Abstract
NRS1 is a murine squamous cell carcinoma that constitutively expresses the co-stimulatory molecule CD80 at a high level yet grows as a tumour in syngeneic C3H mice. We examined the effect of gene transfer of the 4-1BB ligand (4-1BBL) into NRS1 cells. Introduction of the 4-1BBL gene efficiently elicited anti-tumour immune responses in syngeneic mice which acquired specific immunity against wild-type tumour. T-cell depletion studies showed that CD8+, but not CD4+ T cells were essential for tumour eradication. Our results suggest that the transduced 4-1BBL is more effective than the spontaneously expressed CD80 for generation of primary anti-tumour CD8+ T-cell responses. In addition to CD80 and CD86, the host-derived 4-1BBL is also involved in the secondary anti-tumour responses. This study indicates the complicated contribution of 4-1BBL, CD80 and CD86 on tumour and host cells in anti-tumour immune responses and a possible therapeutic application of 4-1BBL for human tumour vaccination and gene therapy.
Introduction
The role of co-stimulation in anti-tumour immune responses has been reported in a number of in vitro and in vivo experimental systems.1–4 The most extensively characterized T-cell co-stimulation is the signal through the binding of CD28 on T cells with its ligands, CD80 and/or CD86 (CD80/86) on antigen-presenting cells (APC).5 Accumulating reports have shown that immunizing mice with CD80/86-transduced tumour cells elicits protective and sometimes curative immunity against wild-type tumours.2,3,6–9 However, in non-immunogenic or low-immunogenic tumours, transduction of CD80 alone failed to facilitate anti-tumour immune responses.6 Additional or other co-stimulatory signals seem to be required for inducing more efficient anti-tumour effector cells in such tumours.
Recently, a number of receptor–ligand pairs which belong to the tumour necrosis factor (TNF) and TNF receptor families have been identified.10 4-1BB ligand (4-1BBL) and 4-1BB (CD137) is one such pair.11,12 4-1BB is a type I transmembrane protein expressed on activated CD4+ and CD8+ T cells13,14 and 4-1BBL is a type II surface glycoprotein expressed on several types of APC, such as activated B cells, macrophages and cultured dendritic cells.11,12,15,16 Ligation of 4-1BB in addition to T-cell receptor engagement co-stimulates T-cell proliferation and cytokine production as well as CD28-mediated co-stimulation. Recent reports have demonstrated the distinct or unique function of 4-1BB co-stimulation. 4-1BBL/4-1BB interaction provides co-stimulatory signals to T cells independent of CD28 signalling.16,17 4-1BBL co-stimulatory signals preferentially induce CD8+ T-cell proliferation and interferon-γ (IFN-γ) production and 4-1BB-mediated proliferation of CD8+ T cells appears to be interleukin-2 (IL-2) independent.18 Administration of monoclonal antibodies (mAb) against 4-1BB eradicated several established murine tumours.19 More recently, the synergistic effect between 4-1BBL and CD80-transduced tumour cells has been demonstrated.20,21 We, here, examined the effect of 4-1BBL transduction into a CD80+ NRS1 murine squamous cell carcinoma and investigated the requirements for tumour eradication.
Materials and methods
Mice
Female C3H/HeN (C3H) mice and athymic BALB/c nu/nu mice, 5–8 weeks old, were purchased from Shizuoka Laboratory Animal Centre (Hamamatsu, Shizuoka, Japan). All murine experiments were reviewed and approved by the Animal Use Committees of the National Children's Medical Research Centre (Tokyo, Japan).
Tumour cells
NRS1 was a cell line established from the spontaneously developed murine squamous cell carcinoma of C3H/HeN (H-2k) origin (kindly provided by Dr K. Ando, National Institute of Radiological Science, Japan).22,23 A plasmacytoma cell line, X5563, originated from the C3H/HeN mice was obtained from Dr Y. Sasakura (Kanagawa Dental College, Japan). All tumours were briefly expanded in vitro in RPMI-1640 medium containing 10% fetal calf serum, l-glutamine and antibiotics and were frozen to decrease experimental variation.
Vectors and 4-1BBL transfection
Murine 4-1BBL cDNA was generated by reverse transcription–polymerase chain reaction (RT–PCR) from total RNA of concanavalin A-activated splenocytes from a BALB/c mouse and subsequently subcloned into the BCMGSneo expression vector.24 Primers used to generate a full-length 4-1BBL cDNA11 were: sense, 5′-GATCCTCGAGATGGACCAGCACACACTTGA-3′, including nucleotides 53–73 of murine 4-1BBL cDNA and a XhoI cloning site; anti-sense, 5′-GATCGCGGCCGCTCATTCCCATGGGTTGTCGG-3′, including nucleotides 1035–1015 of murine 4-1BBL cDNA and a NotI cloning site. The sequence of a product for full-length murine 4-1BBL cDNA was verified by DNA sequencing. NRS1 cells were transfected with 10 µg of murine 4-1BBL-BCMGSneo expression vector using 100 µg of lipofectin (Gibco-BRL, Gaithersburg, MD). After 24 hr, the transfected cells were selected in culture medium containing 1 mg/ml G418 (Sigma, St Louis, MO) and resistant cells were selected. After drug selection, NRS1 cells were cloned and selected for high cell surface expression. Three clones expressing high levels of cell surface 4-1BBL (1.10, 5.13 and 1.9) were used for the following experiments.
Monoclonal antibodies and flow cytometry
Anti-CD80 [(RM80, rat immunoglobulin G2a (IgG2a)] and anti-CD86 (PO3, rat IgG2b) mAbs were generated as described. A hybridoma producing anti-4-1BBL mAb (TKS-1, rat IgG2a) was generated as described previously.25 Fluorescein isothiocyanate- or phycoerythrin-conjugated anti-CD80 (16-10A1), CD86 (GL1), CD54 (3E2), H-2Kk (36-7-5) and I-Ak (11-5.2) mAbs and appropriated fluorochrome-conjugated control hamster, rat, or mouse immunoglobulin were obtained from PharMingen (San Diego, CA). Methods of immunofluorescent staining, flow cytometry and data analysis have been described previously.26 Flow cytometry was performed using a FACScan and cellquest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Tumour inoculation and evaluation of tumour growth
For primary tumour challenges, parental NRS1 and 4-1BBL+ NRS1 cells at 5 × 104, 5 × 105, or 5 × 106 cells/mouse were injected subcutaneously into the shaved left back of syngeneic C3H or BALB/c nu/nu mice using 26-gauge needles on plastic 1 ml syringes. Tumour size was evaluated twice a week by measuring two perpendicular diameters using a calliper. Tumour volumes were estimated using the following equation: tumour volume (mm3)=[long length (mm) × (short length (mm))2]/2. For rechallenge experiments, mice were primarily inoculated with irradiated (80 Gy) NRS1 or 4-1BBL+ NRS1 (5 × 106 cells/mouse) into the right back. After 30 days, immunized mice were rechallenged with either parental NRS1 (5 × 105 cells) or X5563 (5 × 105 cells) into the left back and then the tumour incidence after 5 weeks was evaluated.
In the experiments to see the contributions of 4-1BBL and CD80/86 for tumour rejection of either 4-1BBL+ NRS1 or parent NRS1, 100 µg each of anti-4-1BBL (TKS-1), anti-CD80 (RM80), anti-CD86 (PO3), a mixture of anti-CD80 and CD86 mAbs, or control rat immunoglobulin (Sigma) was injected intraperitoneally (i.p.) on alternated days for 2 weeks after tumour inoculation, the tumour growth was then evaluated as described above.
In vivo depletion of CD4+ or CD8+ T cells
For in vivo depletion of T cells, 1 mg of either anti-CD4 (GK1.5) or anti-CD8 (53.6.72) mAb was administered to the mice i.p. for three consecutive days (days – 5, 4 and 3) prior to tumour inoculation and on day 3 and 7 postinoculation. GK1.5 and 53.6.72 hybridomas were obtained from the American Type Culture Collection (Rockville, MD). These mAbs were purified from ascites by standard procedures using caprylic acid extraction. In the preliminary experiments, depletion of the respective populations was verified up to day 14 by flow cytometry.
Results
4-1BBL-transfection into NRS1 cells
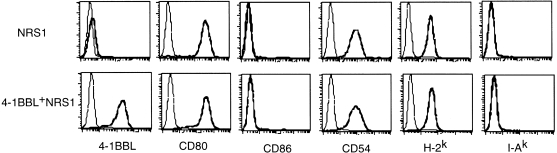
Cell surface expression of several molecules, which may contribute to anti-tumour immune responses, was determined. NRS1 cells express H-2, but not I-A molecules and also express high amounts of CD80 as well as CD54, but not CD86 (Fig. 1). Cell surface expression of 4-1BBL was hardly detectable by flow cytometry, although the mRNA for 4-1BBL was detectable by RT–PCR (not shown). We transfected the murine 4-1BBL cDNA into NRS1 cells and obtained three stable clones expressing murine 4-1BBL at high levels (4-1BBL+ NRS1). Transduction of 4-1BBL gene into NRS1 cells did not affect expression for CD80, CD86, CD54, H-2 and I-A molecules. Representative results (clone 5.13) from three clones are shown in Fig. 1. No difference in the growth rate in vitro was observed between parental NRS1 and the 4-1BBL+ NRS1 clones (not shown).
Figure 1.
Expression of surface molecules on NRS1 and 4-1BBL-transfected NRS1 cells. Parental NRS1 and 4-1BBL-transfected NRS1 (clone 5.13) cells were stained with either fluorescein isothiocyanate- or phycoerythrin-conjugated anti-4-1BBL, anti-CD80, anti-CD86, anti-CD54, anti-H-2Kk and anti-I-Ak, or an appropriate fluorochrome-conjugated immunoglobulin control. Samples were analysed by flow cytometry. Data are displayed as histograms (4-decade scale) with the immunoglobulin control as the lighter line.
Tumorigenicity of 4-1BBL-transduced NRS1
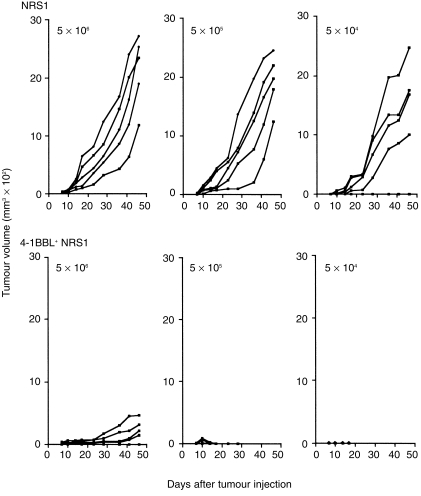
To analyse the effect of 4-1BBL expression on tumour rejection, C3H mice were injected subcutaneously (s.c.) with either parental NRS1 or 4-1BBL+ NRS1 cells at 5 × 104, 5 × 105, or 5 × 106 cells/mouse and tumour growth was observed. All mice inoculated with parental NRS1 cells developed tumours when more than 5 × 105 parental NRS1 cells were injected (Fig. 2). In contrast, the tumours of mice injected with 5 × 105 and 5 × 104 4-1BBL+ NRS1 cells completely regressed after transient growth for 1–2 weeks. No tumours were observed during the experimental period up to day 48 in these mice. Thus, we selected the optimal cell number to be 5 × 105 cells for primary inoculation. Similar results were obtained in the other two clones, 1.10 and 1.9 (data not shown). We therefore used clone 5.13 in the following experiments. To investigate whether 4-1BBL and CD86 are inducible, or CD80 is stably expressed on NRS1 tumour cells in vivo, we removed the tumour nodules after NRS1 inoculation and examined the expression for CD80, CD86 and 4-1BBL by flow cytometry. We failed to detect a substantial induction of 4-1BBL and CD86 in single cell suspensions of tumour cells and observed the consistently high CD80 expression (not shown).
Figure 2.
Reduced tumorigenicity of 4-1BBL-transfected NRS1 cells in syngeneic mice. C3H mice in groups of five were injected s.c. with the indicated number of NRS1 and 4-1BBL+ NRS1 cells (clone 5.13) and tumour growth was measured as described in the Materials and methods. The results are expressed as tumour volume of each mouse. Similar results were obtained in two other 4-1BBL+ NRS1 clones.
We next examined the specificity of the induced anti-tumour responses. One of 11 mice immunized with 5 × 106 irradiated NRS1 cells was protected from the challenge of NRS1 cells, suggesting that NRS1 tumours are relatively immunogenic (Table 1). Similarly, immunization with 5 × 106 irradiated 4-1BBL+ NRS1 eradicated the challenge with NRS1 cells in one of six mice, but permitted the tumour growth of syngeneic X5563 plasmacytoma in all five mice. This suggests that the acquired anti-tumour immunity by the inoculation of either NRS1 or 4-1BBL− NRS1 is specific for NRS1 tumours. However, we cannot evaluate whether the expression of 4-1BBL confers the enhancement of specific immunity.
Table 1.
4-1BBL+ NRS1-inoculated mice induce specific immunity against NRS1 tumours
| Primary inoculation | Challenge | Tumour incidence |
|---|---|---|
| NRS1 | NRS1 | 1/11 |
| 4-1BBL-NRS1 | NRS1 | 1/6 |
| 4-1BBL-NRS1 | X5563 | 5/5 |
Mice were primary inoculated with irradiated NRS1 or 4-1BBL+ NRS1 (5×106 cells) into the right back. After 30 days, immunized mice were challenged with either NRS1 or X5563 (5×105 cells) into the left back. The tumour incidence after 5 weeks was evaluated.
Requirements for tumour rejection by 4-1BBL transduction
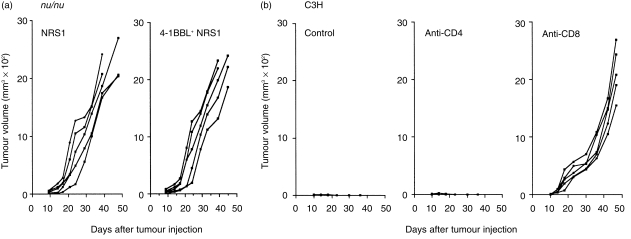
To determine the T-cell-dependence in rejection of 4-1BBL+ NRS1, athymic nude mice were injected with either parental NRS1 or 4-1BBL+ NRS1 tumours. Both tumours grew progressively in all mice and no obvious difference in tumour growth rate was observed (Fig. 3a). These results suggest that rejection of the 4-1BBL+ NRS1 tumour is T-cell-dependent. To further investigate the requirements for either CD4 or CD8 T cells, we injected anti-CD4 or anti-CD8 mAb to eliminate each subset of T cells in vivo and assessed tumour growth of 4-1BBL+ NRS1 cells. Tumour growth occurred on all mice depleted of CD8 T cells, while the on those mice depleted of CD4 T cells the inoculated tumours were consistently eradicated, as was observed in the control mice (Fig. 3b). This result indicates that CD8 T cells, but not CD4 T cells, are essential for tumour rejection of 4-1BBL+ NRS1.
Figure 3.
Rejection of the 4-1BBL+ NRS1 tumour requires CD8+ T cells. (a) Athymic nu/nu mice in groups of five were injected s.c. with 5 × 105 cells/mouse of either NRS1 or 4-1BBL+ NRS1 (clone 5.13) and tumour growth was measured as described in the Materials and methods. (b) C3H mice in groups of five were injected i.p. a total of five times with 500 µg of either immunoglobulin control, anti-CD4 (GK1.5), or anti-CD8 (53.6.72) mAb before and after tumour inoculation as described in the Materials and methods. Each group of mice was injected s.c. with 5 × 105 cells of 4-1BBL+ NRS1 cells at day 0 and tumour size was measured. The results are expressed as tumour volume of each mouse. Similar results were obtained in two independent experiments.
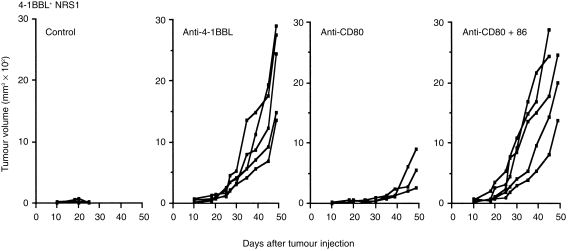
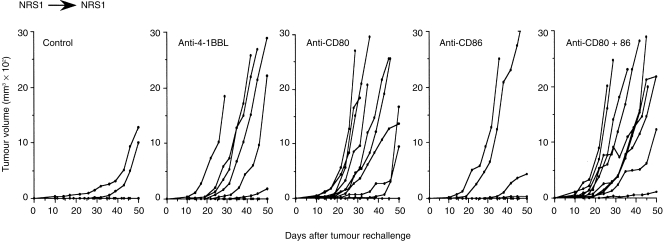
To determine whether the ability to reject 4-1BBL+ NRS1 cells depends only on the transduced 4-1BBL molecule or also on the other co-stimulatory molecules on tumour and host cells, we administered anti-4-1BBL, anti-CD80, both anti-CD80 and anti-CD86 mAbs, or control rat immunoglobulin for 2 weeks from the day of tumour inoculation. All mice treated with anti-4-1BBL mAb developed tumours and the tumour growth rate appears to be similar to that observed in the parental NRS1 as shown in Fig. 2 (Fig. 4), suggesting that 4-1BBL is essential for the rejection of 4-1BBL+ NRS1 tumours. Despite the high level of CD80 expression on tumour cells, the treatment with anti-CD80 mAb alone showed a minimal effect on tumour rejection and the treatment with anti-CD80 together with anti-CD86 mAb completely eradicated the tumour in all mice. Thus, not only CD80 but also CD86 contributes to the eradication of 4-1BBL+ NRS1 tumour cells. Since CD80 and 4-1BBL were expressed on 4-1BBL+ NRS1 tumour cells and these molecules are also inducible on host-derived APC, we cannot distinguish the derivation of these molecules in the above experiments. We next performed a similar mAb treatment at the time of NRS1 rechallenge after the immunization with NRS1. As shown in Fig. 5, most mice (eight of 10) rejected the inoculated tumours in the control group, whereas the administration of anti-4-1BBL mAb reduced the rate of tumour rejection. The administration of anti-CD80 mAb alone, or both CD80 and CD86 mAbs clearly reduced anti-tumour responses and permitted a rapid tumour growth, while the treatment with anti-CD86 mAb alone did not have a clear affect. These results suggest that the host-derived 4-1BBL expressed on APC in host mice may contribute to generation of secondary anti-tumour responses. Furthermore, CD80 on tumour cells is definitely involved in the secondary anti-tumour responses.
Figure 4.
Rejection of the 4-1BBL+ NRS1 tumours requires 4-1BBL and both CD80 and CD86. One hundred micrograms each of control rat immunoglobulin, anti-4-1BBL (TKS-1), anti-CD80 (RM80), or a combination of both anti-CD86 and CD80 mAbs was administered i.p. every other day for 2 weeks after the inoculation with 4-1BB+ NRS1 (5 × 105 cells/mouse). Tumour size was measured. The results are expressed as tumour volume of each mouse. Similar results were obtained in two independent experiments.
Figure 5.
Host-derived 4-1BBL are involved in the eradication of NRS1 tumours at the secondary challenge. C3H mice in groups of 10 were immunized with irradiated NRS1 cells by s.c. injection of 5 × 106 cells. After 30 days, live NRS1 cells (5 × 105 cells/mouse) were injected s.c. and 100 µg each of control rat immunoglobulin, anti-4-1BBL, anti-CD80, anti-CD86, or both anti-CD80 and CD86 mAbs were administered i.p. every other day for 2 weeks. The results are expressed as tumour volume of each mouse. Tumour size was measured. The results are expressed as tumour volume of each mouse. Similar results were obtained in another independent experiment.
Discussion
In this study, we have shown that the inoculation of parental NRS1 cells expressing high levels of CD80 was not able to elicit the primary anti-tumour immune responses. However, the 4-1BBL-transfected tumour cells efficiently induced the primary anti-tumour responses. The primary rejection of 4-1BBL− NRS1 tumour cells was shown to require CD8+ T cells but not CD4+ T cells. The NRS1 tumour seems to be relatively immunogenic, since the immunization with irradiated NRS1 cells mostly eradicated the challenge of NRS1 tumour cells (Table 1 and Fig. 5).
At present, two reports have shown the effect of 4-1BBL transduction on tumour immunity.20,21 Melero et al.20 first investigated the effects of 4-1BBL gene transduction using a P815 mastcytoma and an AG104A sarcoma and the co-operation with CD80. In highly immunogenic P815 tumour cells, single transfection of either CD80 or 4-1BBL alone was enough to induce tumour eradication. On the other hand, in poorly immunogenic AG104A tumour cells, both CD80- and 4-1BBL transfection alone failed to induce tumour eradication but co-transfection with these two molecules successfully induced tumour rejection. Guinn et al.21 also reported the co-operation of 4-1BBL with CD80 and CD86 using a B lymphoma A20. Recent observations using 4-1BBL-deficient mice also indicate the preferential importance of 4-1BB co-stimulation for generating CD8 T-cell responses.27,28 Our results also suggest that 4-1BBL on tumour seems to be a more potent co-stimulator for generation of primary cytotoxic T lymphocytes (CTL), since the blockade of 4-1BBL, but not CD80 entirely diminished the effect of 4-1BBL transduction into CD80+ NRS1 cells.
In an earlier study, it was postulated that tumour-specific CD8+ CTL may be primed directly by the CD80/86-transduced tumours.29 However, more recent studies have suggested that tumour rejection requires the B7 family molecules provided by the host and expressed on the tumour.30–34 At present, the involvement of host-derived 4-1BBL for tumour rejection has not been directly demonstrated, but it is possible that host-derived 4-1BBL may also be involved in tumour rejection, since an inducible expression of 4-1BBL on activated APC, such as B cells, macrophages, and dendritic cells, has been reported.11,15,16,35 Blockade of 4-1BBL by mAb completely reverted the effect of 4-1BBL transduction in the primary inoculation, suggesting that the induced anti-tumour responses seems to be mediated by 4-1BBL–4-1BB interactions between tumour and host T cells, although we cannot completely negate the involvement of the host-derived 4-1BBL. Interestingly, in the secondary responses, the host-derived 4-1BBL is substantially involved, since the administration of anti-4-1BBL mAb at the secondary challenge with 4-1BBL− NRS1 tumours diminished the effect of immunization (Fig. 5). Although 4-1BB can function independently of CD28 co-stimulation,16 CD28 co-stimulation may amplify the effect of 4-1BB co-stimulation. Indeed, simultaneous blockade of CD80 and CD86, but not of CD80 alone, clearly invalidated the effect of 4-1BBL transduction. Since we have not observed the inducible expression of CD86 on NRS1 tumour cells in vivo, the effect of anti-CD86 mAb may result from the blockade of CD28–CD86 interactions between host T cells and APC. Therefore, anti-tumour immunity against 4-1BBL+ NRS1 cells can be achieved by direct antigen presentation with 4-1BBL and CD80 co-stimulation provided from tumour cells and indirect tumour antigen presentation through host APC expressing CD86.
Despite the high levels of CD80 expression on parental NRS1 cells, a primary inoculation of live NRS1 cells failed to elicit anti-tumour immunity and the treatment with anti-CD80 mAb did not clearly affect the tumorigenicity by 4-1BBL+ NRS1 cells. However, the acceleration of tumour growth by the anti-CD80 mAb administration at the secondary challenge indicated a definitive contribution of CD80. This may be explained by the increased ratio of direct presentation provided by CD80+ NRS1 tumour in the secondary responses. Huang et al.34 reported that the primary inoculation of CD80+ tumour vaccines results in some degree of direct presentation to CD8+ T cells, but the dominant mechanism of CTL priming is provided by host APC. However, the repeated immunization with CD80+ tumour cells can efficiently expand the directly primed CD8+ CTL population. This notion supports our results. The detailed discrimination between tumour- and host-derived co-stimulatory molecules required for the primary and secondary responses will require further studies using CD80/86- or 4-1BBL-deficient mice and mAbs.
In this study, we demonstrated that introduction of 4-1BBL may induce efficient primary immune responses against tumours which fails to elicit anti-tumour immune responses despite the high CD80 expression. Our results suggest that 4-1BBL may be more effective than CD80 for direct antigen presentation by tumour cells in the primary anti-tumour responses. In addition, we first demonstrated that the host-derived 4-1BBL co-stimulation was also involved in tumour rejection. Our study shows that 4-1BB co-stimulation will become a potent therapeutic target for tumour vaccination and gene therapy.
Acknowledgments
We thank Koichi Ando (National Institute of Radiological Science) and Yuuichi Sasakura (Kanagawa Dental College). We also thank Hiromitsu Matsuda (University of Juntendo) for excellent technical assistance for flow cytometry. This work was supported by grants from the Core Research for Evolutionary Science and Technology (CREST), the Organized Research Combination System of Science and Technology Bureau and the Ministry of Health, Japan.
Abbreviations
- 4-1BBL
4-1BB ligand
- APC
antigen-presenting cells
- CD80/86
CD80 and/or CD86
- CTL
cytotoxic T lymphocytes
REFERENCES
- 1.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. CD28 interaction with B7 co-stimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–60. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 3.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–70. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 4.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–82. [PubMed] [Google Scholar]
- 5.Allison JP. CD28–B7 interactions in T-cell activation. Curr Opin Immunol. 1994;6:414–9. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, McGowan P, Ashe S, Johnson J, Li Y, Hellström I, Hellström KE. Tumor immunogenicity determinants the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523–32. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, McGowan P, Hellström I, Hellström KE, Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994;153:421–8. [PubMed] [Google Scholar]
- 8.Ramarathinam L, Castle M, Wu Y, Liu Y. T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med. 1994;179:1205–14. doi: 10.1084/jem.179.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Hellström KH, Hellström I, Chen L. Antitumor immunity elicited by tumor cells transfected with B7-2, a second ligand for CD28/CTLA-4 costimulatory molecules. J Immunol. 1995;154:2780–94. [PubMed] [Google Scholar]
- 10.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin RG, Din WS, Davis-Smith T, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB. a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–41. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 12.Alderson MR, Smith CA, Tough CA, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–27. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 13.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad, Sci USA. 1989;86:1963. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollok KE, Kim Y-J, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. analysis of expression and function. J Immunol. 1993;150:771–81. [PubMed] [Google Scholar]
- 15.Pollok KE, Kim Y-J, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T cell antigen binds to mature B cells and macrophages, and cotimulates anti-µ-primed splenic B cells. Eur J Immunol. 1994;24:367–74. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- 16.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28– T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–9. [PubMed] [Google Scholar]
- 17.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–62. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuford WW, Klussman K, Tritchler DD, et al. 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nature Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 20.Melero I, Bach N, Hellström KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–21. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. 10.1002/(sici)1521-4141(199803)28:03<1116::aid-immu1116>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Guinn B, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–10. [PubMed] [Google Scholar]
- 22.Usui S, Urano M, Koike S, Kobayashi Y. Effect of PSK, a protein polysaccharide, on pulmonary metastasis of C3H mouse squamous cell carcinoma. J Natl Cancer Inst. 1976;56:185–7. doi: 10.1093/jnci/56.1.185. [DOI] [PubMed] [Google Scholar]
- 23.Pe MB, Ikeda H, Inokuchi T. Tumour destruction and proliferation kinetics following periodic, low power light, haematoporphyrin oligomers mediated photodynamic therapy in the mouse tongue. Oral Oncol, Eur J Cancer. 1994;30B:174–8. doi: 10.1016/0964-1955(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 24.Karasuyama H, Tihyama N, Tada T. Autocrine growth and tumorgenicity of interleukin 2-dependent helper T cells transfected wih IL-2 gene. J Exp Med. 1989;169:13–25. doi: 10.1084/jem.169.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano HAN, Nohara C, Yagita H, KO. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–66. [PubMed] [Google Scholar]
- 26.Lanier LL, Recktenwald DJ. Multicolor immunofluorescence and flow cytometry. Methods: a Companion. Meth Enzymol. 1991;2:192–9. [Google Scholar]
- 27.Tan JT, Whitmire JK, Ahmed R, Pearson T, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–68. [PubMed] [Google Scholar]
- 28.Tan JT, Whitmire JK, Murali-Krishna K, et al. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol. 2000;164:2320–5. doi: 10.4049/jimmunol.164.5.2320. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz RH. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 30.La Motte RN, Sharpe AH, Bluestone JA, Mokyr MB. Host B7-1 and B7-2 costimulatory molecules contribute to the eradication of B7-1 transfected P815 tumor cells via a CD8+ T cell-dependent mechanism. J Immunol. 1999;162:4817–23. [PubMed] [Google Scholar]
- 31.Gajewski TF, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: Superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909–17. [PubMed] [Google Scholar]
- 32.Yang G, Mizuno MT, Hellstrom KE, Chen L. B7-negative versus B7-positive P815 tumor. Differential requirements for priming of an antitumor immune response in lymph nodes. J Immunol. 1997;158:851–8. [PubMed] [Google Scholar]
- 33.La Motte NL, Sharpe AH, Bluestone JA, Mokyr MB. Importance of B7-1-expressing host antigen-presenting cells for the eradication of B7-2 transfected P815 cells. J Immunol. 1998;161:6552–8. [PubMed] [Google Scholar]
- 34.Huang AYC, Bruce AT, Pardoll DM, Levitsky HI. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J Exp Med. 1996;183:769–76. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeBenedette MA, Chu NR, Pollok KE, Hurtado J, Wade WF, Kwon BS, Watts TH. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J Exp Med. 1995;181:985–92. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]