Abstract
The mucosal immunogenicity of a number of plant lectins with different sugar specificities was investigated in mice. Following intranasal (i.n.) or oral administration, the systemic and mucosal antibody responses elicited were compared with those induced by a potent mucosal immunogen (cholera toxin; CT) and a poorly immunogenic protein (ovalbumin; OVA). After three oral or i.n. doses of CT, high levels of specific serum antibodies were measured and specific IgA was detected in the serum, saliva, vaginal wash, nasal wash and gut wash of mice. Immunization with OVA elicited low titres of serum IgG but specific IgA was not detected in mucosal secretions. Both oral and i.n. delivery of all five plant lectins investigated [Viscum album (mistletoe lectin 1; ML‐1), Lycospersicum esculentum (tomato lectin; LEA), Phaseolus vulgaris (PHA), Triticum vulgaris (wheat germ agglutinin (WGA), Ulex europaeus I (UEA‐1)] stimulated the production of specific serum IgG and IgA antibody after three i.n. or oral doses. Immunization with ML‐1 induced high titres of serum IgG and IgA in addition to specific IgA in mucosal secretions. The response to orally delivered ML‐1 was comparable to that induced by CT, although a 10‐fold higher dose was administered. Immunization with LEA also induced high titres of serum IgG, particularly after i.n. delivery. Low specific IgA titres were also detected to LEA in mucosal secretions. Responses to PHA, WGA and UEA‐1 were measured at a relatively low level in the serum, and little or no specific mucosal IgA was detected.
Introduction
Mucosal and particularly oral administration of antigens is frequently ineffective at stimulating strong and sustained immune responses. In many cases a number of high doses are required and the responses induced may be of short duration. 1 Delivery systems and adjuvants can enhance the responses elicited to mucosally administered antigen, for example by protecting the antigen or by specific targeting to the epithelium. One strategy for antigen targeting is the use of molecules such as plant lectins, which bind specifically to mucosal epithelial cells.
Plant lectins are proteins possessing at least one non‐catalytic domain, which binds reversibly to a specific mono‐ or oligosaccharide.2 A recent definition distinguishes four major types of plant lectins according to their structure: merolectins, hololectins, chimerolectins and superlectins. 3 Merolectins consist of a single carbohydrate‐binding domain. Hololectins include the majority of characterized plant lectins, and contain two or more carbohydrate‐binding domains that are identical or highly homologous and bind either the same or structurally related sugars. Chimerolectins are fusion proteins composed of a carbohydrate‐binding domain and an unrelated non‐binding domain, which may have a catalytic or other biological activity (e.g. mistletoe lectin 1; ML‐1). Superlectins are fusion proteins of two structurally different carbohydrate‐binding domains and recognize structurally unrelated sugars. The specificity of a lectin is usually expressed in terms of the monosaccharide that best inhibits its effect. 4 In the present work, lectins with a specificity for fucose (UEA‐1), N‐acetylglucosamine (LEA, WGA), galactose (ML‐1) and a lectin with complex specificity (PHA) were investigated.
A number of plant lectins have been found to be stable in the rodent gut and to interact with the mucosal epithelium after feeding. 5 Of particular interest are studies that have shown selective labelling of antigen‐sampling M cells in the mouse Peyer’s patch by fucose‐specific lectins. 6,7 There is also recent evidence for translocation of plant lectins across the gut in both mice and humans. 8,9 The finding that certain plant lectins interact with the mucosal epithelium and are translocated across the gut may be exploited in vaccine delivery to induce mucosal and systemic immunity. Mitogenic plant lectins including phytohaemagglutinin (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM) are routinely used for activation of lymphocytes in vitro. 10 High levels of specific serum IgG were induced by oral administration of tomato lectin (LEA) to mice. 11 De Aizpurua and Russell‐Jones demonstrated enhanced immunogenicity of the hapten dinitrophenyl (DNP) when conjugated to plant lectins. 12 The latter authors suggested that proteins with lectin/lectin‐like properties are effective mucosal immunogens and proposed a relationship between receptor binding in the gut and mucosal immunogenicity. However, despite a number of studies on lectin binding and evidence that plant lectins conjugated to antigens/haptens may enhance immune responses following oral 12 and intranasal 13 delivery, there are relatively little data on the comparative mucosal immunogenicity of plant lectins. As there is interest in the use of lectins as mucosal vaccine targeting agents, the immunogenicity of these molecules may be an important factor. Therefore the present work investigated the local and systemic antibody responses to a number of mucosally delivered plant lectins.
Materials and methods
Antigens
Cholera toxin (CT), ovalbumin (OVA; type V, hen egg) and wheat germ agglutinin (WGA) were obtained from Sigma (Poole, UK). PHA from kidney bean was prepared as described previously. 14Ulex europaeus I (UEA‐1) and Lycospersicum esculentum (tomato lectin; LEA) were obtained from Vector Laboratories (Peterborough, UK). ML‐1 was isolated as described previously; 15 the preparation contained more than 95% ML‐1.
Animals
Seven‐week‐old female BALB/c mice (Harlan Olac, Bicester, UK) were given free access to a commercial stock diet (Labsure, Manea, UK) and water.
Mucosal immunization schedule
Groups of mice (n = 10) were bled 1 week prior to the first immunization. On days 1, 14 and 35, phosphate‐buffered saline (PBS), CT, OVA or plant lectins were orally or intranasally (i.n.) administered. For oral delivery, mice were intubated with 10 µg CT or 100 µg OVA in 100 µl sterile 0·1 m sodium bicarbonate via curved oral dosing needles (20 g × 25 mm; International Market Supply, Cheshire, UK). For i.n. immunization, mice were dosed through fine tips attached to a pipette; 1 µg CT and 10 µg OVA were delivered in 30 µl PBS (15 µl per nostril). Mice were orally gavaged with 100 µg plant lectin in 100 µl PBS. For i.n. immunization, lectins were delivered at a dose of 10 µg in 30 µl PBS.
Collection of blood and mucosal secretions
Blood
Blood samples were collected on days 13 and 34 after the primary immunization by bleeding from the tail vein following a 10‐min incubation at 37°. On days 49 and 50, animals were terminally anaesthetized (hypnorm plus diazepam) to allow collection of salivary and vaginal secretions. Mice were then killed by anaesthetic overdose followed by exsanguination. Blood was immediately collected, centrifuged and the serum stored at –20°.
Mucosal secretions
Absorbent cellulose wicks (Whatman International, Maidstone, UK) were used for collection of saliva and vaginal fluid as described previously. 16 Wash fluid [0·01 m PBS, 50 mm EDTA, 5 mm PMSF, 5 µg/ml Aprotinin (ice‐cold)] was used for elution of antibody from wicks and for nasal and intestinal washes.
Saliva was collected by the insertion of a wick tip into the mouth for 2 min. 16 Antibody was extracted from wicks into 400 µl mucosal wash fluid. The mean weights of saliva collected from groups of mice ranged from 2·79 to 5·94 mg. Vaginal fluid was collected by repeated flushing and aspiration of 50 µl of wash fluid and insertion of a wick for 2 min. Antibody was extracted from wicks into 400 µl wash fluid. The mean weights of vaginal secretions collected from groups of mice ranged from 42·2 to 57·2 mg. Nasal washes were collected from decapitated animals by back‐flushing 0·5 ml of mucosal wash fluid from the trachea. Intestinal wash was obtained by flushing the small intestine with 10 ml of ice‐cold wash fluid. All secretions were stored at –20° until required for analysis.
Detection of specific antibodies by enzyme‐linked immunosorbent assay (ELISA)
ELISA assays were set up to enable measurement of specific IgG, IgA and IgG subtypes. Sera (from 1 : 100) and mucosal secretions (from 1 : 2) were titrated in the appropriate dilution buffer.
Microtitre plates (Immunolon 4; Dynex Technologies, Middlesex, UK) were coated with 100 µl per well of 1 µg/ml antigen in carbonate–bicarbonate buffer, pH 9·6, and incubated at 4° overnight. After washing, plates were blocked with 2% gelatin/dilution buffer and incubated at 37° for 1 hr. Plates were washed, and samples were added and serially diluted and incubated at 37° for 1 hr. Biotinylated antiserum in dilution buffer was added and incubated at 37° for 1 hr. After a further series of washes, ExtrAvidin® peroxidase (Sigma) at a dilution of 1 : 750, prepared in dilution buffer, was added and incubated at 37° for 30 min. Plates were washed, 50 µl/well of developing solution [TMB microwell peroxidase substrate (1‐C) Kirkegaard and Perry Laboratories, Gaithersburg, MD] added, and incubated in the dark at room temperature for 30 min. The reaction was stopped by addition of 1 m H2SO4 and the absorbance read at 450 nm.
ELISA dilution buffers were as follows: CT, PBS + 0·1% Tween‐20 (PBST); OVA, PBST; WGA, 100 mm N‐acetylglucosamine/PBST; PHA, 0·1% Fetuin/PBST; UEA‐1, 30 mm l‐fucose/PBST; LEA, chitin hydrolysate 1 : 200 (Vector)/PBST; ML‐1, 100 mm d‐galactose/PBST. Working dilutions of anti‐IgG (1 : 8000) and IgA (1 : 8000) biotinylated capture antisera (Sigma) were determined after preliminary assays with pre‐immune and pooled positive sera. In preliminary assays it was shown that the inclusion of appropriate competing sugars in ELISA reduced cross‐reactions (data not presented). Working dilutions of IgG subtype antisera (Serotec, Kidlington, UK) were as recommended by the manufacturers (IgG1, 1 : 4000; IgG2a, 1 : 4000; IgG2b, 1 : 2000; IgG3, 1 : 2000). Endpoint titres were determined as the dilution of a serum or mucosal sample giving an optical density (OD) value of 0·1 units greater than the mean of control samples at the same dilution.
Total IgA was quantified as specific IgA with the following modifications: plates were coated with goat anti‐mouse IgA (1 : 8000; α‐chain‐specific; Sigma), PBST was used as diluent and 2% gelatin in PBST as blocking solution. Values for total IgA levels were calculated from the linear region of the IgA (IgAκ; Sigma) standard curve. Total IgA endpoint titres were determined as the dilution of a sample giving an OD value of 0·1 units greater than that of buffer alone.
Statistics
Data are expressed as the mean ± standard deviation. An unpaired two‐tailed t‐test was used to test for significance between groups. A paired t‐test for means was used to test for significance in the same group at different time‐points. Where the standard deviations were significantly different between groups, non‐parametric tests (Mann–Whitney to compare two groups, Kruskal–Wallis test with Dunn’s multiple comparison post‐test when comparing more than two groups) were used to assess significance. Kruskal–Wallis non‐parametric test with Dunn’s multiple comparison post‐test was used to assess significance of the total IgA data.
Results
Effect of mucosal administration of lectins on total IgA levels in sera and secretions
Figure 1 presents the levels of total IgA measured in saliva and nasal washes of mice after three doses of antigen. Levels of salivary IgA were significantly increased after three oral (P < 0·05) or i.n. (P < 0·01) doses of CT. None of the other regimes resulted in a significant rise in total salivary IgA levels. Intranasal delivery of both CT (P < 0·05) and ML‐1 (P < 0·001) led to a significant increase in total nasal wash IgA levels. There was no clear effect of any of the antigens on total IgA levels in vaginal wash, gut wash or serum (data not presented).
Figure 1.
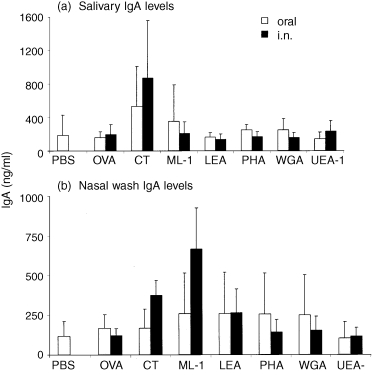
Total IgA levels (ng/ml) measured in the saliva (a) and nasal washes (b) of mice after three oral or i.n. doses of antigen. Mice were immunized i.n. with 10 µg OVA/plant lectin or 1 µg CT, or orally with 100 µg OVA/plant lectin or 10 µg CT. Data represent the mean ± SD.
Specific antibody responses
CT‐specific IgG was detected in 3/10 orally immunized and 1/10 i.n. immunized mice after a single dose of CT. After the second dose, antibody was detected in all mice in both groups, and titres increased further in the i.n. group after the third dose (P < 0·01). There was a significantly higher CT‐specific IgG titre in the i.n. immunized group (P < 0·01) than in the oral group after three doses of CT (Fig. 2). High titres of CT‐specific IgG1 were detected in both groups (not significantly different). In contrast, higher titres of CT‐specific IgG2a (P < 0·01) and IgG2b (not significant) were detected in the i.n. group. Of the antigens administered, significant levels of specific IgG2b and IgG3 antibodies were only elicited after immunization with CT. After two doses, CT‐specific IgA was detected in 8/10 mice in both groups and in all mice after the third dose (not significantly different). Specific IgA was detected in saliva, vaginal wash, nasal wash and gut wash of mice immunized by both routes. Mean titres were higher following i.n. immunization in saliva (P < 0·01) and nasal wash (P < 0·0001), but titres in vaginal wash were not significantly different between the groups. The titre of CT‐specific IgA in gut wash was significantly higher in the orally immunized animals (P < 0·0001). When adjusted for total IgA, the levels of CT‐specific IgA in serum and all the secretions sampled were relatively similar after oral delivery of CT (Table 1). However, a significantly higher CT‐specific IgA level was found in saliva than in vaginal wash (P < 0·05). After i.n. delivery of CT the adjusted levels of CT‐specific IgA in nasal wash and saliva were similar and both were significantly higher than in serum, gut wash and vaginal wash (P < 0·05).
Figure 2.
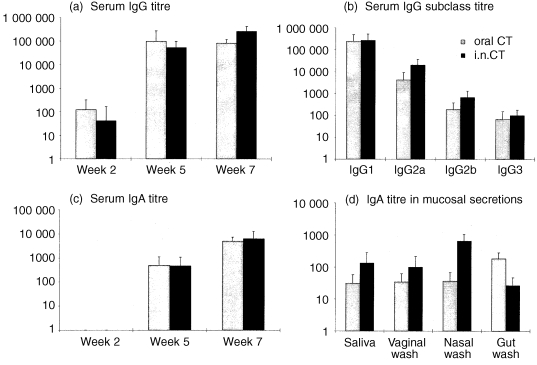
Specific antibody responses measured in mice immunized with CT by the oral or i.n. route. Mice were immunized i.n. with 1 µg CT or orally with 10 µg CT. Data represent the mean ± SD. (a) Serum IgG titres after one, two and three doses; (b) IgG antibody subclass titres 2 weeks after the third dose; (c) serum IgA titres after one, two and three doses; (d) IgA titres in mucosal secretions of mice 2 weeks after the third dose of CT.
Table 1.
IgA antibody response in sera and secretions of mice after oral and i.n. delivery of CT, ML‐1 and LEA. Values were adjusted for total IgA titres to give an indication of the relative levels of antibody at the various sites. Data were calculated by dividing the reciprocal specific IgA titres for each antigen by the reciprocal total IgA titres measured in the same samples
| Saliva | Vaginal wash | Nasal wash | Gut wash | Serum | |
|---|---|---|---|---|---|
| CT oral | 0·21 | 0·031 | 0·128 | 0·104 | 0·049 |
| CT i.n. | 0·313 | 0·037 | 0·55 | 0·005 | 0·025 |
| ML‐1 oral | 0·056 | 0·059 | 0·047 | 0·057 | 0·05 |
| ML‐1 i.n. | 0·458 | 0·078 | 0·203 | 0·003 | 0·017 |
| LEA oral | 0·002 | 0·016 | 0·003 | 0·005 | 0·019 |
| LEA i.n. | 0·045 | 0·009 | 0·131 | 0·004 | 0·017 |
After three immunizations with OVA, specific IgG was detected in 4/10 orally immunized and 5/10 i.n. immunized animals (Table 2). The maximum titre was 1 : 100 and there were no significant differences between the groups. OVA‐specific serum IgA was detected in a small number of mice at a maximum titre of 1 : 200. OVA‐specific IgA was not detected in saliva, vaginal wash or nasal wash but was measured in the gut wash of 1/10 orally immunized mice.
Table 2.
Mean reciprocal antibody titres measured in sera and secretions of mice after three oral or i.n. doses of OVA, PHA, WGA and UEA‐1. The number of responding mice and total number of mice in each group are in parentheses. Mice were immunized i.n. or orally with 10 µg or 100 µg OVA/lectin, respectively. Endpoint titres were determined as the lowest dilution of test sample giving an absorbance of greater than 0·1 units higher than control samples at the same dilution
| Lectin (route) | IgG serum | IgG1 serum | IgG2a serum | IgG2b serum | IgG3 serum | IgA serum | IgA saliva | IgA vagina | IgA nasal | IgA gut |
|---|---|---|---|---|---|---|---|---|---|---|
| OVA oral | 40 (4/10) | – | – | – | – | – | – | – | – | 1·6 (1/10) |
| OVA i.n. | 50 (5/10) | – | – | – | – | 70 (6/10) | – | – | – | – |
| PHA oral | 290 (6/10) | 241 (6/10) | 10 (1/10) | – | – | 450 (9/10) | – | – | – | 1·6 (2/10) |
| PHA i.n. | 800 (10/10) | 841 (10/10) | – | – | – | 410 (7/10) | – | – | – | 2 (4/10) |
| WGA oral | 500 (10/10) | 131 (5/10) | – | – | – | 200 (9/10) | – | – | – | – |
| WGA i.n. | 130 (9/10) | – | – | – | – | 190 (8/10) | – | – | – | – |
| UEA‐1 oral | 3770 (9/10) | 671 (8/10) | – | – | – | 200 (7/10) | – | – | – | 1·2 (3/10) |
| UEA‐1 i.n. | 673 (6/10) | 101 (6/10) | – | – | – | 173 (9/10) | – | – | 0·4 (2/10) | 0·9 (2/10) |
Of the five plant lectins investigated, the highest specific antibody titres were elicited by immunization with ML‐1 (Fig. 3). After a single dose, ML‐1‐specific IgG was detected in 1/10 orally immunized mice but not in i.n. immunized animals. In addition, after the first dose 3/10 orally immunized mice were killed due to their poor condition; the seven remaining animals also suffered weight loss (data not presented). A similar decrease in body weight was measured in the i.n. immunized animals and one animal was killed after the second dose. ML‐1‐specific IgG was detected in all mice in both groups after the second dose and the titre increased after the final dose. The mean titres after two and three doses were not significantly different between the groups. Assays for ML‐1‐specific IgG subclasses in sera from orally immunized mice detected IgG1 and IgG2a only. A high titre of ML‐1‐specific IgG1 was detected in both orally and i.n. immunized mice (not significantly different), but specific IgG2a was only detected at a low level in both groups. ML‐1‐specific serum IgA was detected in 2/7 orally immunized but not in i.n. immunized mice after a single dose. After two and three doses, ML‐1‐specific IgA was detected in all mice in both groups, with significantly higher titres in the orally immunized animals (P < 0·05). This fourfold higher specific IgA titre contrasts with the IgG data, where similar titres were measured in the two groups. The specific serum IgA response elicited to orally administered ML‐1 was the highest recorded for any antigen in this study. After three doses, specific antibody was detected in the saliva of 4/7 orally immunized and 9/9 i.n. immunized mice (no significant difference between groups). ML‐1‐specific IgA was also detected in vaginal washes of all mice in both groups (not significantly different). The highest ML‐1‐specific IgA titres in nasal washes were from i.n. immunized animals (P < 0·01) and in gut washes from the orally immunized animals (P < 0·01). This indicates that mucosal administration of ML‐1 induced a disseminated mucosal antibody response. When the titres were adjusted for total IgA (Table 1), the levels of ML‐1‐specific IgA in serum and in the various secretions were not significantly different after oral delivery of ML‐1. However, after i.n. delivery the levels of ML‐1‐specific salivary IgA were significantly higher than in sera and gut washes (P < 0·01). In addition, the adjusted ML‐1‐specific IgA levels in nasal washes were higher than in gut washes (P < 0·01).
Figure 3.
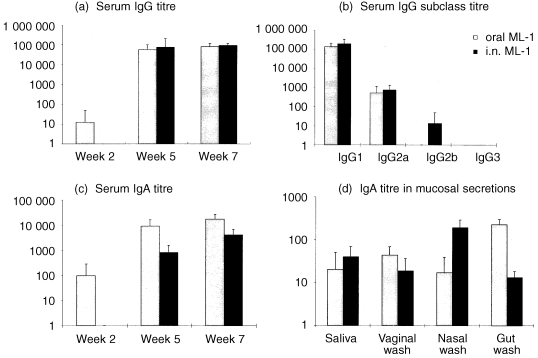
Specific antibody responses measured in mice immunized with ML‐1 by the oral or i.n. route. Mice were immunized i.n. with 10 µg ML‐1 or orally with 100 µg ML‐1. Data represent the mean ± SD. (a) Serum IgG titres after one, two and three doses; (b) IgG antibody subclass titres 2 weeks after the third dose; (c) serum IgA titres after one, two and three doses; (d) IgA titres in mucosal secretions of mice 2 weeks after the third dose of ML‐1.
After a single dose of LEA, specific IgG was detected in 9/10 i.n. immunized (mean titre 8200) and 1/10 orally immunized (mean titre 160) mice (P < 0·0001). The LEA‐specific IgG titres after two (P < 0·01) and three (P < 0·05) doses were also significantly higher in the i.n. immunized mice (Fig. 4). LEA‐specific IgG1 and IgG2a antibodies were measured, but only low levels of IgG2b were detected. After three doses, the titres of LEA‐specific IgG1 were significantly (P < 0·05) higher in the i.n. immunized mice. In contrast to the specific IgG responses to LEA, low levels of LEA‐specific serum IgA were measured and titres were not significantly different between the groups. Significantly higher LEA‐specific salivary IgA titres were detected in the i.n. immunized group (P < 0·0001), although the titres were relatively low. LEA‐specific IgA was also detected in vaginal washes with similar titres in both groups. Higher LEA‐specific IgA titres (P < 0·0001) were measured in nasal washes of i.n. immunized mice. In addition, LEA‐specific IgA was detected in the gut mucus of all animals in both groups (titres not significantly different). After correction for total IgA titres (Table 1), significantly higher levels of LEA‐specific IgA were found in serum than in nasal washes or saliva of mice after oral delivery (P < 0·01). After i.n. administration, higher adjusted levels of LEA‐specific IgA were found in nasal washes than in serum, vaginal washes or gut washes (P < 0·05). In addition, there was a higher LEA‐specific IgA level in saliva than in vaginal washes (P < 0·05).
Figure 4.
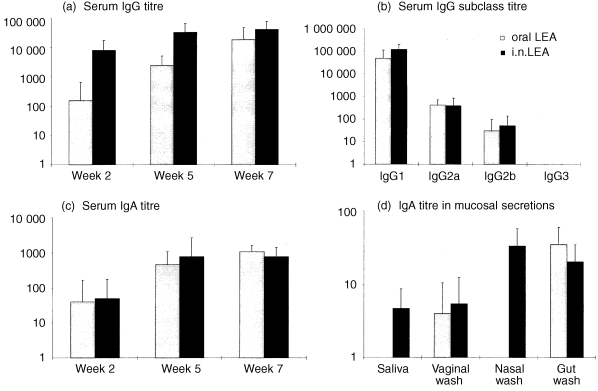
Specific antibody responses measured in mice immunized with LEA by the oral or intranasal route. Mice were immunized i.n. with 10 µg LEA or orally with 100 µg LEA. Data represent the mean ± SD. (a) Serum IgG titres after one, two and three doses; (b) IgG antibody subclass titres 2 weeks after the third dose; (c) serum IgA titres after one, two and three doses; (d) IgA titres in mucosal secretions of mice 2 weeks after the third dose of LEA.
After three doses of PHA, specific IgG (Table 2) was detected in 6/10 orally immunized and 10/10 i.n. immunized mice, with a higher titre in the i.n. group (P < 0·05). Of the PHA‐specific IgG subclasses assayed for, only IgG1 was measured at significant levels, with a higher mean titre (P < 0·05) in the i.n. group. PHA‐specific serum IgA was not detected after a single dose, but was detected after three doses with similar titres in both groups. PHA‐specific IgA was measured in the gut wash of a small number of mice in both groups but not in the saliva, vaginal wash or nasal wash.
After a single dose of WGA, specific IgG was detected in 5/10 orally immunized and 0/10 i.n. immunized mice. After three doses, WGA‐specific IgG was detected in 10/10 orally immunized and 9/10 i.n. immunized mice, with no significant difference in mean titres (Table 2). Of the WGA‐specific IgG subclasses, only IgG1 was measured and only in orally immunized mice. After three doses, WGA‐specific serum IgA was detected with similar titres in both groups. Specific IgA was not detected in mucosal secretions.
After three doses of UEA‐1, specific IgG (Table 2) was measured with the highest titres in orally immunized mice, but the mean titres were not significantly different between the groups. UEA‐1‐specific IgG1 was detected in both groups (difference not significant); IgG2a, IgG2b and IgG3 were not detected. Specific serum IgA was measured in a number of mice after three doses but UEA‐1‐specific IgA was not detected in the saliva or vaginal wash in either group. UEA‐1‐specific IgA was detected in the nasal wash from 2/10 i.n. immunized animals but not in orally immunized mice. Specific IgA was measured in the gut mucus of a small number of mice in both groups.
Discussion
The present data demonstrate that certain plant lectins are strong mucosal immunogens, stimulating systemic and mucosal antibody responses after oral or i.n. delivery. While delivery of all five lectins stimulated higher antibody titres than OVA, the range of responses was very wide. This indicates that lectin or lectin‐like properties do not necessarily confer potent mucosal immunogenicity. DNP conjugates of a number of plant lectins with different sugar specificities (including UEA‐1 and PHA) were of comparable efficacy after oral delivery, 12 so lectin–antigen conjugates may effectively stimulate immune responses even if the carrier lectin used is itself not highly immunogenic. The latter study did not measure anti‐plant lectin responses directly, as sugar specific binding to IgG interfered with the ELISA. In the present study the use of competing sugars in the assays facilitated a determination of specific anti‐lectin antibody responses.
CT and Escherichia coli heat‐labile enterotoxin (LT) are powerful mucosal immunogens, small doses of antigen generating strong antitoxin secretory and systemic antibody responses. 17–19 The B subunits of CT and LT are also effective mucosal immunogens, but less so than the holotoxins. CT consists of structurally and functionally distinct A and B subunits. 20 The B subunit consists of five identical polypeptides that bind with high affinity to GM1 ganglioside cell‐surface receptors and promote entry of the A subunit into the cell. Toxicity is recognized as a problem with the holotoxins, but non‐toxic mutants have been constructed 21,22 that are effective mucosal immunogens and adjuvants. ML‐1 is a type‐2 ribosome inactivating protein (type‐2 RIP), containing a toxophoric (toxic following binding of the B chain to the cell membrane) N‐glycosidase A subunit, responsible for the ribosome‐inactivating activity, and a carbohydrate‐binding B subunit. That ML‐1 is comparable to CT as a mucosal immunogen is an important finding because CT is among the most powerful mucosal immunogens identified. 17,18 The highest titres of specific serum IgA and gut wash IgA were measured in animals immunized orally with ML‐1. Both i.n. and oral delivery also induced specific antibody in the nasal wash, saliva and vagina. At subtoxic concentrations, mistletoe lectins possess immunomodulatory properties. 23,24 The lectins induced mRNA expression and enhanced secretion of proinflammatory cytokines in cultures of human peripheral blood monocytes. 23 Additionally, ML‐1 stimulated natural killer cells and granulocytes in animal models. 24
After oral immunization with 100 µg ML‐1, a number of mice were killed due to their poor condition. Intranasal administration of 10 µg ML‐1 adversely affected growth of the animals. However, i.n. delivery of 1 µg ML‐1 did not result in deaths or significant weight losses and induced comparable immune responses to the 10 µg dose (E. C. Lavelle, G. Grant, A. Pusztai, U. Pfüller & D. T. O'Hagan, manuscript in preparation). This indicated that the lectin can have adverse affects but that lower doses of the lectin may have less deleterious consequences while retaining efficacy. Recent work found that rats could be fed with diets containing up to 200 mg ML‐1/kg body weight without major toxicity symptoms, 25 indicating that oral administration of ML‐1 was less toxic in rats than parenteral delivery in mice. 26 That ML‐1, the most toxic lectin, was also the strongest mucosal immunogen may parallel the case with CT and LT, where the holotoxins are more immunogenic than the non‐toxic B subunits. 27 There was no indication of toxicity in the case of the other lectins delivered in the present study.
Apart from suggestions that the binding activity of lectins can confer immunogenicity, there is little information on what determines the mucosal immunogenicity of lectins. CT has been shown to exert a number of immunomodulating effects, including the enhancement of antigen presentation by an effect on costimulation, promoting B‐cell isotype switch differentiation, and enhancing T‐cell priming efficiency against unrelated antigens. 27 B subunit of Escherichia coli heat‐labile enterotoxin (LTB) binds to GM1 ganglioside and galactoproteins on the brush border of intestinal epithelial cells, is taken up by receptor‐mediated endocytosis, and is transcytosed to the basolateral side of the enterocyte. 28 It was suggested that LTB taken up across villous enterocytes entered the circulation to induce an IgG response in the spleen, while uptake across the Peyer’s patch follicle‐associated epithelium induced a local IgG and IgA response in the Peyer’s patch and intestinal lymphoid follicles. The receptors to which lectins bind in the gut are poorly understood but may be a decisive factor in determining mucosal immunogenicity.
LEA is stable in the digestive tract 11,29 and binds to rat intestinal villi. Data from the present study confirms that LEA is immunogenic by the oral route and demonstrates that it also stimulates systemic and mucosal responses when delivered i.n. Higher responses were measured in the i.n. immunized mice, particularly after a single antigen dose, possibly due to strong binding of the orally delivered lectin to mucus in the gut. 30
Mouse intestinal M cells are distinguished by expression of particular oligosaccharide epitopes. The mouse Peyer’s patch M‐cell membranes selectively express carbohydrate epitopes bearing α‐(1‐2)‐linked fucose. 6,8 In the murine large intestine, M cells are distinguished by expression of carbohydrate epitopes bearing terminal α‐(1‐3)‐linked galactose. Probes that recognized α‐linked galactose, bound to the follicle‐associated epithelium (FAE) of hamster nasal‐associated lymphoid tissue (NALT) 13 and were efficiently endocytosed, and i.n. immunization with lectin‐antigen conjugates elicited an increased specific serum IgG response. The specificity of UEA‐1 for mouse Peyer’s patch M cells stimulated interest in its use for oral vaccine targeting. Coating of microspheres with UEA‐1 led to increased binding to M cells, while binding to enterocytes was unaffected. 31 The present work does not indicate that UEA‐1 is a potent mucosal immunogen; however, the higher titres induced by oral delivery may reflect greater uptake by the intestinal M cells. Interaction of UEA‐1 with mucus may have prevented binding and uptake of the lectin. Strong binding of WGA and UEA‐1 to goblet cells in the intestine of rats has been reported. 32
The highest levels of specific IgA in secretions were detected in animals with high levels of circulating antibody, particularly IgG. Thus there was no indication of the induction of a strong local response in the absence of a systemic response. An interesting finding was the strong induction of a serum IgA response by orally delivered ML‐1 (ratio of specific IgG : IgA, 4·63 compared with LEA, 17·27, or CT, 16). Oral and i.n. administration of CT or LEA induced comparable levels of specific circulating IgA, while significantly higher levels of serum IgG were elicited by i.n. administration. This indicates that, as with CT, the route of lectin delivery influences the type and magnitude of the response. No correlation was found between the sugar specificity of lectins and immunogenicity. While LEA induced a strong serum IgG response, WGA was poorly immunogenic. The inhibitory carbohydrate for both lectins is N‐acetyl‐d‐glucosamine. However, binding of lectins to monosaccharides does not imply that these are their true receptor molecules, lectins may have a higher affinity for oligosaccharides containing two or more monosaccharide units. 3 The immune responses to LTB and K99 pili were abolished by cofeeding galactose, lactose or sorbitol, 12 which are structurally similar to galactose, suggested to be the specific sugar determinant of GM1 ganglioside, to which LTB binds, and GM2 ganglioside, to which K99 can bind. The suggestion that receptor‐mediated binding is required to induce immune responses following oral feeding is supported by recent findings on fimbriae from enterotoxigenic E. coli in pigs. 33
A number of antigens (CT, ML‐1, and LEA in particular) stimulated high titres of IgG1, but only CT stimulated high titres of IgG2a. In the case of CT a similar IgG1 titre was measured in i.n. and orally immunized mice, while there was a 16‐fold higher IgG2a titre in the i.n. immunized mice and the titres of IgG2b and IgG3 were also higher. Such a bias was not noted with the other antigens. A predominance of IgG1 and IgG2b in the serum of mice immunized i.n. with CT has been reported previously. 34 The oral administration of CT alone or together with a co‐administered protein antigen induced a strong T‐helper type 2 (Th2)‐type response in both Peyer’s patches and spleen. 34,35 Although IgG subclass antibodies are not a conclusive marker for type 1 or type 2 responses, the present data indicate that the responses to lectins are more skewed towards a type 2 response than in the case of CT.
This is the first detailed study of the immunogenicity of plant lectins following i.n. administration. The data indicate that i.n. delivery of a 10‐fold lower dose is more effective than oral delivery. This was not the case for WGA and UEA‐1, but these responses were low and highly variable. The greater efficacy of i.n. compared with oral delivery of CT has been documented recently. 36 Intranasal administration of horseradish peroxidase (HRP) conjugates of the lectins BSI‐B4 and WGA elicited an enhanced serum IgG response to HRP but a local IgA response was not measured. 13 The present findings that PHA, WGA and UEA‐1 do not elicit strong local antibody responses may indicate a potential as vaccine carriers, as a local IgA anti‐lectin response might reduce the response to booster immunizations. In summary, highly immunogenic plant lectins have been identified that, when delivered by oral or i.n. routes, can effectively stimulate systemic and mucosal antibody responses.
Acknowledgments
We thank the Chiron Corporation for supporting this work. The practical assistance of W. Buchan, K. Angel, W. Pickford and T. Walker is much appreciated.
References
- 1.Nossal GJV. Vaccines. In: Paul WE, editor. Fundamental Immunology. 4. Philadelphia, PA: Lippincott‐Raven Publishers; 1999. pp. p.p1387–1425. [Google Scholar]
- 2.Peumans WJ, Van Damme EJM. Lectins as plant defense proteins. Plant Physiol. 1995;109:347. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S. Plant lectins: a special class of plant proteins. In: Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S, editors. Handbook of Plant Lectins: Properties and Biomedical Applications. Chichester, UK: John Wiley and Sons; 1998. p. p.3. [Google Scholar]
- 4.Goldstein IJ, Poretz RD. Isolation and chemical properties of lectins. In: Liener IE, Sharon N, Goldstein IJ, editors. The Lectins: Properties, Functions, and Applications in Biology and Medicine. Orlando, FL: Academic Press; 1986. p. p.33. [Google Scholar]
- 5.Pusztai A, Bardocz S. Biological effects of plant lectins on the gastrointestinal tract: metabolic consequences and applications. Trends Glycoscience Glycotechnol. 1996;8:149. [Google Scholar]
- 6.Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin‐binding sites defines mouse intestinal M‐cells. J Histochem Cytochem. 1993;41:1679. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Schumacher U, Adam E. Lectin histochemistry reveals the appearance of M‐cells in Peyer’s patches of SCID mice after syngeneic normal bone marrow transplantation. J Histochem. 1998;46:143. doi: 10.1177/002215549804600202. [DOI] [PubMed] [Google Scholar]
- 8.Clark MA, Jepson MA, Simmons NL, Hirst BH. Selective binding and transcytosis of Ulex europaeus lectin I by mouse Peyer’s patch M cells in vivo. Cell Tissue Res. 1995;282:455. doi: 10.1007/BF00318877. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Yu L‐G, Campbell BJ, Milton JD, Rhodes JM. Identification of intact peanut lectin in peripheral venous blood. Lancet. 1998;352:1831. doi: 10.1016/S0140-6736(05)79894-9. [DOI] [PubMed] [Google Scholar]
- 10.Heegaard PMH, Muller K. Lectins and the immune system. EOS J Immunol Immunopharmacol. 1988;8:239. [Google Scholar]
- 11.Naisbett B, Woodley J. The potential use of tomato lectin for oral drug delivery. IV. Immunological consequences. Int J Pharm. 1995;120:247. [Google Scholar]
- 12.Di Aizpurua HJD, Russell‐jones GL. Identification of classes of proteins that provide an immune response upon oral feeding. J Exp Med. 1988;167:440. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannasca PJ, Boden JA, Monath TP. Targeted delivery of antigen to hamster nasal lymphoid tissue with M‐cell directed lectins. Infect Immun. 1997;65:4228. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardocz S, Grant G, Pusztai A, Franklin MF, Carvalho A de FFU. The effect of phytohemagglutinin at different dietary concentrations on the growth, body composition and plasma insulin of the rat. Br J Nutr. 1996;76:613. doi: 10.1079/bjn19960067. [DOI] [PubMed] [Google Scholar]
- 15.Eifler R, Pfüller K, Göckeritz W, Pfüller U. Improved procedures for isolation of mistletoe lectins and their subunits: lectin pattern of the European mistletoe. In: Basu J, Kundu M, Chakrabarty P, editors. Lectins: Biology, Biochemistry, Clinical Biochemistry. New Delhi, India: Wiley Eastern Limited; 1994. p. p.9.144. [Google Scholar]
- 16.Ugozzoli M, O'hagan DT, Ott GS. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. 1998;93:563. doi: 10.1046/j.1365-2567.1998.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elson CJ, Ealding W. Generalised systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736. [PubMed] [Google Scholar]
- 18.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat‐labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 19.Di Tommaso A, Saletti G, Pizza M, et al. Induction of antigen‐specific antibodies in vaginal secretions by using a non‐toxic mutant of heat‐labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 21.Pizza M, Domenighini M, Hol W, et al. Probing the structure activity relationship of Escherichia coli LT‐A by site‐directed mutagenesis. Mol Microbiol. 1994;14:51. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 22.Douce G, Turcotte C, Cropley I, et al. Mutants of Escherichia coli heat‐labile toxin lacking ADP‐ribosyltransferase activity act as non‐toxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajto T, Hostanska K, Frei K, Rordorf C, Gabius HJ. Increased secretion of tumor necrosis factor‐alpha, interleukin‐1, and interleukin‐6 by human mononuclear‐cells exposed to beta‐galactoside‐specific lectin from clinically applied mistletoe extract. Cancer Res. 1990;50:3322. [PubMed] [Google Scholar]
- 24.Hajto T, Hostanska K, Gabius HJ. Modulatory potency of the beta‐galactoside‐specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 1989;49:4803. [PubMed] [Google Scholar]
- 25.Pusztai A, Grant G, Gelencsér E, et al. Effects of an orally administered mistletoe (type‐2 RIP) lectin on growth, body composition, small intestinal structure, and insulin levels in young rats. J Nutr Biochem. 1998;9:31. [Google Scholar]
- 26.Gossrau R, Franz H. Histochemical response of mice to mistletoe lectin I (MLI) Histochemistry. 1990;94:531. doi: 10.1007/BF00272618. [DOI] [PubMed] [Google Scholar]
- 27.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera‐like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 28.Lindner J, Geczy AF, Russell‐jones GJ. Identification of the site of uptake of the E.coli heat‐labile enterotoxin, LTB. Scand J Immunol. 1994;40:564. doi: 10.1111/j.1365-3083.1994.tb03505.x. [DOI] [PubMed] [Google Scholar]
- 29.Kilpatrick DC, Pusztai A, Grant G, Graham C, Ewen SWB. Tomato lectin resists digestion in the mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 1985;185:299. doi: 10.1016/0014-5793(85)80927-3. [DOI] [PubMed] [Google Scholar]
- 30.Lehr C‐M. Bioadhesion technologies for the delivery of peptide and protein drugs to the gastrointestinal tract. Crit Rev Ther Drug Carrier Syst. 1994;11:119. [PubMed] [Google Scholar]
- 31.Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 targets microspheres to mouse Peyer’s patch M‐cells in vivo. Vaccine. 1998;16:536. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Schumacher U. The influence of diets and gut microflora on lectin binding patterns of intestinal mucins in rats. Lab Invest. 1995;73:558. [PubMed] [Google Scholar]
- 33.Van den Broek W, Cox E, Goddeeris BM. Receptor‐dependent immune responses in pigs after oral immunization with F4 fimbriae. Infect Immun. 1999;67:520. doi: 10.1128/iai.67.2.520-526.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu‐amano J, Kiyono H, Jackson RJ, et al. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinaro M, Staats HF, Hiroi T, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL‐4. J Immunol. 1995;155:4621. [PubMed] [Google Scholar]
- 36.Douce G, Giuliani MM, Giannelli V, Pizza MG, Rappuoli R, Dougan G. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine. 1998;16:1065. doi: 10.1016/s0264-410x(98)80100-x. [DOI] [PubMed] [Google Scholar]


