Abstract
Human immunoglobulin preparations are used therapeutically for various disorders. Such therapy is generally safe but adverse effects occasionally occur in recipients. It has been suggested that antibodies to cytokines present in clinical immunoglobulin products may contribute to undesirable effects in recipients. Therefore, we investigated intravenous and intramuscular immunoglobulin products for the presence of cytokine‐specific neutralizing antibodies. Using validated bioassays, we detected neutralizing activity against human granulocyte–macrophage colony‐stimulating factor (GM‐CSF), interferon‐α2a (IFN‐α2a) and interleukin‐1α (IL‐1α) in immunoglobulin products. We found no neutralization of granulocyte colony‐stimulating factor, macrophage colony‐stimulating factor, stem cell factor, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐6, IL‐9, IL‐10, IL‐12, tumour necrosis factor‐α, oncostatin M (OSM) and IFN‐γ. Most batches which neutralized IFN‐α2a activity also neutralized other IFN‐α subtypes, IFN‐ω and IFN‐β. Most products (94%) neutralized the biological activity of GM‐CSF. No correlation between batches and their ability to neutralize bioactivities of GM‐CSF, IFN‐α2a and IL‐1α was found. This neutralizing activity could be traced to plasma pools used for manufacture of immunoglobulins. The neutralization was mediated by specific cytokine antibodies contained within immunoglobulin products as it was present in specific immunoglobulin G (IgG) fractions eluted from cytokine affinity chromatography columns. Specific binding of such IgG fractions to cytokines in immunoblots and in enzyme‐linked immunosorbent assays (ELISAs) was observed. This contrasts with the broad non‐specific recognition of cytokine proteins observed using unfractionated immunoglobulins in ELISAs. This is the first comprehensive study showing the presence of neutralizing antibodies against GM‐CSF, IL‐1α, or IFN‐α2a in immunoglobulin products.
Introduction
Human immunoglobulin preparations are increasingly being used for the therapy of various disorders including primary/secondary immune deficiencies, infections and a range of haematological, neurological and autoimmune diseases where inflammatory processes are critical to the pathophysiology. Most therapeutic immunoglobulins are prepared by cold ethanol fractionation from plasma and are intended for intravenous (i.v.) or intramuscular (i.m.) administration. The i.v. immunoglobulin preparations usually undergo additional processing. In addition, ‘specific’ immunoglobulin preparations are prepared from plasma containing relatively high titres of particular antibodies, e.g. anti‐D, anti‐tetanus, etc. These specific immunoglobulin products are mainly intended for intramuscular use. In general, immunoglobulin products are safe and efficacious for treatment of licensed indications, however, there are some reports associating at least some immunoglobulin preparations with induction of adverse effects in recipients of these products.
Cytokines are important mediators of inflammatory reactions and immune responses. They also influence many physiological systems. The control of their production and action is complex. In particular, cytokine activities can be significantly modulated by cytokine‐binding proteins such as soluble cytokine receptors, cytokine antibodies, and α2‐macroglobulin which may interfere with interactions between cytokines and their cognate receptors.
Several reports have described the presence of autoantibodies which bind interferon‐α (IFN‐α), 1 interleukin‐1α (IL‐1α), 2 IL‐2 3 and IL‐6 4 in the serum of healthy individuals. Cytokine autoantibodies have also been detected in patients in various disease states. For example, IL‐1α antibodies have been demonstrated in non‐destructive chronic polyarthritis, 5 IL‐8 autoantibodies in adult respiratory distress syndrome and heparin‐associated thrombocytopenia, 6,7 IL‐10 autoantibodies in chronic inflammatory arthritis or bullous pemphigoid, 8 and IFNα autoantibodies in autoimmune disease, 9−11 cancer, 12 viral diseases 13−16 and also in recipients of allogenic bone marrow transplants. 17 In general, the occurrence of autoantibodies to cytokines appears to be sporadic. However, in myasthenia gravis, especially thymoma‐associated disease, a high incidence (>80%) of IFNα and IL‐12 autoantibodies, 18 but rarer occurrence of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) autoantibodies, has been found. 19 The clinical significance of the development of antibodies to cytokines remains uncertain.
A recent study showed GM‐CSF, IL‐5 and IL‐10 binding antibodies in pharmaceutical human i.v. immunoglobulin preparations and concluded that the presence of cytokine antibodies in immunoglobulin preparations is responsible for undesirable immunomodulatory effects. 20,21 In another study, it was suggested that high levels of IFN‐γ‐specific antibodies present in i.v. immunoglobulin (as detected by binding assays) contributed to the inhibition of proliferation evident in mixed lymphocyte reactions. 22 However, there remains in many cases, a lack of evidence demonstrating the authenticity of these autoantibodies. There is an over‐reliance on antibody binding tests alone and there are scarce data, if any, confirming that the observed inhibitory effects of immunoglobulin preparations on functional responses of specific cytokines are due to bona fide specific cytokine antibodies. In this study, we tested immunoglobulin preparations for the presence of antibodies against a range of cytokines using validated assays for detection of neutralizing antibodies. In addition, we have affinity‐isolated specific neutralizing antibodies against GM‐CSF, IL‐1α and IFN‐α, from immunoglobulin preparations having inhibitory effects on their biological activity. However, we found no evidence of any neutralizing activity against granulocyte colony‐stimulating factor (G‐CSF), macrophage colony‐stimulating factor (M‐CSF), stem cell factor (SCF), IL‐1β, IL‐2, IL‐3, IL‐4, IL‐6, IL‐9, IL‐10, IL‐12, oncostatin M (OSM), tumour necrosis factor‐α (TNF‐α) and IFN‐γ in any of the immunoglobulin preparations tested.
MATERIALS and METHODS
Test materials
Several batches of pharmaceutical‐grade human i.v. immunoglobulin preparations and specific immunoglobulin preparations designated A, B, C and D, etc. from different manufacturers were randomly selected for analysis from samples received by the National Institute for Biological Standards and Control (NIBSC) for batch‐release purposes.
Cytokines and antibodies
Recombinant cytokines used in various assays were generously donated by Hoffman‐La Roche (Basle, Switzerland), Chiron Corporation (Emeryville, CA), Schering‐Plough Ltd (Bloomfield, NJ), Immunex Corporation (Seattle, WA) and Amgen (Thousand Oaks, CA). The polyclonal sheep antibodies to recombinant human GM‐CSF, IL‐1α, IL‐2, IL‐3 and other cytokines were generated and characterized for specificity at NIBSC using established protocols. In biological assays, World Health Organization (WHO) international standards (IS), WHO reference reagents (RR) or reference preparations (available from NIBSC) for the cytokines GM‐CSF, G‐CSF, M‐CSF, IL‐1α, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐5, IL‐6, IL‐9, IL‐10, IL‐11, IL‐12, TNF‐α, SCF, leukaemia inhibitory factor (LIF), OSM, thrombopoietin (TPO), IFN‐α1, IFN‐α2a, consensus IFN, lymphoblastoid IFN, IFN‐α2b, IFN‐β, IFN‐γ and IFN‐ω were used.
Bioassays for cytokines
The effect of various immunoglobulin preparations on the biological activity of IL‐1, IL‐2, IL‐6, TNF‐α, IL‐10 and IL‐12 was evaluated using assays based on the murine thymoma cell line, NOB‐1 (for IL‐1), the murine cytotoxic T‐cell line, CTLL‐2 (for IL‐2), the murine plasmacytoma cell line, B9 (for IL‐6), the human rhabdosarcoma cell line, KD4 (for TNF‐α), a murine pro‐B‐cell line transfected with the murine IL‐10 receptor, BaMr (for IL‐10), a murine plasmacytoma cell line, B9‐11, a subclone of B9 cells (for IL‐11) and the human adult T‐lymphocytic leukaemia, KIT 225 cell line (for IL‐12), respectively. 23 In all assays, the effect of immunoglobulin preparations was assessed by performing a dilution series of immunoglobulin preparations in microtitre plate wells and preincubating these with a fixed amount of the appropriate cytokine for 1 hr at 37°. All assays included a dose–response curve of the appropriate cytokine in the presence or absence of the appropriate positive control neutralizing antibody.
Bioassay for GM‐CSF
For effects on the biological activity of GM‐CSF, two human cell lines, TF‐1 (of erythroleukaemic origin) and MO7e (of megakaryocytic origin) which proliferate in response to GM‐CSF were used in assays as described. 23,24 These cell lines also respond to other cytokines (e.g. TF‐1 responds to IL‐3, IL‐5, OSM, SCF, LIF, etc. while MO7e responds to IL‐3, IL‐9, TPO, etc.) and so were an ideal choice for evaluating the effect of different immunoglobulin preparations on proliferation induced by various cytokines. All assays included a dose–response curve of the appropriate recombinant human cytokine [for GM‐CSF, we also used a natural GM‐CSF preparation prepared by stimulating 2 × 106 human peripheral blood mononuclear cells with phytohaemagglutinin (PHA) for 48 hr at 37°].
Anti‐viral assay for IFN
The effects on the anti‐viral activity of IFN were assessed using the human glioblastoma cell line 2D9 in combination with encephalomyocarditis virus (EMCV), which was used as a challenge virus. 25 This assay is responsive to human IFN‐α, ‐β and ‐γ (A. Meager et al., in preparation). For assay, immunoglobulin preparations were serially diluted and IFN preparations were added at a level at which IFN completely protects cells against viral challenge. Following incubation at 37° for 1 hr, the plates were seeded with 6 × 104 2D9 cells per well. The plates were incubated for 24 hr at 37° and the antibody–IFN mixture was removed and replaced by 0·1 ml of EMCV‐containing medium per well (EMCV kills 2D9 cells, if not protected by IFN, within 24 hr of challenge). To terminate the assay, 2D9 cell monolayers were stained with 0·05% amido blue‐black and fixed with 10% formalin solution in acetic acid buffer (A. Meager et al., in preparation). Amido blue‐black stain was eluted in 0·15 ml of 0·05 m NaOH solution per well and absorbances were read at 620 nm.
Statistical analysis
The volume of immunoglobulin preparation giving a 50% response or effective dose‐50 (ED50) obtained from fitting common asymptotes and slopes for all test samples (within an assay) separately for GM‐CSF, IL‐1α, or IFN type 24 was used to derive the volume of immunoglobulin preparations required to neutralize the biological activity of a fixed amount of cytokine. The ED50 is thus a direct reflection of the volume of the preparation required to neutralize a constant amount of cytokine in the assay system described, with a small volume reflecting a high neutralizing capacity.
Binding assays for the detection of cytokine antibodies
Solid‐phase binding assays (enzyme‐linked immuosorbent assays; ELISAs) were used to detect anti‐cytokine antibodies in immunoglobulin preparations as described. 26
Immunoblotting of cytokine antibodies
Sodium dodecyl sulphate–polyacrylamide electrophoresis (SDS–PAGE) under non‐reducing conditions was carried out using 12·5% total acrylamide gels (approximately 2 µg of GM‐CSF/IL‐1α/IFN‐α was loaded per track). Immunoblots were incubated with fractions obtained from affinity purification of immunoglobulin G (IgG) preparations [approximately 5 µg/ml in phosphate‐buffered saline (PBS)/milk] or specific polyclonal antibodies to human GM‐CSF, IL‐1α, or IFN‐α (approximately 1/2000 dilution in PBS/milk) as described. 26,27
Preparation of F(ab′)2 fragments from human i.v. immunoglobulin
F(ab′)2 fragments from human i.v. immunoglobulin were prepared by limited proteolysis with pepsin and isolated using a Protein A affinity column. 27 The identity of the separated F(ab′)2 was confirmed using SDS–PAGE.
Purification of antibodies from immunoglobulin preparations
Affinity columns were prepared as follows: GM‐CSF (5 mg) and IL‐10 (5 mg) were coupled separately to cyanogen‐bromide (CNBr) ‐activated Sepharose 4B (0·5 g; Pharmacia); IL‐1α (0·5 mg) and IFN‐α2a (0·7 mg) were coupled separately to Reacti‐Gel 6X matrix (1 ml; Pierce & Warriner, Chester, UK) following the manufacturer’s instructions.
For each column, 3 ml of a batch of immunoglobulin preparation was loaded for 2 hr at room temperature and the unbound fraction (void volume) was collected. After extensive washing with PBS, pH 7·4, bound antibodies were eluted with 0·1 m glycine, pH 2·7. 27 The GM‐CSF column bound fraction was neutralized with 0·1 m Tris, pH 8·8. In the case of IL‐1α and IFN‐α2a columns, the specific fraction from the column was eluted with 0·1 m glycine, pH 2·7, maintained at pH 2·7 (to prevent formation of immune complexes between the leached ligand and the eluted specific antibody component which were causing interference in the highly sensitive bioassays) and subjected immediately to size exclusion high‐performance liquid chromatography (HPLC) at pH 2·7 for separation of the immunoglobulin peak from the leached cytokine. The HPLC column used was TosoHaas TSK G3000SWXL (7·8 × 300 mm) eluted at 1 ml/min. The sample was then applied at 100 µl per injection and 0·5 ml fractions were collected, which were immediately neutralized by the addition of 1 m Tris–HCl pH 8·8. Fractions containing immunoglobulin were pooled and concentrated.
Both the void volume and the eluted fractions isolated from immunoglobulin preparations using different columns were stored at 4° overnight and subsequently analysed in appropriate assays. The IL‐10 affinity column was used as a negative control for the GM‐CSF column.
Results
Cytokine‐binding activity in human immunoglobulin preparations
Several batches of immunoglobulin products from different manufacturers were tested for the presence of cytokine antibodies using binding assays which had previously been validated for use with human sera/plasma samples. 18,19, 26 Preliminary experiments showed the presence of binding antibodies to GM‐CSF, IFN‐α2a and IL‐10 in immunoglobulin preparations and confirmed the results of other studies. 8,20, 28 However, since all tested batches of all immunoglobulin products appeared to be positive for GM‐CSF, IFN‐α2a and IL‐10, we conducted further assays using immobilized IL‐2, IL‐3, IL‐10, IFN‐α2a, SCF and GM‐CSF. When using unfractionated immunoglobulin preparations, a general indiscriminate recognition of all six cytokines was found which suggested that at least some of the apparent binding to cytokines was non‐specific and spurious. Therefore, ELISAs were considered inappropriate for detection of antibodies specific for cytokines in immunoglobulin preparations. Further studies were conducted using biological assays which have the ability to distinguish between neutralizing and non‐neutralizing antibodies against cytokines and, from the clinical perspective, can produce results that are meaningful and functionally relevant. In addition, other immunochemical procedures were used to identify and purify genuine antibodies (neutralizing and non‐neutralizing) against cytokines in immunoglobulin batches.
Cytokine‐neutralizing activity in human immunoglobulin preparations
The same i.v. immunoglobulin preparations that had been evaluated in ELISAs were tested in cytokine‐specific bioassays for the presence of neutralizing activity against a range of cytokines. We found no evidence of any inhibitory effects on the biological activity of G‐CSF, M‐CSF, SCF, IL‐2, IL‐3, IL‐4, IL‐6, IL‐9, IL‐10, IL‐12, OSM and TNF‐α. Weak neutralization of IL‐5, IL‐11, LIF and TPO, evident only at very high concentrations of immunoglobulin preparations (ED50 >2 mg/ml), were considered to be artefactual. However, some, but not all, preparations, exhibited significant reproducible neutralization of the anti‐viral effect of IFN‐α2a but not IFN‐γ. Some preparations also weakly neutralized the anti‐viral effect of IFN‐β. A majority of preparations showed reproducible inhibition of biological responses induced by GM‐CSF and/or IL‐1α (Table 1). We therefore extended our investigation by evaluating batches of ‘specific’ i.v. immunoglobulin and i.m. immunoglobulin preparations, e.g. anti‐D, anti‐hepatitis B virus (HBV), anti‐rabies virus, anti‐varicella zoster and anti‐tetanus for inhibitory activity in bioassays for GM‐CSF, IL‐1α, IFN‐α2a and other cytokines (Table 1). Variable levels of anti‐GM‐CSF, anti‐IL‐1α and/or anti‐IFN‐α2a activities were detectable in several, but not all, batches of such specific preparations (Table 1) similar to their occurrence in normal i.v. immunoglobulin preparations. As with i.v. immunoglobulin preparations, a very slight neutralization of IL‐5, IL‐11, LIF and TPO was also observed, but there was no inhibition of G‐CSF, M‐CSF, SCF, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐6, IL‐9, IL‐10, IL‐12, OSM, IFN‐γ and TNF‐α activities (data not shown).
Table 1.
Relative levels of cytokine neutralizing activity in human immunoglobulin products expressed as 104 × ED50 in the specified assay system. In a number of cases, two or more assays were carried out and the reported value is the geometric mean of all values obtained
| Manufacturer code | Batch no. | IFN‐α2a | GM‐CSF | IL‐1α |
|---|---|---|---|---|
| (a) Normal i.v. immunoglobulin products | ||||
| A | 1 | 776 | 1494 | 306 |
| 2 | 407 | 44 | ND | |
| 3 | – | 124 | 360 | |
| B | 1 | 249 | 93 | 400 |
| 2 | – | 96 | 352 | |
| 3 | 570 | 110 | 647 | |
| 4 | – | 149 | 642 | |
| C | 1 | 2259 | 121 | 307 |
| 2 | 1612 | 208 | 300 | |
| 3 | 265 | 223 | 431 | |
| 4 | 1830 | 126 | 516 | |
| D | 1 | 1873 | 646 | 645 |
| 2 | 2751 | 139 | 478 | |
| E | 1 | 437 | 51 | 569 |
| 2 | 1159 | 17 | 492 | |
| 3 | 517 | 17 | 1364 | |
| 4 | 396 | 99 | 999 | |
| F | 1 | 949 | 215 | 460 |
| 2 | 898 | 172 | 742 | |
| 3 | 4549 | 66 | 1088 | |
| 4 | 958 | 76 | 494 | |
| (b) Specific immunoglobulin products* | ||||
| F (anti‐D) | 1 | 37 | 59 | 550 |
| 2 | 368 | 15 | 262 | |
| 3 | 716 | 47 | 547 | |
| 4 | – | 559 | 510 | |
| 5 | 858 | 45 | 511 | |
| 6 | 2498 | 149 | 306 | |
| 7 | 262 | 23 | 247 | |
| C (anti‐D) | 1 | – | 68 | 254 |
| 2 | 833 | 240 | 233 | |
| 3 | 713 | 158 | 192 | |
| 4 | 861 | 285 | 256 | |
| 5 | ND | ND | 184 | |
| 6 | ND | ND | 234 | |
| 7 | ND | ND | 197 | |
| F (hepatitis B) | 1 | 1360 | 31 | 1663 |
| 2 | 1395 | 5 | ND | |
| 3 | – | 53 | 482 | |
| 4 | ND | ND | 1086 | |
| C (hepatitis B) | 1 | 5490 | 378 | 1558 |
| 2 | 4941 | 250 | 286 | |
| F (rabies) | 1 | 1546 | 690 | – |
| 2 | – | 1026 | ND | |
| 3 | – | – | 17 | |
| 4 | ND | ND | 516 | |
| C (rabies) | 1 | – | 1419 | 106 |
| F (zoster) | 1 | 90 | 774 | 375 |
| 2 | 7 | 7 | 641 | |
| 3 | 16 | 14 | 971 | |
| 4 | 2024 | 1 | 66 | |
| 5 | 48 | 8 | 998 | |
| 6 | ND | ND | 625 | |
| C (zoster) | 1 | – | 12 | 237 |
| 2 | – | 14 | 746 | |
| 3 | – | 16 | 726 | |
| F (tetanus) | 1 | – | 27 | ND |
| 2 | – | 864 | 288 | |
| 3 | ND | ND | 518 | |
| C (tetanus) | 1 | 7524 | 1743 | 149 |
| V (tetanus) | 1 | 111 | 156 | ND |
Preparations given in parentheses; ND, not done; –, no neutralizing antibodies.
F(ab′)2 fragments prepared from a number of batches of immunoglobulin preparations that neutralized the biological activity of GM‐CSF, IFN‐α2a and IL‐1α were found to retain cytokine neutralization activity showing that inhibition was not mediated by Fc interaction with cytokines or cells.
Neutralization of the biological activity of GM‐CSF by human immunoglobulin preparations
Of the 21 batches of i.v. immunoglobulin preparations tested, 20 batches showed neutralization of GM‐CSF in the TF‐1 cell‐based assay (Table 1). A dose‐dependent neutralization of the biological effect of GM‐CSF by immunoglobulin preparation(s) is illustrated in Fig. 1. The majority of specific preparations tested (90%) also neutralized GM‐CSF‐induced proliferation of the TF‐1 cell‐line (Table 1). Such neutralization was batch‐related and was also observed with another human leukaemic cell‐line, MO7e, which also proliferates in response to GM‐CSF. This neutralization of GM‐CSF biological activity was not due to any toxic activity of the particular immunoglobulin preparations tested, since proliferation of TF‐1 cells stimulated by other cytokines was unaffected (data not shown).
Figure 1.
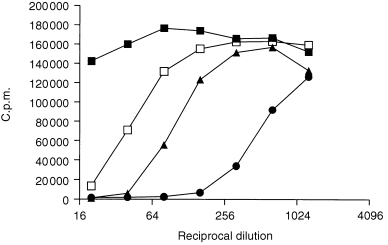
Neutralization of the biological effect of GM‐CSF by different i.v. immunoglobulin preparations. For this, the TF‐1 cell‐line was stimulated with approximately 1·0 IU/ml of GM‐CSF in the presence or absence of various immunoglobulin products. Symbols for individual products are: A, ▪ B, ▴ C, □ and E, •.
In further experiments, we found that batches of immunoglobulin preparations which inhibited Escherichia coli‐derived rDNA human GM‐CSF were also capable of inhibiting human GM‐CSF protein produced from Chinese hamster ovary (CHO) cells, yeast (this differs from the native protein in the substitution of a leucine residue for arginine at position 23 of the amino acid sequence) and natural protein prepared from PHA‐stimulated human peripheral blood mononuclear cells (Fig. 2).
Figure 2.
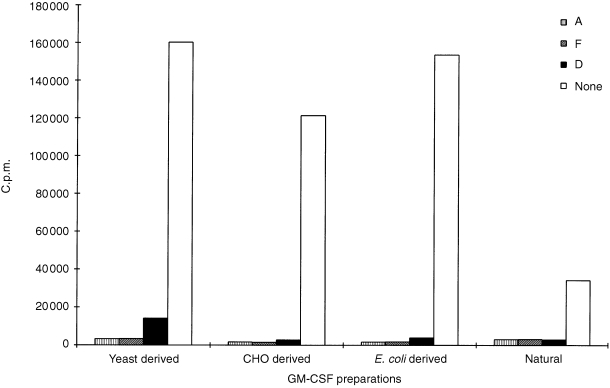
Neutralization of the biological effect of different GM‐CSF preparations by immunoglobulin products. Recombinant DNA‐derived GM‐CSF produced by CHO cells, yeast, E. coli, or conditioned medium from PHA‐stimulated human peripheral blood mononuclear cells (containing natural GM‐CSF) was used. For this, the TF‐1 cell‐line was stimulated with approximately 1·0 IU/ml of GM‐CSF (exception was conditioned medium) and cultured alone (none) or in the presence of products (A, F and D at 1/20 dilution).
Neutralization of IL‐1α bioactivity by human immunoglobulin preparations
Seventeen of the 20 batches of human i.v. immunoglobulin preparations tested showed moderate neutralization of the biological effect of IL‐1α on NOB‐1 cells. Of the 36 specific preparations evaluated, most (89%) showed neutralization of IL‐1α responses. Several anti‐D preparations from one manufacturer contained potent neutralizing activity against IL‐1α. Such neutralization was specific and not due to toxic effects of immunoglobulin products on the NOB‐1 cell‐line since no neutralization was evident with IL‐1β (which has the same biological activity on NOB‐1 cells as IL‐1α).
Neutralization of the anti‐viral effect of IFN by human immunoglobulin preparations
Of the 34 batches of human i.v. immunoglobulin preparations tested, 25 batches showed significant and reproducible inhibition of the anti‐viral effect of recombinant human (rh) IFN‐α2a. Of the 55 specific preparations evaluated, 22 showed significant neutralizing activity against IFN‐α2a (Table 1). Interestingly, anti‐varicella zoster and anti‐D preparations from one manufacturer appeared more likely to contain neutralizing activity against IFN‐α2a than other specific products. The 2D9 cell‐line used for anti‐viral assays is sensitive to all IFN types and therefore we investigated the specificity of inhibitory activity contained in different immunoglobulin preparations for different IFN types and subtypes. Immunoglobulin preparations that neutralized IFN‐α2a were found to neutralize other IFN‐α subtypes, including a mixture of heterogeneous IFN‐α (of leucocyte and lymphoblastoid cell origin) and IFN‐ω. IFN‐β was less effectively neutralized by i.v. immunoglobulin preparations. Only one batch of specific preparation was found to contain anti‐IFN‐β activity. However, IFN‐γ was not detectably neutralized by any of the immunoglobulin preparations tested (data not shown).
Affinity purification and characterization of specific GM‐CSF, IL‐1α and IFN‐α antibodies in human immunoglobulin preparations
To isolate GM‐CSF neutralizing antibodies in human immunoglobulin preparations, we affinity purified three immunoglobulin preparations (previously shown to neutralize GM‐CSF bioactivity) using human GM‐CSF coupled to CNBr‐activated Sepharose and assessed the eluted fractions for effects on the biological activity of GM‐CSF. From these products, we were able to isolate a specific antibody fraction which bound to the GM‐CSF column and was eluted at pH 2·7. This antibody component contained neutralizing activity for GM‐CSF comparable to the original immunoglobulin preparation. The void volume (eluted with PBS) from the GM‐CSF column did not contain antibodies capable of neutralizing GM‐CSF. However, neutralizing GM‐CSF antibodies were present in the void volume eluted from an affinity column which was prepared by coupling human IL‐10 to CNBr‐activated Sepharose (instead of GM‐CSF and used as a control column, Fig. 3a). Using this IL‐10 column, a specific antibody fraction could not be isolated from the whole immunoglobulin preparations (the pH 2·7 fraction contained no detectable IgG) even though the immunoglobulin preparations were found to be capable of binding immobilized IL‐10 in ELISAs (but incapable of neutralizing IL‐10 bioactivity, Fig. 3b). Similarly, a specific antibody fraction could not be isolated following purification of immunoglobulin preparations using an IL‐3‐coupled affinity column. These results clearly contrasted with results obtained from purification of immunoglobulin preparations using the GM‐CSF affinity column. With immunoglobulin preparations shown to be non‐neutralizing for GM‐CSF, a specific GM‐CSF antibody component (pH 2·7 eluted fraction) which did not impair the biological activity of GM‐CSF but which contained only binding antibodies (non‐neutralizing) to GM‐CSF was recovered. These results demonstrated that some immunoglobulin preparations contain antibodies with specificity for GM‐CSF capable, in some instances (but not all), of neutralizing GM‐CSF‐induced biological activity.
Figure 3.
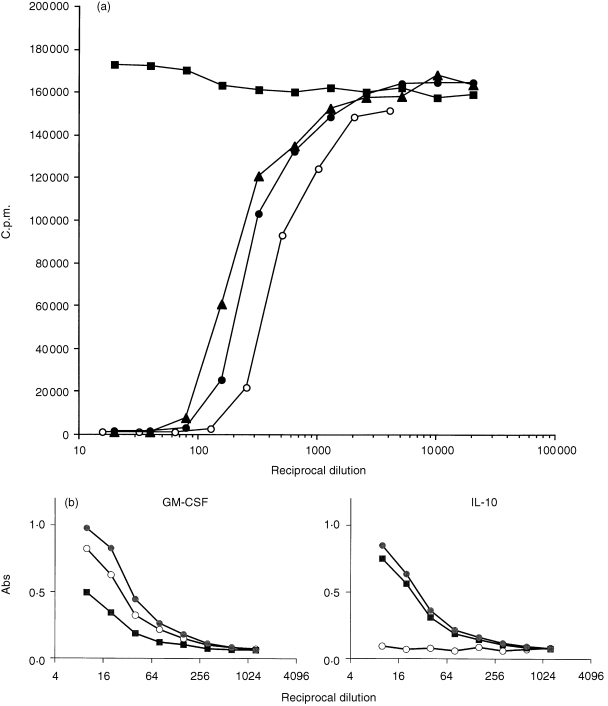
(a) Effect of an immunoglobulin preparation and specific antibody fraction isolated from the same immunoglobulin preparation using a GM‐CSF affinity column on the biological activity of GM‐CSF. The immunoglobulin preparation is denoted by • and the specific antibody fraction is denoted by ○. Results obtained with the void volumes (eluted with PBS) from the GM‐CSF column denoted by ▪ and the IL‐10 column (which was used as a control column for simultaneous purification of the immunoglobulin preparation) denoted by ▴ are also shown.(b) Data from an ELISA using GM‐CSF or IL‐10 preparation as antigen. Immunoglobulin preparation is denoted by •, a specific antibody fraction isolated from the same immunoglobulin preparation using a GM‐CSF affinity column is denoted by ○ and the void volume (eluted with PBS) from the GM‐CSF column is denoted by ▪.
Immunoblotting experiments showed that the specific GM‐CSF antibody fractions from immunoglobulin preparations (both neutralizing and non‐neutralizing) strongly recognized human GM‐CSF protein which migrated as three close bands at approximately 15 000 MW. These bands were also detected using a specific polyclonal sheep anti‐GM‐CSF serum. There was no recognition of human IL‐10 with the specific human GM‐CSF antibody fraction (Fig. 4a). These results confirm that some immunoglobulin preparations contain antibodies specific for GM‐CSF. In binding assays, we found that the specific antibody fractions purified using the GM‐CSF column recognized only GM‐CSF but not other cytokines such as IL‐10, IFN‐α2a. This contrasted with the indiscriminate recognition detectable using ELISAs with unfractionated immunoglobulin preparations or the fraction containing the void volume component (Fig. 3b).
Figure 4.
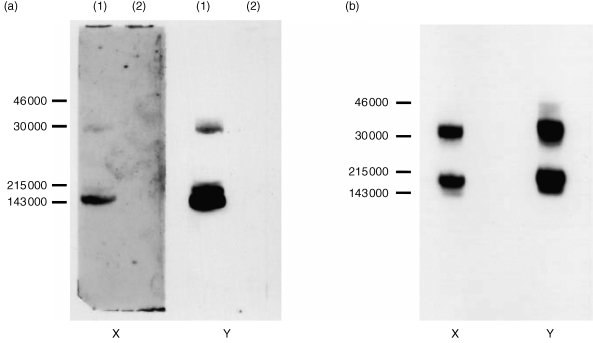
(a) Immunoblot of GM‐CSF (lane 1) and IL‐10 (lane 2) preparations using specific antibody fraction eluted from the immunoglobulin preparation using a GM‐CSF affinity column (X) and a specific sheep anti‐GM‐CSF serum (Y).(b) Immunoblot of an IL‐1α preparation using specific antibody fraction isolated from the immunoglobulin preparation using an IL‐1α affinity column (X) and a specific sheep anti‐IL‐1α serum (Y).
Antibodies specific for IL‐1α or IFN‐α2a were also affinity‐isolated from human immunoglobulin preparations which neutralized the biological activity of these cytokines. Initial experiments showed that the eluted fractions from columns of human IL‐1α or IFN‐α2a coupled to Reactigel contained small amounts of ligand (leached from the matrix) which interfered in bioassays for these cytokines. To separate the ligand from the specific antibody component, we therefore subjected the glycine‐eluted fractions to an additional purification step using size‐exclusion HPLC (carried out at pH 2·7). Using this approach, we were able to isolate specific IL‐1α or IFN‐α antibody components containing neutralizing activity against IL‐1α or IFN‐α2a consistent with the neutralizing activity profile of immunoglobulin preparations used as starting material. Using immunoblotting, we found that the specific IL‐1α antibody fractions from immunoglobulin preparations and the sheep anti‐IL‐1α serum strongly recognized the human IL‐1α protein, which migrated at approximately 18 000 MW (Fig. 4b). A confirmation of IFN specificity by immunoblot was also obtained with the IFN‐α2a‐specific antibody fraction.
Presence of cytokine‐neutralizing antibodies in plasma pools used for preparation of immunoglobulin products
Immunoglobulin products are fractionated from one or more plasma pools and so we evaluated plasma pools used to prepare immunoglobulin products for the presence of neutralizing activity against GM‐CSF, IL‐1α and IFN‐α2a. Results showed that at least one of the plasma pools contributing to immunoglobulin products that neutralized GM‐CSF, IL‐1α and/or IFN‐α2a activity also exhibited neutralization of these cytokines. The effect of a neutralizing i.m. immunoglobulin product and the corresponding plasma pools on the biological activity of GM‐CSF is shown in Fig. 5. However, plasma pools used to prepare immunoglobulin products devoid of neutralizing activity were found to be negative for neutralization against these cytokines.
Figure 5.
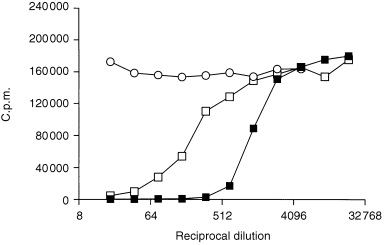
Effect of an immunoglobulin product, F (anti‐varicella zoster) and the plasma pools (from which the product was prepared) on the biological activity of GM‐CSF. Immunoglobulin product is denoted by ▪ and the plasma pools are denoted by □ and ○.
Discussion
In this study, we used specific bioassays to identify and measure neutralizing antibodies to particular cytokines in batches of human immunoglobulin preparations. We found that some batches of human immunoglobulin preparations contain specific neutralizing antibodies against some, but not all, cytokines. In particular, the biological activities of GM‐CSF, IL‐1α and IFN‐α2a, were neutralized by some, but not all, of the batches tested. The levels of neutralizing activity found among the neutralizing immunoglobulin batches to each of these three cytokines varied considerably. In some cases, all three cytokines were neutralized to some degree by a single batch while in others only one or two cytokines were neutralized. For these three cytokines, a specific antibody fraction which bound specific cytokine on immunoblots and in binding assays and neutralized biological activity in appropriate bioassays could be affinity purified from whole immunoglobulin preparations. We did not detect significant neutralizing activity against IFN‐γ, TNF‐α, IL‐2, IL‐3, IL‐4, IL‐5, IL‐6, IL‐9, IL‐10, IL‐11, IL‐12, G‐CSF, M‐CSF, SCF, OSM, LIF and TPO in any batch of the preparations tested. Specific antibodies for IL‐3 and IL‐10 could not be isolated by affinity chromatography despite detecting apparent binding of immunoglobulin preparations to these cytokines using ELISAs. We consider the results of ELISAs (when not supported by data generated using other techniques) to represent artefactual non‐specific binding which cannot be meaningfully interpreted as indicating the presence of genuine antibodies which recognize these cytokines.
A significant number of immunoglobulin preparations contained neutralizing GM‐CSF antibodies. In particular, i.m. immunoglobulin preparations enriched in anti‐varicella zoster (88% of total) produced by different manufacturers showed marked neutralization of GM‐CSF‐induced proliferation. Several anti‐D (90% of total) and anti‐hepatitis B (100%) products also contained antibodies with low to moderate levels of GM‐CSF neutralizing activity. These antibodies had characteristics similar to those of neutralizing anti‐GM‐CSF that spontaneously occurred in myasthenia gravis patients, or those that were induced in immunocompetent colorectal carcinoma patients following therapy with GM‐CSF. 19,24, 26 Moreover, these antibodies neutralized the biological activity of natural GM‐CSF and rDNA‐derived GM‐CSF produced by yeast, CHO cells and E. coli expression systems showing that the activity was not influenced by glycosylation or source of the cytokine. 19,24
Out of the immunoglobulin preparations tested in the IL‐1 bioassay which responds to IL‐1α and IL‐1β, 29 several contained neutralizing antibodies against IL‐1α but not IL‐1β. Some i.m. immunoglobulin preparations, e.g. anti‐D, contained highly potent neutralizing IL‐1α antibodies. However, no apparent association between levels of IL‐1α neutralizing activity and the specificity of i.m. immunoglobulin was evident.
In the case of IFN‐α, the level of neutralizing activity found among the i.v. immunoglobulin batches studied varied considerably with many having no detectable activity. For i.v. immunoglobulin products which neutralized IFN‐α2a, other IFN‐α subtypes such as α1, α8, the IFN‐α‐related consensus IFN, and heterogeneous mixtures of IFN‐α subtypes, such as leucocyte and lymphoblastoid IFN, were also neutralized. This neutralization profile is consistent with the highly conserved amino acid sequences of IFN‐α subtypes and the expected small antigenic differences that exist among them. 30 However, IFN‐ω, a component of leucocyte IFN which is approximately 60% homologous to IFN‐α subtypes and thus more antigenically distinct, 30 was also effectively neutralized by all of the batches of i.v. immunoglobulin which neutralized IFN‐α2a. This suggests that either common epitopes between IFN‐α and IFN‐ω were recognized by the neutralizing antibodies, or that separate sets of neutralizing antibodies specific for each type of IFN coexist in i.v. immunoglobulin batches. Similar considerations can be applied to IFN‐β, which is approximately 30% and 60% related to IFN‐α and IFN‐ω, respectively. 30 In comparison to i.v. immunoglobulin preparations, the neutralization profiles found for different i.m. immunoglobulin batches appeared to be more variable. Only one batch which strongly neutralized IFN‐β as well as IFN‐α subtypes and IFN‐ω was found. In most cases, IFN‐α subtypes together with IFN‐ω, but not IFN‐β, were neutralized to a similar extent. A positive correlation among i.m. immunoglobulin batches and their ability to neutralize the anti‐viral activity of IFN‐α2a and consensus IFN (r = 0·97) and IFN‐α2 and IFN‐ω (r = 0·60) could be demonstrated. However, one batch was identified which neutralized IFN‐α subtypes but not IFN‐ω, strongly suggesting the presence of antibodies which recognize different epitopes on the IFN molecules. Neutralizing activity against IFN‐γ, which is completely unrelated to IFN‐α, was not detected in any batch of the immunoglobulin preparations tested. This finding concurs with one study, 28 but contradicts other reports 22,31 which have claimed the presence of anti‐IFN‐γ activity in immunoglobulin preparations. Inhibition of IFN‐γ activity by normal sera/plasmas in bioassays other than anti‐viral assays has also been previously reported. 32 To our knowledge, neutralization of IFN‐γ anti‐viral activity has not been demonstrated, and in our opinion, the specificity of the alternative bioassays used by other groups is insufficient to rule out non‐specific inhibitory effects of immunoglobulin preparations.
We found no association between antigen specificity of the immunoglobulin preparations and neutralizing IFN‐α2a antibodies. High levels of these antibodies occurred in 77% of the total anti‐D products and 80% of the total anti‐varicella zoster i.m. immunoglobulin preparations tested, although the latter was restricted to products from one manufacturer only. Very few batches (< 20%) of immunoglobulin products of other antigen specificities contained appreciable levels of anti‐IFN‐α‐neutralizing activity.
The reason for the development of antibodies against a particular array of cytokines in individuals is unclear. There is evidence that neutralizing antibodies against IL‐1α can occur as the result of pathology, since antibodies with such neutralizing characteristics have been found in non‐destructive chronic polyarthritis. 5 The mechanism for induction of antibodies against this predominantly cytosolic protein (and not detected in the circulation or in body fluids except during severe disease) has not been established. Similarly, the origin and/or mechanism of development of antibodies against GM‐CSF and/or IFN‐α, which are secreted by several cell types during immune and/or inflammatory responses, remains enigmatic. To date, neutralizing autoantibodies against GM‐CSF and/or IFN‐α have only rarely been found in patients with autoimmune disease. 18,19 However, it remains difficult to pinpoint those individuals who have circulating neutralizing autoantibodies against GM‐CSF, IL‐1α and/or IFN‐α as a significant proportion of immunoglobulin products fractionated from the plasma of healthy donors contained neutralizing antibodies to these cytokines. Therefore, it may be that individuals with underlying infections or subclinical manifestations or those who are undergoing immune or inflammatory responses who present as normal healthy donors may have circulating neutralizing antibodies against GM‐CSF, IL‐1α and/or IFN‐α and consequently contribute significant amounts of neutralizing activity against these cytokines to plasma pools. In the case of specific products, it is possible that cytokine antibodies occur in the plasma of individuals following immunization with a specific antigen, e.g. Rh D + erythrocytes, or following exacerbated immune responses to infectious viruses, e.g. varicella zoster. However, as yet there are no reports demonstrating the occurrence of GM‐CSF or IL‐1α antibodies in such circumstances and only one report showing high levels of anti‐IFN‐α activity in a patient with disseminated varicella zoster infection, 14 which may support this hypothesis. The sporadic occurrence of neutralizing antibodies in any individual(s) with high antibody levels to varicella zoster who are the likely contributors to the plasma pool may therefore lead to the presence of antibodies in particular preparations. This was confirmed in further studies in which plasma pools used for fractionation of the final immunoglobulin product were tested for the presence of GM‐CSF and/or IFN‐α neutralizing activity. At least one of the plasma pools used for production of neutralizing i.m. immunoglobulin (or i.v. immunoglobulin) preparations was always found to contain neutralizing antibodies for GM‐CSF, IL‐1α and/or IFN‐α. In contrast, plasma pools used to produce non‐neutralizing products were devoid of any neutralizing activity. These results tend to suggest that the neutralizing activity of the immunoglobulin products is a sum of the antibody activities contributed by plasma of only a few donors with highly potent antibodies. Therefore, it appears that the donor population, the relative size of plasma pools and possibly even the type of immunoglobulin preparation (e.g. antigen specificity) are factors which determine and contribute to the variable degree of neutralization of GM‐CSF, IL‐1α, or IFN‐α bioactivity seen with different immunoglobulin preparations.
Affinity chromatography experiments using immobilized cytokines showed that the binding and neutralizing capacity of immunoglobulin preparations was due to a small amount of cytokine‐specific IgG present in those batches with anti‐cytokine activity. Specific IgG fractions for GM‐CSF, IL‐1α and IFNα/ω could be isolated from immunoglobulin preparations showing binding/neutralization for the relevant cytokine. Isolation of GM‐CSF antibody from immunoglobulin preparations was straightforward and posed no problems. Purification of IFN‐α and IL‐1α antibodies was technically more problematical and it was necessary to maintain conditions that were appropriate for preventing immune complex formation (between ligand that was leaching off the affinity column and antibody) and enabling availability of adequate amounts of free antibody. For other cytokines (e.g. IL‐10, IL‐3), a specific antibody fraction could not be isolated. This suggests that some batches of immunoglobulin products contain specific IgG for some cytokines with distinct binding/neutralizing characteristics. In contrast, the spurious binding seen with all immunoglobulin products in ELISAs is probably an artefact, i.e. not mediated by specific IgG–cytokine interaction.
Further characterization clearly showed the specificity of the purified antibody components for their respective cytokine only and not other cytokines. In immunoblots, these antibodies showed strong sole recognition of the specific cytokine from which they were affinity isolated but not other cytokines; the recognition profile was comparable to specific polyclonal antisera raised to these cytokines. The specificity of the purified IgG components for particular cytokines was also demonstrated using ELISAs. Further confirmation of the specificity was obtained from data derived from functionally relevant bioassays. Only the purified antibody component (not the fractions from the void volume) specifically inhibited the biological effects of either GM‐CSF, IFN‐α, or IL‐1α in their respective bioassays. In addition, there was no evidence of any cross‐reactivity with other cytokines by any of the GM‐CSF‐ or IL‐1α‐specific antibody fractions in appropriate cell‐based assays. The IFN‐α2a specific antibody also only neutralized structurally related IFN‐α subtypes and IFN‐ω similar to the neutralizing profile of the unfractionated immunoglobulin preparations.
Reports for the existence of antibodies against cytokines are conflicting. For example, the presence of autoantibodies in sera against IL‐2, IL‐8, IL‐10 and TNF‐α has been shown in some studies but not in others. 3,8,20,21,33,34 Similarly, the presence of antibodies against IL‐10 in immunoglobulin preparations has been noted in one study but not in another. 8,20 Such discrepancies between reported results may be related either to the different, and often poorly comparable, assay methods used for antibody detection, differences in sensitivity and specificity of assays, or inconclusive data derived from inappropriately validated assay systems. 1 ELISAs are commonly used for identification of antibodies because of their relative ease and simplicity. Such assays, however, may provide erroneous results when screening ‘sticky’ samples (e.g. immunoglobulin preparations) that can cause considerable interference in assays. Careful consideration should be given to the methods that have been employed for antibody detection when correctly interpreting results from studies relating to detection of cytokine antibodies. Selection of appropriate and valid methods is critical for reliable detection and measurement of antibodies. 1,26, 35 Inclusion of additional immunochemical procedures (based on different scientific principles) is essential to confirm the presence of ‘real’ antibodies to cytokines. Additionally, the use of functional assays which can identify neutralizing antibodies are necessary if an assessment of the potential for compromising the biological activity of the cytokine is to be obtained.
Acknowledgments
We are grateful to Maryvonne Brasher for her assistance in procuring the immunoglobulin products and Jenni Haynes for processing the manuscript.
References
- 1.Ross C, Hansen MB, Schyberg T, Berg K. Autoantibodies to crude human leucocyte interferon (IFN), native human IFN, recombinant human IFN‐alpha 2b and human IFN‐gamma in healthy blood donors. Clin Exp Immunol. 1990;82:57. doi: 10.1111/j.1365-2249.1990.tb05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svenson M, Hansen MB, Bendtzen K. Distribution and characterization of autoantibodies to interleukin 1 alpha in normal human sera. Scand J Immunol. 1990;32:695. doi: 10.1111/j.1365-3083.1990.tb03212.x. [DOI] [PubMed] [Google Scholar]
- 3.Monti E, Pozzi A, Tiberio L, et al. Purification of interleukin‐2 antibodies from healthy individuals. Immunol Lett. 1993;36:261. doi: 10.1016/0165-2478(93)90098-m. [DOI] [PubMed] [Google Scholar]
- 4.Hansen MB, Svenson M, Diamant M, Bendtzen K. High‐affinity IgG autoantibodies to IL‐6 in sera of normal individuals are competitive inhibitors of IL‐6 in vitro. Cytokine. 1993;5:72. doi: 10.1016/1043-4666(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 5.Jouvenne P, Fossiez F, Garrone P, et al. Increased incidence of neutralizing autoantibodies against interleukin‐1 alpha (IL‐1 alpha) in nondestructive chronic polyarthritis. J Clin Immunol. 1996;16:283. doi: 10.1007/BF01541394. [DOI] [PubMed] [Google Scholar]
- 6.Kurdowska A, Miller EJ, Noble JM, et al. Anti‐IL‐8 autoantibodies in alveolar fluid from patients with the adult respiratory distress syndrome. J Immunol. 1996;157:2699. [PubMed] [Google Scholar]
- 7.Amiral J, Marfaing‐koka A, Wolf M, et al. Presence of autoantibodies to interleukin‐8 or neutrophil‐activating peptide‐2 in patients with heparin‐associated thrombocytopenia. Blood. 1996;88:410. [PubMed] [Google Scholar]
- 8.Menetrier‐caux C, Briere F, Jouvenne P, et al. Identification of human IgG autoantibodies specific for IL‐10. Clin Exp Immunol. 1996;104:173. doi: 10.1046/j.1365-2249.1996.d01-646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panem S, Check IJ, Henriksen D, Vilcek J. Antibodies to alpha‐interferon in a patient with systemic lupus erythematosus. J Immunol. 1982;129:1. [PubMed] [Google Scholar]
- 10.Suit BE, Axelrod D, Moutsopoulos HM, Decker JL, Hooks JJ. Detection of anti‐interferon antibodies in systemic lupus erythematosus. Clin Exp Rheumatol. 1983;1:133. [PubMed] [Google Scholar]
- 11.Prummer O, Frickhofen N, Digel W, Heimpel H, Porzsolt F. Spontaneous interferon‐alpha antibodies in a patient with pure red cell aplasia and recurrent cutaneous carcinomas. Ann Hematol. 1991;62:76. doi: 10.1007/BF01714905. [DOI] [PubMed] [Google Scholar]
- 12.Trown P, Kramer M, Dennin RJ, et al. Antibodies to human leukocyte interferons in cancer patients. Lancet. 1983;I:81. doi: 10.1016/s0140-6736(83)91737-3. [DOI] [PubMed] [Google Scholar]
- 13.Mogensen KE, Daubas PH, Gresser I, Sereni D, Varet B. Patient with circulating antibodies to α‐interferon. Lancet. 1981;ii:1227. doi: 10.1016/s0140-6736(81)91460-4. [DOI] [PubMed] [Google Scholar]
- 14.Pozzetto B, Mogensen KE, Tovey MG, Gresser I&. Characteristics of autoantibodies to human interferon in a patient with varicella‐zoster disease. J Infect Dis. 1984;150:707. doi: 10.1093/infdis/150.5.707. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Toda G, Hashimoto N, et al. Naturally occurring anti‐interferon‐alpha 2a antibodies in patients with acute viral hepatitis. Clin Exp Immunol. 1991;85:80. doi: 10.1111/j.1365-2249.1991.tb05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fall LS, Chams V, Le‐coq H, et al. Evidence for an antiviral effect and interferon neutralizing capacity in human sera; variability and implications for HIV infection. Cell Mol Biol Noisy Grand. 1995;41:409. [PubMed] [Google Scholar]
- 17.Prummer O, Bunjes D, Wiesneth M, et al. Antibodies to interferon‐alpha: a novel type of autoantibody occurring after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;17:617. [PubMed] [Google Scholar]
- 18.Meager A, Willcox N, Vincent A, Newsom‐davis J. Spontaneous neutralizing antibodies to interferon‐alpha and interleukin‐12 in thymoma‐associated autoimmune disease. Lancet. 1997;350:1596. doi: 10.1016/s0140-6736(05)64012-3. [DOI] [PubMed] [Google Scholar]
- 19.Meager A, Wadhwa M, Bird C, et al. Spontaneously occurring neutralizing antibodies against Granulocyte‐Macrophage Colony‐Stimulating Factor (GM‐CSF) in patients with autoimmune disease. Immunology. 1999;97:526. doi: 10.1046/j.1365-2567.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenson M, Hansen MB, Ross C, et al. Antibody to Granulocyte‐Macrophage Colony‐Stimulating Factor is a dominant anti‐cytokine activity in human IgG preparations. Blood. 1998;91:2054. [PubMed] [Google Scholar]
- 21.Bendtzen K, Hansen MB, Ross C, Svenson M. High‐avidity autoantibodies to cytokines. Immunol Today. 1998;19:209. doi: 10.1016/s0167-5699(98)01252-3. [DOI] [PubMed] [Google Scholar]
- 22.Denys C, Toungouz M, Dupont E. Increased in vitro immunsuppressive action of anti‐CMV and anti‐HBs intravenous immunoglobulins due to higher amounts of interferon‐gamma specific neutralizing antibodies. Vox Sang. 1997;72:247. doi: 10.1046/j.1423-0410.1997.7240247.x. [DOI] [PubMed] [Google Scholar]
- 23.Wadhwa M, Thorpe R. Assays for Cytokines. In: Thomson A, editor. The Cytokine Handbook. 3. London: Academic Press; 1998. p. p.855. [Google Scholar]
- 24.Wadhwa M, Bird C, Fagerberg J, et al. Production of neutralizing GM‐CSF antibodies in carcinoma patients following GM‐CSF combination therapy. Clin Exp Immunol. 1996;104:351. doi: 10.1046/j.1365-2249.1996.11704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Däubener W, Wanagat N, Pilz K, et al. A new, simple, bioassay for human IFN‐γ. J Immunol Methods. 1994;168:39. doi: 10.1016/0022-1759(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa M, Hjelm Skog AL, Bird C, et al. Immunogenicity of GM‐CSF products in patients undergoing combination therapy with GM‐CSF. Clin Can Res. 1999;5:1353. [PubMed] [Google Scholar]
- 27.Johnstone AP, Thorpe R. Immunochemistry in Practice. 3. Oxford: Blackwell Scientific; 1997. [Google Scholar]
- 28.Ross C, Svenson M, Hansen MB, Vejlsgaard GL, Bendtzen K. High avidity IFN‐neutralizing antibodies in pharmaceutically prepared human IgG. J Clin Invest. 1995;95:1974. doi: 10.1172/JCI117881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gearing AJH, Bird CR, Bristow A, Poole S, Thorpe R. A simple sensitive bioassay for interleukin‐1 which is unresponsive to 103U/ml of interleukin 2. J Immunol Methods. 1987;99:7. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- 30.Meager A. Interferons alpha, beta and omega. In: Mire‐sluis AR, Thorpe R, editors. Cytokines. London: Academic Press; 1998. p. p.361. [Google Scholar]
- 31.Toungouz M, Denys C, Dupont E. Blockade of proliferation and tumor necrosis factor‐alpha production occurring during mixed lymphocyte reaction by interferon‐gamma‐specific natural antibodies contained in intravenous immunoglobulins. Transplantation. 1996;62:1292. doi: 10.1097/00007890-199611150-00020. [DOI] [PubMed] [Google Scholar]
- 32.Turano A, Balsari A, Viani E, et al. Natural human antibodies to gamma interferon interfere with the immunomodulating activity of the lymphokine. Proc Natl Acad Sci USA. 1992;89:4447. doi: 10.1073/pnas.89.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sioud M, Dybwad A, Jespersen L, et al. Characterization of naturally occurring autoantibodies against tumour necrosis factor‐alpha (TNF‐alpha): in vitro function and precise epitope mapping by phage epitope library. Clin Exp Immunol. 1994;98:520. doi: 10.1111/j.1365-2249.1994.tb05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylvester I, Yoshimura T, Sticherling M, et al. Neutrophil attractant protein‐1‐immunoglobulin G immune complexes and free anti‐NAP‐1 antibody in normal human serum. J Clin Invest. 1992;90:471. doi: 10.1172/JCI115883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Meide PH, Schellekens H. Anti‐cytokine autoantibodies: epiphenomenon or critical modulators of cytokine action. Biotherapy. 1997;10:39. doi: 10.1007/BF02678216. [DOI] [PubMed] [Google Scholar]


