey M. T cell populations in the pig intestinal lamina propria – memory cells with unusual phenotypic characteristics. Immunology. 1999;96:66–73. doi: 10.1046/j.1365-2567.1999.00658.x. 10.1046/j.1365-2567.1999.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salmon M, Pilling D, Borthwick N, et al. The progressive differentiation of primed T-cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892–89. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 39.Hammerberg C, Schurig GG. Characterization of monoclonal-antibodies directed against swine leukocytes. Vet Immunol Immunopathol. 1986;11:107–12. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- 40.Lunney JK, Walker K, Goldman T, et al. Overview of the First International Workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1994;43:193–220. doi: 10.1016/0165-2427(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 41.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal-antibodies reactive with porcine PBL. J Immunol. 1984;133:368–75. [PubMed] [Google Scholar]
- 42.Thacker E, Summerfield A, McCullough K, et al. Summary of workshop findings for porcine myelomonocytic markers. Vet Immunol Immunopathol. in press. [DOI] [PubMed]
- 43.Dato ME, Wierda WG, Kim YB. A triggering structure recognized by G7 monoclonal-antibody on porcine lymphocytes and granulocytes. Cell Immunol. 1992;140:468–74. doi: 10.1016/0008-8749(92)90212-8. [DOI] [PubMed] [Google Scholar]
- 44.Denham S, Shimizu M, Bianchi A, et al. Monoclonal antibodies recognizing differentiation antigens on porcine B cells. Vet Immunol Immunopathol. 1994;43:259–67. doi: 10.1016/0165-2427(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 45.Nasu K, Whyte A, Green SJ, Evans PC, Kilshaw PJ. Alpha-Galactosyl-mediated activation of porcine endothelial cells – studies on CD31 and VE-cadherin in adhesion and signaling. Transplantation. 1999;68:86167. doi: 10.1097/00007890-199909270-00020. [DOI] [PubMed] [Google Scholar]
- 46.Zuckermann FA, Binns RM, Husmann R, et al. Analyses of monoclonal-antibodies reactive with porcine CD44 and CD45. Vet Immunol Immunopathol. 1994;43:293–305. doi: 10.1016/0165-2427(94)90151-1. [DOI] [PubMed] [Google Scholar]