Abstract
Polymophonuclear cells (PMN) of healthy donors do not express major histocompatibility complex (MHC) class II antigens or the T-cell costimulatory molecules CD80 or CD86. Expression of these receptors, however, is seen in patients with chronic inflammatory diseases. We now report that, by culturing PMN of healthy donors with autologous serum, interferon-γ (IFN-γ) and granulocyte–macrophage colony-stimulating factor (GM-CSF), de novo synthesis of MHC class II, CD80 and CD86 could be induced. MHC class II-positive PMN acquired the capacity to present staphylococcus enterotoxin to peripheral T cells, apparent as induction of interleukin-2 (IL-2) synthesis and proliferation of the T cells. Moreover, the PMN also processed tetanus toxoid (TT) and induced proliferation of TT-specific T cells in a MHC class II-restricted manner. Taken together, these data indicate that PMN can be activated to function as accessory cells for T-cell activation.
Introduction
Polymorphonuclear cells (PMN) are considered to be short-lived, terminally differentiated cells that act in the first line of defence against bacterial infection. Under pathological conditions, however, PMN may have an extended life span that is concurrent with alterations of receptor expression and function.1,2 Similar observations were made with cultured PMN: within hours after onset of culture, PMN undergo apoptosis, CD16 is lost from the surface and the phagocytic activity declines.3–5 Addition of cytokines, particularly interferon-γ (IFN-γ), preserves the expression of CD16 and the phagocytic activity, and extends the life span.6–9 Moreover, CD64 is up-regulated, as are major histocompatibility complex (MHC) class II antigens.5,10,11
Constitutive expression of MHC class II is restricted to professional antigen-presenting cells (APC) such as dendritic cells, B cells, monocytes or macrophages. There is, however, evidence that MHC class II antigen expression can be induced in a number of cells, including eosinophils,12 keratinocytes,13 synovial fibroblasts,14 tubular epithelial cells15 and others, by culturing the cells in the presence of cytokines, notably IFN-γ, granulocyte–macrophage colony-stimulating factor (GM-CSF) or interleukin (IL)-3.16 Those cytokines also induce synthesis of MHC class II on PMN of healthy donors within 24–48 hr of culture. Of note is the variability in response when cells of different donors were compared.10,16,17
When studying MHC class II expression in vivo, we found MHC class II expression on PMN of patients with active Wegener's granulomatosis in close correlation with disease activity.18 Moreover, MHC class II was up-regulated in patients receiving GM-CSF,19–21 whereas in patients with acute bacterial infections no MHC class II was found, despite up-regulation of other activation markers, including CD64.5
The role of surface-associated MHC class II on PMN is still elusive. There is evidence that MHC class II-positive PMN are able to present the superantigen staphylococcus enterotoxin E (SEE) to T cells in an MHC class II-dependent manner.17 Moreover, PMN pulsed with peptide was shown to activate antigen-specific memory T cells.22 In the present work we found that PMN cultured with IFN-γ acquired not only MHC class II antigens, but also B7.1 and B7.2 (CD80 and CD86), important costimulatory molecules required for T-cell signalling. When pulsed with tetanus toxoid C fragment (TT), PMN thus activated induced proliferation and IL-2 release of TT-specific T-cell lines in a MHC class II-restricted manner.
Materials and methods
Culture media and reagents
AIM-V and RPMI-1640 were obtained from Gibco (Eggenstein, Germany). PolymorphPrep™ was purchased from Nycomed (Oslo, Norway), SEE from Serva (Heidelberg, Germany) and TT from Calbiochem (Marburg, Germany). IL-2 and IFN-γ were obtained from Boehringer Mannheim (Mannheim, Germany) and GM-CSF from Sigma (Deisenhofen, Germany).
For inhibition experiments, monoclonal antibodies (mAbs) to the human leucocyte antigens (HLA) ABC (MHC class I), B7.1 (CD80) and intracellular adhesion molecule-1 (ICAM-1) (CD54), CD14 and CD16 were purchased from Coulter Immunotech (Hamburg, Germany); HLA DR-DP-DQ (MHC class II) and anti-B7.2 (CD86) were obtained from Pharmingen (Hamburg, Germany). They were used at a concentration of 1 µg/ml. For cytofluorometry, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labelled murine mAbs to CD66b (ex-CD67)–FITC, B7.1 (CD80)–PE, and CD18–PE were purchased from Coulter Immunotech. mAbs to HLA DP-DQ-DR–PE, CD64 (FcγRI)–PE, and CD54 (ICAM-1)–PE were obtained from Dianova (Hamburg, Germany); the PE-labelled murine mAb to B7.2 (CD86) was obtained from Pharmingen and another clone from Serotec (purchased from Biozol, Eching, Germany). Propidium iodide used for staining apoptotic and dead cells was purchased from Sigma. Cytofluorometric characterization of lymphocytes was performed using FITC- and PE-labelled mAbs purchased from Coulter Immunotech (CD4–FITC/CD8–PE, CD3–FITC/CD25–PE; CD19 and CD20). For comparison, isotypic mouse immunoglobulin G1 (IgG1)–FITC, IgG2a–FITC and IgG2b–PE were used. Anti-CD83 and a mAb to dendritic cells (clone x-11) were obtained from Coulter Immunotech. All antibodies were used in the concentrations recommended by the supplier (0·5–2 µg/ml).
Purification of cells
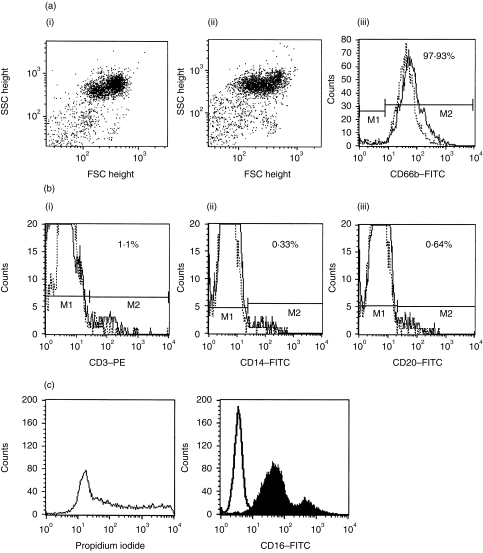
PMN were separated from heparinized blood of healthy donors by PolymorphPrep, according to the manufacturer's instructions. Contaminating red blood cells were removed by two or three hypotonic lysis steps (0·2% NaCl for 30 seconds followed by addition of an equal volume of 1·6% NaCl). Purity of the cell preparation was assessed by cytofluorometry with CD66b as marker for PMN. Usually 95–98% of cells were CD66b+. A small proportion (1–2%) of cells were monocytes (positive for CD14, but not CD66b), and 1–3% were CD3+ cells. CD83+ cells or cells bearing x-11, a marker for dendritic cells,23 were not found. For further purification, the cells were seeded on plastic dishes (see below); the non-adherent cells were 99% CD66b+, as judged by fluorescence-activated cell sorter (FACS) analysis (example shown in Fig. 1). By histological examination after Giemsa staining, > 99% of cells were identified as PMN, 90–95% were viable (as judged by Trypan Blue exclusion and propidium iodide staining) and still expressed CD16 (Fig. 1).
Figure 1.
Characterization of cells after isolation with Polymorphprep (PMP) and adherence on plastic plates. Figure 1(a) shows dot-plot analysis (FSC against SSC) of cells directly after PMP isolation (I) and after 2 hr of culture to plastic dishes (II). Cells were not gated and 98% of the cells expressed CD66b as a PMN marker (III) (solid line represents before adherence and dotted line represents after adherence). Two markers (M) were set: M1 to analyse further the CD66b– population and M2 for CD66b+ cells. Figure 1(b) shows characterization of CD66b– cells (cells within M1) (solid lines in Fig. 1b, I, II and III). The majority of cells expressed CD3 (I); only 0·33% of cells were positive for the monocyte marker CD14 (II) and 0·64% were positive for the B-cell marker CD20 (III). No CD83+ or X-11+ cells could be detected (results not shown). Values are corrected for immunoglobulin G1 (IgG1)- and IgG2a-negative controls (dotted lines in Fig. 1b, I, II and III). The data shown are representative of five experiments. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
T cells and T-cell lines
Peripheral T cells were isolated by sedimentation of heparinized blood on Ficoll® (Gibco); from the mononuclear cell (MNC) fraction, T cells were isolated by rosetting to sheep erythrocytes, yielding > 99% CD3+ cells. T-cell lines with specificity for TT were established by culturing T cells (106), isolated from a recently immunized healthy donor, with TT (1 µg/ml) and irradiated autologous MNC (106) and Epstein–Barr virus (EBV)-transformed autologous B cells (105) as feeder cells. After 2 days, IL-2 (30 U/ml) was added and after 7 days in culture, TT and irradiated MNC and EBV-transformed B-cells were again added. Four days later the T cells were diluted to 1 × 106/ml in RPMI-1640 containing IL-2 (30 U/ml) and grown for a further 14 days. The T cells were then restimulated with TT and irradiated feeder cells (106 MNC and 105 EBV-transformed B cells per 106 T cells) for 14 days. To select TT-specific T cells, cells were subcultured (100 cells/well) on irradiated MNC (105) in the presence or absence of TT or other test antigens. Cells with a high proliferative response to TT and no reactivity with other antigens (protease 3, elastase or bovine serum albumin [BSA]; 1 µg/ml of each), MNC or autologous PMN, were chosen for further propagation. The cell lines were restimulated every 14–18 days with TT (1 µg/ml) and autologous MNC/EBV-transformed B cells and IL-2. Between 15 and 20% of T cells were CD8+, the others CD4+. Contamination of T cells with monocytes or B cells (presumably derived from the feeder cells) was less than 1%, as judged by FACS analysis. For designated experiments, CD4+ and CD8+ cells were separated by the use of anti-CD4- or anti-CD8-coated beads (Miltenyi Biotech, Bergisch-Gladbach, Germany) strictly following the protocol supplied by the manufacturer.
The CD4+ T-cell clones D89/4 and A37/2 were provided by D. Kabelitz and restimulated every 14–18 days with phytohaemagglutinin, as described in ref. 24.
Cytofluorometry
Cells were double labelled with anti-CD66b–FITC as a PMN marker and PE-labelled antibodies to HLA DP-DQ-DR, or CD80, CD86, CD64, CD54, respectively, using standard procedures. Cells were analysed by FACSCalibur® and CellQuest® software (Becton-Dickinson, Heidelberg, Germany). Results are expressed as percentage of positive cells in the respective gate or quadrant.
For staining of intracellular antigens, PMN were first incubated in FACS buffer containing 0·1% saponin and 4% paraformaldehyde (PFA) for 20 min. Then the antibody to DP, DQ, DR was added (0·4 µg/106 PMN).
Stimulation of PMN
Purified PMN were cultured in AIM-V with 2·5% heat-inactivated (30 min at 56°) autologous normal human serum (NHS), 50 U/ml of GM-CSF and 100 U/ml of IFN-γ. Alternatively, supernatants of the T-cell lines or of PHA-stimulated MNC were added (10–30% v/v) and harvested 24–36 hr after stimulation. After 24 hr the PMN were removed, reducing the contamination with CD14+/CD66b− cells to 1% or less, as tested by cytofluorometry and Giemsa staining (see Fig. 1).
Coculture experiments
Prestimulated PMN (1 × 105) in 100 µl were added per well of a 96-well flat-bottom plate (Greiner, Nürtingen, Germany). Then, 1 × 105 T cells (T-cell lines A37/2 or D89/4) (100 µl) were added to each well together with SEE (1–10 ng) or TT (200 ng), respectively. All experiments were carried out in the absence of exogenous IL-2. After coincubation for 4 days at 37° with 5% CO2, proliferation was tested by adding 1 µCi of 3H-thymidine ([3H]TdR, specific activity 20 Ci/mmol; Amersham Life Science, Braunschweig, Germany) for 14–16 hr. [3H]TdR incorporation into DNA was measured and expressed as counts per min (c.p.m.). The values represent the mean ± SD of 6–12 parallel wells. The probability that the mean values were different was calculated by using the t-test for unpaired samples.
Determination of cytokines
Supernatants derived from PMN/T-cell cocultures were collected after 2 or 3 days of culture. After filtration through a 0·22-µm filtration unit, the supernatant was analysed for IL-2 and IL-4 by using separate enzyme-linked immunosorbent assays (ELISAs, both purchased from Coulter-Immunotech) according to the manufacturer's instructions.
RNA isolation and reverse transcription–polymerase chain reaction (RT–PCR)
Total cellular RNA was isolated using the RNeasy kit from Qiagen (Hilden, Germany). For RT, 2 µg of RNA was incubated with 50 pmol random primer, 1 U RNase inhibitor, 10 pmol dNTP and 20 U Moloney Murine Leukemia Virus (MMuLV) reverse transcriptase (all purchased from Boehringer Mannheim) for 60 min at 37°, followed by 15 min at 94°. PCR for MHC class II and B7.2 was carried out in a Perkin-Elmer (Überlingen, Germany) thermocycler as follows: 10 pmol dNTP was added to 5 µl of cDNA, followed by 50 pmol of the primers and 1 U Taq DNA polymerase in 2 mm MgCl2. After preheating to 94° for 10 min, 30 cycles for MHC class II and 35 cycles for B7.2 were performed (30 seconds at 94°, 30 seconds at 60°, 60 seconds at 72°) followed by a final extension step at 72° for 10 min. For B7.1, the following conditions were used: 2 mm MgCl2, 10 pmol dNTP, 50 pmol of each primer and 1 U Taq polymerase in a total volume of 50 µl. After preheating for 10 min at 94°, 35 amplification cycles were performed (1 min at 94°, 1 min at 50°, 2 min at 72°) and a final extension for 10 min at 72°. For comparison, RNA isolated from EBV-transformed B cells was processed in parallel. The following primers were used: HLA-DR: sense: 5′-CGGATCCTTCGTGTCCCCAC-3′; antisense: 5′-CTCCCCAACCCCGTAGTTGTGTCTGCA-3′, amplifying a 270-bp fragment;25 B7.2-specific primers (sense: 5′-AGGACAAGGGCTTGTATCAA-3′ and antisense 5′-ATTGCTCGTAACATCAGGGA-3′) were used as described in ref. 25 yielding a 330-bp PCR product. The B7.1-specific primers (sense: 5′-TTGGATTGTCATCAGCCCTGC-3′ and antisense 5′-ATTTTCTTCTCCTTTTGCCAGTAG-3′) yielded a 318-bp fragment, as described by HUSAR, version 0·8 (EMBL databank). The primers were synthesized by ARK Scientific Biosystems (Darmstadt, Germany). The PCR products were analysed by gel electrophoresis (1·5% agarose) and staining with Sybr green (Molecular Probes, Leiden, the Netherlands). As a size marker, DNA molecular weight marker VI (Boehringer Mannheim) was used. For sequencing, the PCR product was extracted by using the QIAquick gel extraction kit (Qiagen); sequencing was performed using the ABI PRISM™ Big Dye™ Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Warrington, UK).
Confocal laser microscopy
The cells were centrifuged onto a PolyPrep® slide (Sigma) and fixed with ice-cold ethanol for 1 min. After blocking with 1% BSA, a FITC-labelled antibody to MHC class II antigen was used. The slides were examined by confocal microscopy (Leica, Bensheim, Germany) using Windows TC as software.
Results
Expression of MHC class II, CD80 and CD86 on PMN
PMN of healthy donors do not express MHC class II antigens. Surface expression as well as intracellular MHC class II, however, were seen when PMN had been cultured with IFN-γ and GM-CSF (Fig. 2). In addition, PMN also acquired CD80 and CD86. Moreover, an increase in expression of ICAM-1 (CD54) was seen, but a decrease in lymphocyte function-associated antigen-3 (LFA-3) (CD58) (examples are shown Fig. 3; data from different experiments are summarized in Table 1). Essentially similar data were obtained when PMN were cocultured with T cells or T-cell-derived supernatants.
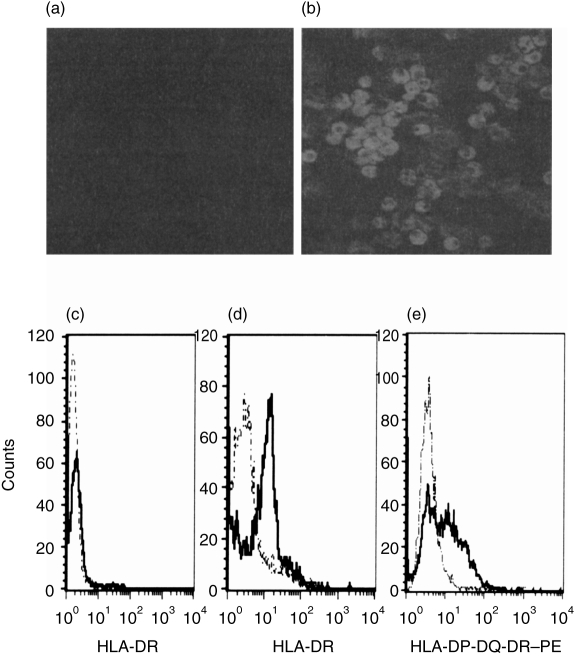
Figure 2.
Expression of major histocompatibility complex (MHC) class II antigens on polymorphonuclear cells (PMN). Freshly isolated PMN (a) and PMN cultured for 48 hr in medium containing interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml) (b) were examined by confocal laser microscopy for the presence of MHC class II antigens (upper panel) using a fluorescein isothiocyanate (FITC)-labelled antibody to MHC class II. By cytofluorometry (lower panel), no MHC class II was seen on untreated PMN (c) (the broken lines show the isotype control, the solid lines represent fluorescence of an FITC-labelled anti-MHC class II). After culturing the PMN for 48 hr in the presence of IFN-γ (100 U/ml) and GM-CSF (50 U/ml), MHC class II antigens were detectable intracellularly in saponin-treated PMN (d) and on the surface of untreated cells (e). HLA, human leucocyte antigen.
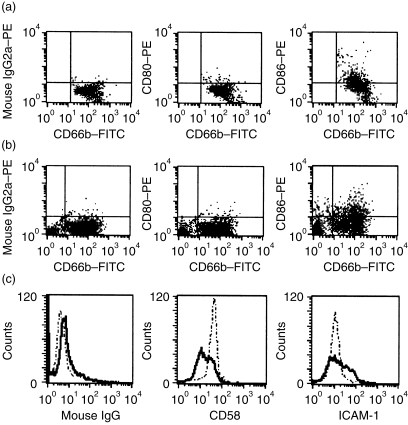
Figure 3.
Surface expression of costimulatory molecules CD80, CD86, CD58 and CD54 on polymorphonuclear cells (PMN). (a) PMN were treated with interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml) for 48 hr; CD80 and CD86 were analysed by cytofluorometry using PE-labelled antibodies to CD80 or CD86 and a fluorescein isothiocyanate (FITC)-labelled antibody to CD66b. The proportion of double-positive cells (right upper quadrant) was estimated. (b) PMN were cocultured with peripheral T cells for 48 hr. (c) Surface expression of CD58 and CD54 was analysed on cytokine-treated PMN (solid line); the broken line represents the surface expression of untreated PMN. ICAM-1, intracellular adhesion molecule-1; IgG, immunoglobulin G; PE, phycoerythrin.
Table 1.
Expression of major histocompatibility complex (MHC) class II antigen, CD80, CD86, CD54, and CD58 on polymorphonuclear cells (PMN) upon stimulation for 24 hr with T-cell supernatants (30% v/v) or interferon-γ (IFN-γ) (100 U/ml)+granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml)
| MHC class II | CD80 | CD86 | CD54 | CD58 | ||
|---|---|---|---|---|---|---|
| IFN-γ+GM-CSF | ||||||
| 0 hr | Range | 0–1·5 | 0 | 0–2·2 | 22–73 | 25–91 |
| Median | 0·07 (n = 12) | 1·1 (n = 5) | 45 (n = 4) | 82 (n = 4) | ||
| 24 hr | Range | 3·5–8·5 | 0·4–6·7 | 0·4–9·5 | 82–380 | 12–35 |
| Median | 5·5 (n = 12) | 2·5 (n = 5) | 3·5 (n = 5) | 127 (n = 3) | 24 (n = 4) | |
| 48 hr | Range | 6·5–23·5 | 0·4–11·0 | 0·4–11·5 | ND | ND |
| Median | 8·5 (n = 12) | 3·5 (n = 7) | 5·9 (n = 7) | |||
| T-cell supernatant | Range | 12·5–42·9 | 1·5–12·5 | 8·5–48·5 | ND | ND |
| 48 hr | Median | 22·5 (n = 7) | 6·6 (n = 5) | 19·5 (n = 5) | ||
Data for MHC II CD80 and CD86 were calculated from the % of positive PMN; data for CD54 and CD58 were calculated from the mean fluorescence of the cells.ND, not determined.
MHC class II expression varied widely when cells of different donors were compared, as described previously.10,17 In the majority of experiments, MHC class II expression was higher when PMN had been cultured with T-cell-derived supernatants instead of the cytokines IFN-γ and GM-CSF (data from multiple experiments are summarized in Table 1). The same was true for CD80 and CD86: with the cytokines only a very small percentage of cells became positive; this percentage increased considerably upon culture of PMN with T-cell supernatants. MHC class II-specific mRNA was found in freshly isolated PMN and also in stimulated PMN, as was mRNA for CD80 and CD86 (Fig. 4).
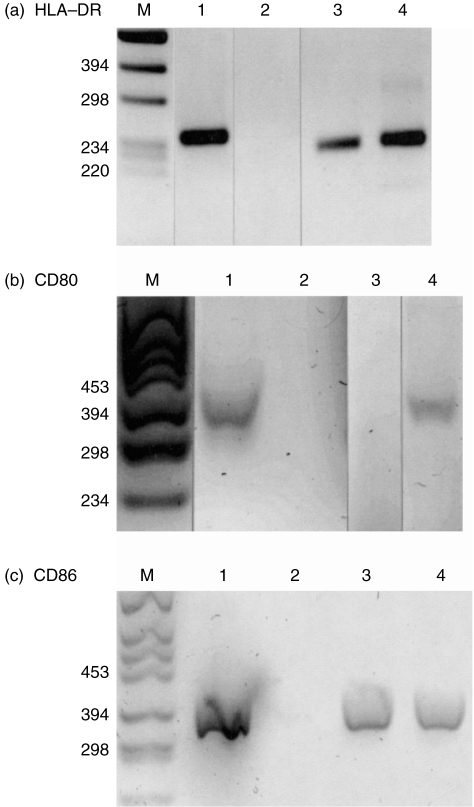
Figure 4.
Detection of human leucocyte antigen (HLA)-DR, CD80 and CD86 by reverse transcription–polymerase chain reaction (RT–PCR): RNA derived from Epstein–Barr virus (EBV)-transfected B cells (lane 1) or from polymorphonuclear cells (PMN) cultured in the absence (lane 3) or presence of cytokines interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml) for 16 hr (lane 4) was amplified by RT–PCR with primers specific for HLA-DR (a), CD80 (b) and CD86 (c). In lane 2 the products of a control amplification (using water instead of the RNA) are shown; M is molecular weight marker VI.
Superantigen presentation by PMN to T lymphocytes
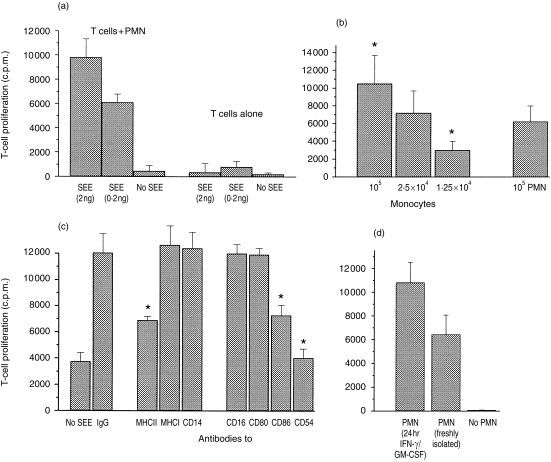
The major problem when analysing antigen or superantigen presentation by PMN is the exclusion of contaminating monocytes or dendritic cells which, as professional APC, might contribute significantly, even in low numbers. Our isolation procedure using PolymorphPrep followed by incubation with IFN-γ and GM-CSF for 24 hr and harvesting of the non-adherent cells yielded 99% pure PMN. Thus, the number of potentially contaminating monocytes was well below 1%. With those PMN, proliferation of peripheral T cells and of the T-cell clones A37/2 and D89/4 was assessed. Proliferation was found to be dependent on the concentration of SEE (only data for peripheral T cells are shown in Fig. 5). With 105 T cells, 2 ng of SEE and 105 PMN, a 250 ± 76-fold (n = 5) higher proliferation was seen than without PMN. When comparing IFN-γ-pretreated PMN and monocytes of the same donor with regard to their capacity to induce T-cell proliferation, it was observed that 2·5 × 104 monocytes were required to induce approximately the same extent of T-cell proliferation as 105 PMN (Fig. 5b). This clearly argues against contaminating monocytes as APC.
Figure 5.
Induction of T-cell proliferation by polymorphonuclear cells (PMN) and staphylococcus enterotoxin E (SEE). Proliferation of peripheral T cells (1 × 105) was measured as incorporation of 3H-thymidine and is depicted on the y-axes as counts per minute (c.p.m.). All data are expressed as mean±SD (n = 6). Unless indicated otherwise, PMN or monocytes (105) had been precultured with interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml) for 24 hr. (a) T cells were cultured with SEE at different concentrations, with or without PMN (T cells alone). (b) Monocytes in the concentrations indicated and PMN of the same donor were coincubated with T cells and SEE (1 ng) for 5 days. The asterisk indicates values different from that obtained with 105 PMN (P < 0·05). (c) PMN were cocultured with T cells and SEE in the presence of monoclonal antibodies (0·2 µg/well) or mouse immunoglobulin G (IgG) for 5 days. On the very left, data are shown for PMN plus T cells without SEE. The asterisk indicates values different from that of PMN + T cells + SEE + IgG. (d) PMN of the same donor were either precultured with IFN-γ/GM-CSF for 24 hr and then cocultured with T cells for 5 days, or used immediately after isolation. In the absence of PMN no proliferation was seen.
In another set of experiments, the number of PMN and of monocytes required to induce T-cell proliferation was down-titrated: 1000 PMN per 105 T cells were sufficient to induce proliferation when SEE was present. When the number of PMN was reduced further, proliferation still occurred, but not in all wells, leading to a considerable standard deviation of the mean. With 500 PMN, proliferation occurred in eight to 10 of 12 parallel wells, and with 100 PMN proliferation occurred in six or seven (two independent experiments). This set of data rules out monocytes as accessory cells as, under our experimental conditions, not even 10 monocytes per well were sufficient to induce considerable T-cell proliferation.
The PMN-dependent T-cell proliferation could be inhibited by antibodies to MHC class II as well as by antibodies to CD86 and ICAM-1. Antibodies to MHC class I, CD80, CD14 or CD16 were not inhibitory (Fig. 5c).
Freshly isolated PMN also induced T-cell proliferation (Fig. 5d); this result is in agreement with the finding that during coculture with T cells PMN acquired MHC class II, CD80 and CD86 (Table 2).
Table 2.
Effect of coculturing T cells with staphylococcus enterotoxin E (SEE) and interferon-γ (IFN-γ)-pretreated polymorphonuclear cells (PMN) on T-cell proliferation, T-cell activation and cytokine production
| IL-2 (pg/ml)* | IL-4 (pg/ml)* | CD25† | T-cell proliferation‡ | |
|---|---|---|---|---|
| Day 0 | 0 | 0 | 2·7% | |
| Day 2 | 3033 | 56 | 19·6% | |
| Day 3 | 261 | 0 | ND | |
| Day 5 | ND | ND | ND | 23 402 ± 2550 |
Measured in the cell supernatant; the value represents the mean of duplicate samples; all values have been corrected for spontaneous cytokine release, i.e. measured in the absence of SEE.
Measured by fluorescence-activated cell sorter (FACS) analysis.
Corrected for proliferation in the absence of SEE; the value represents the mean±SD of six parallel wells.
ND, not determined.
As an activation marker of T cells, CD25 expression was measured by cytofluorometry. During coculture, T cells acquired CD25 when both PMN and SEE were present. At that time IL-2, but not IL-4, was found in the cell supernatant (Table 2).
Antigen presentation by PMN to T cells
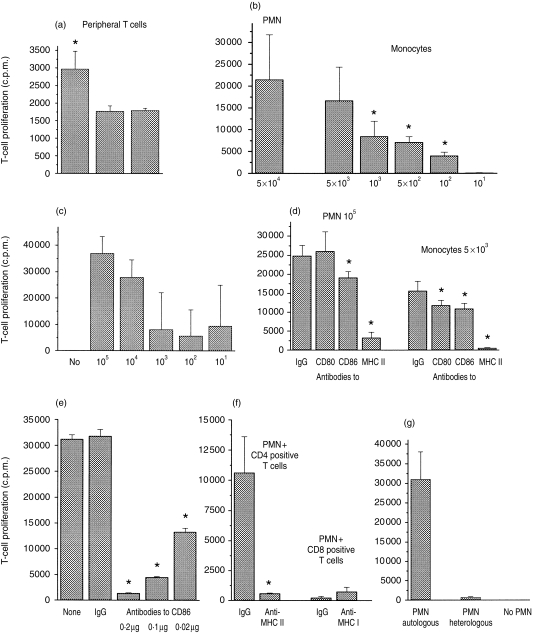
To ascertain whether PMN would also be able to present peptide antigens, peripheral T cells of a recently vaccinated individual were cultured with IFN-γ/GM-CSF prestimulated autologous PMN and TT, which is known to require processing by APC. As expected, with peripheral T cells a very low, but statistically significant, proliferation was seen (Fig. 6a).
Figure 6.
Induction of T-cell proliferation by autologous polymorphonuclear cells (PMN) and tetanus toxoid C fragment (TT). (a) Peripheral, freshly isolated T cells (2 × 104) were cultured in the presence of PMN (2 × 104) + TT (0·2 µg), PMN alone or TT alone. Proliferation was measured after 5 days and expressed as mean±SD (n = 12). The following experiments were carried out with PMN cultured for 24 hr with interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml) or with freshly isolated monocytes and the TT-specific T-cell line (105 cells; all cells obtained from the same donor) and TT (0·2 µg). Proliferation was measured after 5 days and expressed as counts per minute (c.p.m.), mean±SD (n = 12). (b) T cells and TT were cocultured with autologous monocytes or PMN at the numbers indicated. The asterisk indicates values different from those obtained with 5 × 104 PMN. (c) Different numbers of PMN were cocultured with TT-specific T cells and TT. (d) PMN (105) or monocytes (5 × 103) were cultured with T cells and TT in the presence of the monoclonal antibodies (0·2 µg of each). The asterisk indicates values different from those of the respective experiment with immunoglobulin G (IgG). (e) PMN (105) were incubated with TT and T cells for 24 hr. Different amounts of anti-CD86 were then added and proliferation measured after 4 days. The asterisk indicates values different from those obtained with 0·2 µg of IgG. (f) CD4+ or CD8+ TT-specific T cells (5 × 104) were cocultured with PMN (105) and TT with or without antibodies to major histocompatibility complex (MHC) class II or class I antigens, respectively (0·2 µg each). (g) Autologous PMN or PMN of a donor with different DR-type (heterologous) (105 each) were cultured with TT and T cells (105).
For further analysis, T-cell lines with reactivity for TT were established from this individual by repeated cycles of TT-dependent proliferation. T-cell lines were selected that proliferated in response to TT presented by autologous MNC, but not in response to other antigens, MNC or autologous PMN alone.
In a first set of experiments, autologous PMN were pretreated with the cytokines for 24 hr, then different numbers of cells were coincubated with 105 T cells. In parallel, monocytes of the same donor were used. When TT was present, T-cell proliferation occurred; however, 10-fold more PMN than monocytes were required to yield a comparable extent of T-cell proliferation (Fig. 6b). With ≤ 1000 PMN per well and 105 T cells, proliferation still occurred; however, it differed greatly from well to well, with single wells showing no proliferation at all (Fig. 6c).
T-cell proliferation was inhibited by antibodies to MHC class II (mean 59 ± 23%, n = 6), but not by antibodies to MHC class I (for an example see Fig. 6d). Moreover, antibodies to CD86 were inhibitory (0·2 µg of antibody yielded 45 ± 17% inhibition, n = 5) (Fig. 6d, 6e) whereas antibodies to CD80 were not. The latter observation was of particular interest, as antibodies to CD80 are known to inhibit antigen presentation by professional APC, including monocytes (example shown in Fig. 6d). Mouse IgG or antibodies to other PMN surface antigens, including CD16 or CD14, did not affect T-cell proliferation (data not shown).
With CD8+ T cells, no considerable proliferation was obtained (Fig. 6f). Moreover, only autologous PMN, and not PMN of a donor with a different DR-type, were able to induce proliferation (Fig. 6g). Taken together, these data indicate a MHC class II-restricted T-cell proliferation.
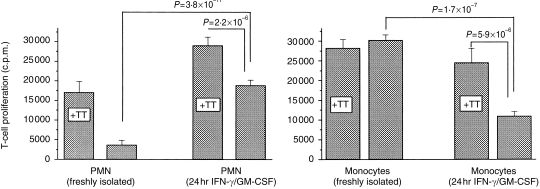
In another set of experiments, processing of TT by PMN was examined. Freshly isolated PMN or PMN precultured with IFN-γ and GM-CSF for 24 hr were incubated with TT for 3 hr. The cells were then washed repeatedly and TT-specific T cells were added. Freshly isolated PMN did not induce T-cell proliferation; for precultured PMN, 3 hr of antigen contact was sufficient to induce T-cell proliferation (Fig. 6). On the other hand, when antigens were incubated together with the TT-specific T cells, proliferation occurred with both freshly isolated PMN and precultured PMN. These results were in line with the observation that PMN acquire MHC class II antigens during coculture with T cells. Monocytes of the same donor, which were tested in parallel, induced proliferation with or without additional TT (Fig. 7). Of interest was that monocytes lost some of their capacity to process antigen when precultured, a phenomenon described previously by others.25
Figure 7.
Antigen-pulse experiments with tetanus toxoid C fragment (TT)-specific T cells and polymorphonuclear cells (PMN) or monocytes. (a) Freshly isolated PMN or PMN (105 each), precultured for 24 hr in interferon-γ (IFN-γ) (100 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 U/ml), were incubated with TT (0·2 µg) for 3 hr. The cells were then washed twice and T cells (105) were added with additional TT (+ TT) or without TT. (b) A similar experiment was performed with freshly isolated monocytes (1 × 104) from the same donor or with monocytes that also had been precultured with IFN-γ (100 U/ml) and GM-CSF (50 U/ml) for 24 hr. Proliferation was measured after 5 days. The results are expressed as counts per minute (c.p.m.) mean±SD (n = 12).
Discussion
PMN are important effector cells in host defence and inflammation. They are considered short-lived cells undergoing spontaneous apoptosis in vivo as well as in culture (reviewed in ref. 4). In recent years it has become increasingly evident that culturing PMN in the presence of cytokines extends their life span.6–9 Cultured PMN then synthesize and release immunomodulatory cytokines by which they may participate in the afferent limb of the immune response.26 The observation that PMN may also express MHC class II antigens when appropriately stimulated,10,17 highlighted the possibility that PMN might function as accessory cells for T-cell activation. More recently, MHC class II expression was found on PMN of patients with active Wegener's disease.18 The close correlation of MHC class II expression to disease activity prompted the present study with the objective of investigating whether or not MHC class II-positive PMN might activate or support activation of T lymphocytes.
To induce MHC class II synthesis, PMN were cultured in the presence of autologous serum. A proportion of PMN (± 60%) survived up to 72 hr, a process depending on protein synthesis, as shown previously.6,7,27,28 When IFN-γ and GM-CSF were also present or, alternatively, T cells or T-cell supernatants, de novo synthesis of MHC class II, CD80 and CD86 was seen, as was up-regulation of ICAM-1. T cells and T-cell supernatants were more efficient, particularly with regard to CD80 and CD86 up-regulation. How T cells activate PMN is currently under investigation. T-cell-derived mediators as well as cell–cell contact might provide signals, for example via β2-integrin/ICAM ligation, molecules constitutively expressed on both cells and likely to interact when cells are in close contact and in a low concentration of serum. Moreover, one has to bear in mind that T-cell lines, although used when they are not proliferating, are not fully quiescent cells. Therefore it is feasible that cytokines are produced and surface receptors activated.
The cytofluorometry data indicate that only a proportion of PMN acquire MHC class II, CD80 and CD86. Whether this represents a specialized subset of PMN is not yet known. The fact, however, that the percentage of PMN acquiring the surface receptors varies with the stimulating agent makes a subset less probable.
The accessory function of MHC class II-positive PMN was studied with either SEE as ‘superantigen’ or TT, the latter known to require processing. PMN and SEE dose-dependently induced T-cell proliferation, which could be inhibited by antibodies to MHC class II; this result is compatible with the view that SEE is presented by MHC class II molecules (Fig. 4c). Preculture of PMN with IFN-γ or GM-CSF was not necessarily required. Coculture of PMN with T cells was sufficient to induce expression of MHC class II, CD80 and CD86. Similarly to professional APC, proliferation could be inhibited by antibodies to MHC class II and also by antibodies to the T-cell costimulatory molecules ICAM-1 and CD86.
PMN were also able to induce proliferation of antigen (TT)-specific T cells. Proliferation only of CD4+ (not CD8+) T cells was induced, and only with autologous, but not heterologous, PMN. Moreover, as for other professional APC, T-cell proliferation was dependent not only on MHC class II, but also on the costimulatory molecules, ICAM-1 and CD86. With antibodies to CD80 no inhibition was seen, in agreement with the observation that CD80 is expressed only on a minor proportion of PMN.
With use of TT we could further demonstrate that PMN were able to process protein and to present it as peptide. Three hours of contact with exogenous TT was sufficient to induce proliferation of TT-specific T cells provided that the PMN had been precultured with IFN-γ and GM-CSF. Freshly isolated PMN could not process TT within that time span, quite in contrast to monocytes of the same donor, which were tested in parallel. These data also rule out that contaminating monocytes or dendritic cells participate in T-cell activation, as these cells would process antigens without preactivation.
When comparing the number of monocytes and PMN required to induce T-cell proliferation, it was observed that four to 10 times more PMN than monocytes were necessary to yield the same extent of T-cell proliferation. However, the cell preparation used in this work never contained more than 1% of contaminating cells and certainly not the 10% of monocytes that would be required to affect the results.
The observation that ≈ 10 times more PMN than monocytes were required to induce a similar extent of T-cell proliferation has to be considered together with the observation that only a proportion of PMN acquired MHC class II and yet a smaller proportion the accessory antigens CD80 and CD86. Thus, when only fully equipped PMN are considered, the ability to process and to present antigen is similar to that of monocytes.
While the data on MHC class II expression and presentation of SEE by PMN are in agreement with previous findings,17 there is discrepancy with respect to presentation of peptide antigens.17,29 The discrepancy cannot be explained at present because of possible differences between the T-cell lines and the experimental procedures used. In the present work we selected highly reactive T cells generated by multiple cycles of restimulation, which then might express a different fine specificity. Ongoing studies with cells from other donors and with other antigens confirm our present data, supporting the conclusion that PMN do present protein antigens that require internal processing. The data are also in agreement with studies of PMN precursors, which can be differentiated to acquire characteristics of dendritic cells when cultured for prolonged time-periods with cytokines.30
Moreover, PMN are equipped with tools of protein degradation, including phagosomes, which, at least in macrophages, are thought to be sufficient for intracellular antigen processing.31 That processed peptides can be presented by PMN is in agreement with a previous observation using synthetic peptides and peptide-specific T cells.22
Whether MHC class II-positive PMN participate in the immune defence or play a role in pathophysiological events, is a matter of speculation. The fact that only a minor proportion of PMN acquire MHC class II might lead to the conclusion that a possible accessory function of PMN would be rather weak. One has, however, to bear in mind that PMN are numerous in the peripheral blood, and that even a low percentage of PMN expressing MHC class II would exceed both circulating monocytes and dendritic cells in number.
Taken together, our data fit into the concept that PMN are not end-differentiated cells, but are still able to synthesize proteins de novo and to acquire new functions. Presentation of antigens to T lymphocytes is obviously within the proficiency of PMN, as precursors of PMN can be driven to acquire dendritic-cell characteristics.30
Acknowledgments
This work was supported by Forschungsförderungsprogramm der Medizinischen Fakultät der Universität Heidelberg.
References
- 1.Keel M, Ungethüm U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin 10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:521–530. [PubMed] [Google Scholar]
- 2.Chitnis D, Dickerson C, Munster AM, Winchurch RA. Inhibition of apoptosis in polymorphonuclear neutrophils from burn patients. J Leukoc Biol. 1996;59:835–8. doi: 10.1002/jlb.59.6.835. [DOI] [PubMed] [Google Scholar]
- 3.Dransfield I, Buckle AM, Savill J, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 4.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 5.Wagner C, Radsak M, Andrassy K, Hänsch GM. Expression of the high affinity receptor for IgG (CD64) on polymorphonuclear neutrophils (PMN), induction by inflammatory cytokines and expression in vivo. Immunobiology. 1998;199:641A. [Google Scholar]
- 6.Klebanoff SJ, Olszowski S, van Voorhis WC, Ledbetter JA, Waltersdorph AM, Schlechte KG. Effects of gamma interferon on human neutrophils, protection from deterioration on storage. Blood. 1992;80:225–34. [PubMed] [Google Scholar]
- 7.Lee A, Whyte MBK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–8. [PubMed] [Google Scholar]
- 8.Biffl WL, Moore EE, Moore FA, Barnett CC, Carl VS, Peterson VM. Interleukin-6 delays neutrophil apoptosis. Arch Surg. 1996;131:24–30. doi: 10.1001/archsurg.1996.01430130026005. [DOI] [PubMed] [Google Scholar]
- 9.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20. [PubMed] [Google Scholar]
- 10.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 11.Gericke GH, Ericson SG, Pan L, Mills LE, Guyre PM, Ely P. Mature polymorphonuclear leukocytes express high-affinity receptors for IgG (Fc gamma RI) after stimulation with granulocyte colony-stimulating factor (G-CSF) J Leukoc Biol. 1995;57:455–61. doi: 10.1002/jlb.57.3.455. [DOI] [PubMed] [Google Scholar]
- 12.Mawhorter SD, Kazura JW, Boom WH. Human eosinophils as antigen-presenting cells, relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology. 1994;81:584–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Nickoloff BJ, Mitra RS, Green J, Zheng XG, Shimizu Y, Thompson C, Turka LA. Accessory cell function of keratinocytes for superantigens. Dependence on lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. J Immunol. 1993;150:2148–59. [PubMed] [Google Scholar]
- 14.Kraft M, Filsinger S, Krämer KL, Kabelitz D, Hänsch GM, Schoels M. Synovial fibroblasts as accessory cells for staphylococcal enterotoxin-mediated T-cell activation. Immunology. 1995;85:461–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz H, Karau A, Filsinger S, Schoels M, Kabelitz D, Richter R, Hänsch GM. Tubular epithelial cells as accessory cells for superantigen-induced T cell activation. Exp Nephrol. 1998;6:67–73. doi: 10.1159/000020506. [DOI] [PubMed] [Google Scholar]
- 16.Smith WB, Guida L, Sun Q, et al. Neutrophils activated by granulocyte–macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–44. [PubMed] [Google Scholar]
- 17.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility complex class II-expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–35. [PubMed] [Google Scholar]
- 18.Hänsch GM, Radsak M, Wagner C, Reis B, Koch A, Breitbart A, Andrassy K. Expression of major histocompatibility class II antigens on polymorphonuclear neutrophils in patients with Wegener's granulomatosis. Kidney Int. 1999;55:1811–8. doi: 10.1046/j.1523-1755.1999.00446.x. 10.1046/j.1523-1755.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 19.Spagnoli GC, Juretic A, Rosso R, Van Bree J, Harder F, Heberer M. Expression of HLA-DR in granulocytes of polytraumatized patients treated with recombinant human granulocyte–macrophage colony-stimulating factor. Hum Immunol. 1995;43:45–50. doi: 10.1016/0198-8859(94)00131-9. [DOI] [PubMed] [Google Scholar]
- 20.Mudzinski SP, Christian TP, Guo E, Cirenza E, Hazlett KR, Gosselin EJ. Expression of HLA-DR (major histocompatibility class II) on neutrophils of patients treated with granulocyte–macrophage colony-stimulating factor for mobilization of stem cells [letter] Blood. 1995;86:2452–3. [PubMed] [Google Scholar]
- 21.Reinisch W, Tillinger W, Lichtenberger C, Gangl A, Willheim M, Scheiner O, Steger G. In vivo induction of HLA-DR on human neutrophils in patients treated with interferon-γ [letter] Blood. 1996;87:3068. [PubMed] [Google Scholar]
- 22.Reali E, Guerrini R, Moretti S, Spisani S, Lanza F, Tomatis R, Traniello S, Gavioli R. Polymorphonuclear neutrophils pulsed with synthetic peptides efficiently activate memory cytotoxic T lymphocytes. J Leukoc Biol. 1996;60:207–13. doi: 10.1002/jlb.60.2.207. [DOI] [PubMed] [Google Scholar]
- 23.Würzner R, Xu H, Franzke A, Schulze M, Peters JH, Götze O. Blood dendritic cells carry terminal complement complexes on their cell surface as detected by newly developed neoepitope-specific monoclonal antibodies. Immunology. 1991;74:132–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Kabelitz D, Wesselborg S. Life and death of a superantigen-reactive human CD4+ T cell clone, staphylococcal enterotoxins induce death by apoptosis but simultaneously trigger a proliferative response in the presence of HLA-DR+ antigen-presenting cells. Int Immunol. 1992;4:1381–8. doi: 10.1093/intimm/4.12.1381. [DOI] [PubMed] [Google Scholar]
- 25.Nadler SG, Rankin BM, Moran-Davis P, Cleaveland JS, Kiener PA. Effect of interferon-γ on antigen processing in human monocytes. Eur J Immunol. 1994;24:3124–30. doi: 10.1002/eji.1830241232. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd AR, Oppenheim JJ. Poly's lament, the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 27.William R, Watson G, Rotstein OD, Parodo J, Bitar R, Marshall JC. The IL-1β-concerting enzymes (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1β. J Immunol. 1998;161:957–62. [PubMed] [Google Scholar]
- 28.Iking-Konert C, Radsak M, Wagner C, Andrassy K, Hänsch GM. Polymorphonuclear neutrophils (PMN) in chronic inflammatory disease: expression on the surface of MHC class II, CD80 and CD86. Immunobiology. 1999;200:664A.. [Google Scholar]
- 29.Prior C, Townsend PJ, Hughs DA, Haslam PL. Induction of lymphocyte proliferation by antigen-pulsed human neutrophils. Clin Exp Immunol. 1992;87:485–92. doi: 10.1111/j.1365-2249.1992.tb03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oehler LO, Majdic WF, Pickl J, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–28. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandra L, Song R, Harding CV. Phagosomes are fully competent antigen-processing organelles that mediate the formation of peptide, class II MHC complexes. J Immunol. 1999;162:3263–72. [PubMed] [Google Scholar]