Abstract
CD98 is a widely expressed cell surface heterodimeric glycoprotein, which is rapidly up‐regulated upon activation of T lymphocytes. Monoclonal antibody (mAb) 80A10 recognizes an epitope on CD98 and in combination with CD3 antibody causes proliferation of peripheral blood T lymphocytes. CD98 co‐stimulatory activity, mediated by either mAb 80A10 or 4F2, a well‐characterized CD98‐specific mAb, is blocked in the presence of the soluble β1 integrin antibody 18D3. Previously we have reported that co‐stimulatory activity of antibodies to integrins α4β1, α5β1, αLβ2 and α4β7 is inhibited by 18D3, whereas co‐stimulation mediated by non‐integrins was unaffected. Thus the non‐integrin CD98 is uniquely sensitive to the inhibitory effects of β1 integrin‐blocking antibodies, which may reflect convergent signalling mechanisms between integrins and CD98. This is consistent with recent reports suggesting that CD98 may regulate integrin‐mediated adhesive events.
Introduction
T‐lymphocyte activation requires a minimum of two signals. Signal 1 is delivered by the CD3 complex and is generated as a consequence of interaction of the T‐cell receptor with its major histocompatibility complex (MHC) –peptide ligand. Signal 2 originates from non‐clonotypic interactions between the T cell and antigen‐presenting cell, and may be mediated by one or more of a number of T‐cell membrane antigens, including CD4, CD26, CD27, CD28, CD43, CD44, CD45, CD82 and 4‐1BB. 1 Integrin family members present on the T‐cell membrane are also able to cause co‐stimulation. Thus immobilized vascular cell adhesion molecule‐1 (VCAM‐1; α4β1, α4β7 ligand, ref. 2), fibronectin (α4β1, α5β1 ligand, refs 3–5) and intercellular adhesion molecule‐1 (ICAM‐1; αLβ2 ligand, ref. 6) in addition to co‐stimulation triggered by anti‐integrin antibodies 2–7 are co‐stimulatory with anti‐CD3 antibody for proliferation of CD4+ T lymphocytes.
We have previously shown that T‐cell co‐stimulation mediated by integrins α4β1, α5β1, α4β7 and αLβ2 can be partially prevented by the presence of the β1 integrin‐specific monoclonal antibody (mAb) 18D3, possibly reflecting common signalling pathways (or at least utilization of a common pool of signalling intermediates) of β1 and non‐β1 integrins. 1 The intrinsic activity of integrins, defined by their ability to bind to ligands, is regulated in a cell‐specific manner, although the factors and molecular mechanisms which modify integrin activity are incompletely understood. In the case of the platelet integrin αIIbβ3, the activated form differs conformationally from the inactive form; whether similar changes underpin the activation of β1 integrins is currently unclear. 8 To further understand these events, we have attempted to identify molecules which interact with integrins in lymphocytes, and which may have an involvement in integrin biology. In this report we describe a functional interaction between CD98 and the integrin co‐stimulation pathway. Our observation that CD98 and integrins are functionally associated in T lymphocytes may be relevant during the generation of signal 2 and is consistent with reports from other laboratories working on monocyte aggregation and fusion 9 and with transfected cell lines 10 concerning interactions between CD98 and integrins published during the course of this work.
Materials and methods
Monoclonal antibodies
Monoclonal antibodies to β1 integrin [18D3, immunoglobulin G1 (IgG1)], CD26 (AC7) and CD98 (80A10, IgG1) and control IgG1 (86C10) were generated in this laboratory, and anti‐CD3 mAb OKT3 and anti‐CD98 mAb 4F2 (IgG2a, initially a generous gift from Dr Barton Haynes, Duke University Medical School, Durham, NC) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The mAb T40/25 is an idiotypic antibody that recognizes T‐cell receptors on HPB‐ALL cells. 11 Antibodies were used as purified IgG.
Cultured cell lines
Human T‐lymphoid cell lines HPB‐ALL and HSB were maintained in RPMI‐1640 medium supplemented with 10% fetal calf serum (FCS; complete medium) at 37° in a 5% CO2 incubator.
Cell surface iodination and immunoprecipitation
HPB‐ALL cells (2·5 × 106 per lane) were surface labelled by lactoperoxidase catalysed radioiodination and lysed in 1% Triton X‐100 lysis buffer containing protease inhibitors leupeptin (10 µg/ml, Sigma, St Louis, Mo), pepstatin A (1 µg/ml, Sigma) and aprotinin (10 µg/ml, Sigma). Clarified lysate was precleared with fixed Staphylococcus aureus (Sigma) and then immunoprecipitated with Protein A agarose beads (Boehringer Mannheim, Germany) precomplexed with goat anti‐mouse immunoglobulins (ICN, Costa Mesa, CA) and relevant mAb, by incubation at 4° for 16 hr. Polypeptides were eluted by boiling in reducing or non‐reducing Laemmli sample buffer, and separated by 11·25% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Dried gels were exposed to Kodak XR film at − 80°.
Competitive binding assay
HSB T cells (106 cells/well) suspended in Tris‐buffered saline (TBS) were incubated in 1·5 ml Eppendorf tubes with the indicated concentration of unlabelled purified mAb at room temperature for 30 min before addition of 125I‐labelled mAb. After a further 30 min, cells were rapidly washed three times with TBS and bound radioactivity was determined by gamma‐counting. Assays were performed in triplicate.
Cell surface staining and flow cytometry
Human peripheral T lymphocytes were stimulated with 10 ng/ml phorbol 12‐myristate 13‐acetate (PMA; Sigma), 1 mg/ml ionomycin (Sigma) or PMA and ionomycin together for 0, 4, 9, 15, or 27 hr 2 × 106 cells/sample were collected, washed twice with fluorescence‐activated cell sorter (FACS) buffer [phosphate‐buffered saline (PBS) containing 0·1% FCS, 0·02% sodium azide], and incubated for 1 hr at 37° with 1 µg/ml purified 80A10 mAb. Following two washes in FACS buffer, cells were incubated with 1 : 100 dilution of fluorescein isothiocyanate (FITC)‐conjugated goat anti‐mouse IgG (ICN) for 1 hr at 4°. Following two washes with FACS buffer, fluorescence was analysed on an Epics Profile flow cytometer (Coulter, Miama, FL).
Purification of human T lymphocytes
Human peripheral blood T cells were isolated by negative selection, to avoid inadvertent activation during the purification procedure, from the peripheral blood of healthy laboratory donors, by passing Ficoll–Hypaque‐prepared peripheral blood mononuclear cells through a T‐cell purification column (R & D Systems, Abingdon, UK). Residual contaminating monocytes and natural killer cells were removed using magnetic beads (Dynal, Bebbington, UK) coated with CD14 and CD57 mAb (Pharmingen, Oxford, UK). In some experiments, T cells were prepared from buffy coats, as described previously. 12
T‐lymphocyte co‐stimulation assay
Co‐stimulation experiments using mAb 80A10 were carried out as previously described. 12 Soluble purified mAb 18D3 or IgG at the indicated concentration was added at the onset to appropriate wells. Data in Fig. 6 describe an experiment in which 96‐well plates were coated with OKT3, diluted in PBS, for 2 hr, washed four times, then coated with co‐stimulatory antibody (4F2, mIgG) in PBS for 4 hr, followed by four PBS washes. All coating steps were carried out at room temp. Purified T lymphocytes (4 × 104 per well) were added in the presence or absence of 10 μg/ml 18D3, and 3 days later were pulsed with 0·5 μCi [3H]thymidine for 5 hr before harvesting onto glass fibre filters and detection.
Figure 6.
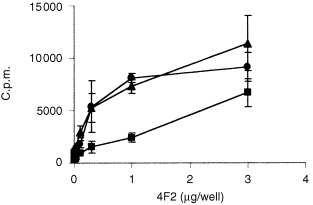
Co‐stimulation mediated by CD98 mAb 4F2 is blocked by soluble β1 integrin mAb 18D3. Purified human T lymphocytes were cultured in 96‐well plates containing immobilized OKT3 in the presence of immobilized 4F2, in the presence of 10 µg/ml soluble 18D3 mAb (▪), 10 μg/ml purified mouse IgG (•), or in the absence of soluble antibody (▴) as described in the Materials and Methods. Incorporation of [3H]thymidine was measured after 3 days. Results are the mean of triplicate wells, and are representative of three experiments.
Results
Generation and initial characterization of antibody 80A10
To further our studies on the α4β1 molecule, we raised a panel of mouse mAbs against the human B‐lymphoblastoid cell line JY. Our initial aim was twofold, first to isolate and to characterize α4β1‐specific mAb which might possess blocking or activating activities towards α4β1, and second to identify mAb which might be useful in identifying antigens which associate with α4β1 integrin, or which might potentially modify its behaviour. The initial screen was designed to select mAb that induced homotypic aggregation of a T‐leukaemic cell line, HPB‐ALL. We have previously used this assay to select mAbs that activate, and in some cases inhibit, the activity of the leucocyte integrins α4β1 and αLβ2. Of 600 wells screened, 20 were positive in this assay (data not shown). We next tested whether the aggregate‐promoting mAb were capable of causing T‐lymphocyte co‐activation. Of the mAb tested, 80A10 was found to induce a strong co‐stimulatory signal (Fig. 1), in contrast to isotype‐matched control mAbs, including 3B11 (phospholipid‐specific), which failed to co‐activate when co‐immobilized with anti‐CD3 (data not shown), and we selected mAb 80A10 for further study. Aggregation promoted by mAb 80A10 is blocked in the presence of the divalent cation chelator ethylenediaminetetraacetic acid (EDTA), a typical feature of integrin‐mediated adhesion. We have previously shown that the presence of anti‐β1 integrin‐specific mAb 18D3 depresses co‐stimulation mediated by antibodies to integrins α4β1, α5β1, α4β7 and αLβ2, but not co‐stimulation mediated by non‐integrins. Because mAb 80A10 causes homotypic aggregation as well as co‐stimulation, and this activity bears the hallmarks of integrin involvement, we tested its sensitivity to inhibition by 18D3. The substantial proliferative response to co‐stimulation with 80A10 was inhibited by 18D3 by 71% (Fig. 1), a reduction comparable to that caused by 18D3 to α4β1 antibody‐driven co‐stimulation. 1 Addition of irrelevant isotype‐matched control mAbs 3B11 (phospholipid‐specific) or IB7 (CD43‐specific) did not reduce mAb 80A10‐mediated co‐stimulation (data not shown), indicating that the inhibitory effect of 18D3 in this system is a property directly dependent on its β1 integrin specificity. Thus the antigen recognized by mAb 80A10 may be an integrin, or a molecule functionally associated with integrins during T‐cell activation.
Figure 1.
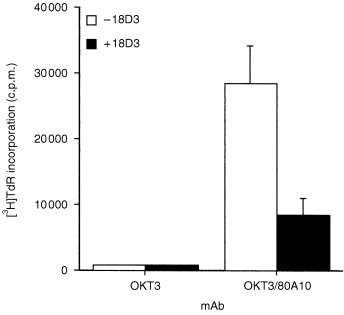
Co‐stimulatory activity of mAb 80A10 is blocked by β1 integrin mAb 18D3. Purified human T lymphocytes were cultured in 96‐well plates containing immobilized OKT3 in the presence and absence of immobilized 80A10, and in the presence or absence of 10 µg/ml soluble 18D3 mAb, as described in the Materials and Methods. Incorporation of [3H]thymidine was measured after 3 days. Results are the mean of triplicate wells, and are representative of three experiments.
Biochemical identification of antigen recognized by mAb 80A10
Immunoprecipitation studies with cell lysates prepared from HPB‐ALL cells showed that mAb 80A10 recognized a broad band of approximately 120 000–200 000 MW under non‐reducing conditions (Fig. 2 lane 2) which resolved into two bands of approximately 80 000 and 40 000 MW under reducing conditions (Fig. 2 lane 4). In lighter exposures, it is apparent that the major density in the heterogeneous band under non‐reducing conditions is at 120 000 MW. From these experiments, we concluded that the 80A10 antigen was not an integrin. In an unrelated separate series of experiments, we had previously reported that Joro 177, a rat mAb raised against a mouse pro‐T‐lymphocyte cell line was able to induce homotypic aggregation of selected mouse haematopoietic cell lines. 13 The characteristics of this aggregation suggested integrin involvement. Isolation of the cDNA clone encoding the antigen recognized by this antibody had identified it as the heavy subunit of the mouse CD98 molecule. 13 The molecular weight of CD98 is 120 000, shifting to 80 000 and 40 000 under reducing conditions. In view of the aggregation‐promoting activity of the CD98 antibody Joro 177, and the similarity in size of CD98 to the 80A10 antigen, we tested whether 80A10 recognizes CD98. Since neither 4F2 (a well‐characterized antibody recognizing human CD98) nor 80A10 are useful reagents for Western blotting (unpublished observations) this question was addressed by immunoprecipitation from surface labelled cells. Sequential immunoprecipitation of labelled cell lysates with 80A10 followed by 4F2, specific for human CD98 (Fig. 3b) or immunoprecipitation of lysates with 4F2 followed by 80A10 (Fig. 3a) indicated that both antibodies recognize the same protein. In contrast, immunoprecipitation with an irrelevant antibody (T40/25, a T‐cell receptor‐specific reagent) followed by either 4F2 or 80A10 yielded bands of 80 000 and 40 000 corresponding to the heavy and light chains of CD98 (Fig. 3c).
Figure 2.
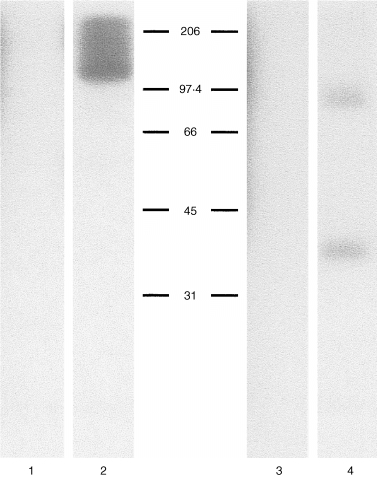
Immunoprecipitation of 80A10 from HPB‐ALL lysates. One per cent Triton X‐100 solubilized 125I‐radiolabelled HPB‐ALL cells (2·5 × 106 cell equivalents) were immunoprecipitated (IP) and analysed by SDS–PAGE on 11·25% polyacrylamide gels under non‐reducing (lanes 1 and 2) or reducing conditions (lanes 3 and 4). Lanes 1 and 3 IP with control isotype‐matched control mAb 86C10, lanes 2 and 4 with mAb 80A10.
Figure 3.

Immunodepletion analysis of mAb 80A10 and mAb 4F2·1% Triton X‐100 solublized 125I‐radiolabelled HPB‐ALL cells (2·5 × 106 cell equivalents) were immunoprecipitated (IP) five times with protein‐A beads conjugated to the indicated antibody for 2 hr intervals. The immunodepleted lysate was split into two samples and IP with either CD98 antibody or control antibody, mAb T40/25 (anti‐T‐cell receptor). Immunoprecipitated polypeptides were analysed by 11·25% SDS–PAGE under reducing conditions. (a) Immunodepletion with mAb 4F2, lanes 1–5; lane 6, blank; lane 7, IP with 80A10; lane 8, IP with T40/25. (b) Immunodepletion with mAb 80A10, lanes 1–5; lane 6, blank; lane 7, IP with mAb 4F2; lane 8, IP with mAb T40/25. (c) Immunodepletion of T40/25, lane 1–5; lane 6, blank; lane 7, IP with 4F2; lane 8 IP with mAb 80A10.
Cross‐blocking of mAb 80A10 with mAb 4F2
Further experiments were undertaken to determine the spatial relation of epitopes recognized by 80A10 and 4F2 antibodies on the CD98 molecule. Binding of antibody 80A10 to HPB‐ALL cells was depressed by either unlabelled 80A10 or by 4F2 but not by the presence of an irrelevant antibody (AC7, specific for CD26, Fig. 4a). Similarly, binding of radiolabelled 4F2 could be inhibited by excess unlabelled 4F2 or 80A10 (Fig. 4b) strongly suggesting that these antibodies recognize identical or spatially related epitopes on CD98 heavy chain.
Figure 4.
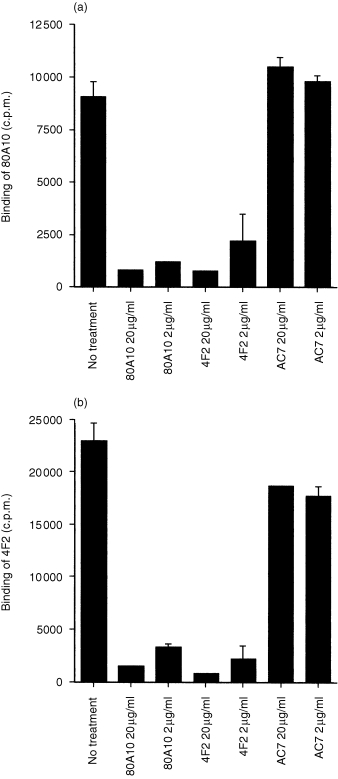
The mAbs 80A10 and 4F2 compete for binding to the same epitope. HSB T cells were incubated with the indicated concentrations of the unlabelled antibodies for 30 min before addition of either 125I‐labelled 80A10 (a) or 125I‐labelled 4F2 (b). Results are mean of triplicate samples.
Binding of 80A10 is increased during T‐cell activation
The expression of CD98 is low on resting T lymphocytes and is up‐regulated in response to activation. We tested whether binding of 80A10 is regulated during T‐cell activation by phorbol ester and calcium ionophore. PMA alone led to a slight increase in 80A10 binding, but stimulation with PMA in combination with ionomycin led to strong binding from 9 hr onwards (Fig. 5), in agreement with an early report that 4F2 (CD98) expression is rapidly induced on human peripheral blood T cells treated with concanavalin A. 14
Figure 5.

Up‐regulation of CD98 expression on mitogen‐activated purified human T lymphocytes. Purified human T lymphocytes were cultured in complete medium in 12‐well plates (2·5 × 106 cells/ml, 2 ml per well) alone or in the presence of PMA (10 ng/ml), ionomycin (1 µg/ml), or in the presence of both, or were untreated (NT). Cells were harvested after 4, 9, 15 and 27 hr, washed and stained with mAb 80A10. Results are expressed as mean fluorescence intensity of flow cytometry histograms. Data are representative of two experiments.
Co‐stimulation of T‐lymphocyte proliferation by CD98 mAb 4F2 and blockade by the β1 integrin antibody 18D3
A striking feature of mAb 80A10 is its sensitivity to inhibition, in the T cell co‐stimulation assay, by β1 integrin mAb 18D3. The prototype mAb to CD98 heavy chain, 4F2, has previously been reported to co‐stimulate T cells 15 and so we tested whether this co‐activation signal was similarly sensitive to 18D3 blockade. A representative experiment (Fig. 6) demonstrates that the presence of soluble mAb 18D3 also diminishes 4F2‐mediated co‐stimulation. Thus the sensitivity of mAb 80A10 co‐stimulation to β1 integrin mAb 18D3 is not a unique property of mAb 80A10, but is a feature shared by the well‐characterized CD98 mAb 4F2. Thus the non‐integrin CD98 co‐stimulatory signal is unique in its sensitivity to blockade by an integrin‐specific mAb, demonstrating functional co‐association between integrins and CD98 in peripheral T lymphocytes, and suggesting convergent signalling pathways between integrins and CD98.
Discussion
We report that the co‐stimulatory monoclonal antibody 80A10 recognizes an epitope on the human CD98 (heavy chain) molecule, an epitope that is spatially related to, or identical to that recognized by the prototypic antibody of the CD98 cluster, 4F2. This places mAb 80A10 in epitope group 4F2‐1 as defined by the Vth International Workshop on Leucocyte Differentiation Antigens. 15 CD98 is a 120 000 disulphide‐linked heterodimer consisting of an 80 000 MW glycosylated subunit which has been molecularly characterized in several laboratories, 13, 16, 17 and a recently cloned 40 000 light chain. 18−20 Sequence analysis has not suggested a function for the heavy chain, whilst the light chain has been demonstrated to function as an amino acid transporter. 18−20 CD98 is expressed on all cultured cell lines, both haematopoietic and non‐haematopoietic, suggesting an important function in maintaining cell viability/proliferation in vitro, of which amino acid transport may be an important component. Amongst leucocytes, expression is restricted to monocytes and lymphocytes, and activation causes a strong and rapid up‐regulation of CD98 expression on T lymphocytes (ref. 14Fig. 5).
It has been appreciated for some time that CD98 antibodies modify the responsiveness of T lymphocytes, conferring either co‐stimulatory or inhibitory effects. 15,21,22 The mechanisms whereby CD98 influences T‐cell activation have until recently received little attention. Our results strongly suggest the involvement of integrins in CD98‐mediated T‐cell activation, and previous work from our laboratory has implicated integrin involvement in CD98‐mediated aggregation of haematopoietic cells. 13 Compelling evidence from work in other systems points to CD98 involvement in regulating adhesive interactions mediated by integrins. Firstly, Ito and colleagues have shown that antibodies to CD98 promote integrin‐mediated virus‐induced cell fusion. 9,23 Secondly, during the course of our work it was reported that transfection of CD98 heavy chain cDNA is sufficient to overcome a dominant negative suppression of integrin activity. 10 Further proof that CD98 controls integrin activity was demonstrated in experiments in which 4F2 antibody promotes β1 integrin‐mediated adhesion of a small cell lung carcinoma cell line to fibronectin and laminin. 10 Thus, CD98 may be a general mechanism for regulating integrin activity.
Our data indicate that uniquely for a non‐integrin, CD98 co‐stimulation is blocked by antibody to β1 integrin. There are two possible explanations for these results. Firstly, CD98 and integrins may co‐stimulate T‐cell activation by essentially separate pathways, except for a common shared intermediate. In this model, the blocking activity of β1 integrin antibody 18D3 is mediated by the sequestration of this common intermediate. The second alternative, which we favour, is that CD98 co‐stimulation occurs because antibody cross‐linking of CD98 molecules leads directly to the activation of the full programme of integrin co‐stimulation, a model supported by evidence from a number of laboratories that CD98 induces integrin activation. 10,13,24
What are the possible mechanisms which could explain CD98 modification of integrin function? CD98 may itself physically associate with integrins, providing a mechanism whereby cross‐linking of CD98 modifies integrin activity as a consequence of their close proximity. Alternatively CD98 cross‐linking may generate activated signalling intermediates which themselves activate integrin signalling pathways. In either case, the blocking activity of antibody 18D3 is effected through the sequestration of essential and perhaps limiting components of the integrin signalling complex, resulting in their incomplete assembly following integrin triggering with either antibody to integrin or antibody to CD98. Work from other laboratories has shown that integrin ligand binding induces association of the β‐chain cytoplasmic tail with cytoskeletal and cytoplasmic proteins which assemble into a focal adhesion plaque. 25 This machinery transduces extracellular adhesive information into altered cytoskeleton architecture and ultimately changes in gene expression. Hypothetically, the action of blocking β1 antibodies such as 18D3 may lead to assembly of partial complexes which act as dominant negatives, mopping up available supplies of one of the 30 or so proteins reported to participate in these plaques. 25−27 Alternatively, CD98 may modify the association of integrins in the plane of the membrane with transmembrane 4 superfamily members CD81, CD82, CD63 and CD53, each of which has been shown to co‐precipitate with α4β1. 28 In this case CD98 antibodies should prove useful reagents in determining whether these proteins have positive or negative regulatory effects on integrin activity.
Currently there is little evidence in the literature on CD98 signal transduction pathways, and few clues which suggest how the integrin‐associated and amino acid‐transporting functions of CD98 may be connected.
In conclusion, we have extended our earlier observation that soluble β1 integrin antibody blocks co‐stimulation mediated by β1 (α4β1, α5β1), β2 (αLβ2) and β7 (α4β7) integrins, but not by non‐integrins CD26, CD28, CD4, CD44, CD45RA, or CD45RO, 1 to include co‐stimulation mediated by non‐integrin CD98, indicating functional association between CD98 and integrins. Future studies will aim to determine the locus of this convergence.
Acknowledgments
We thank Dr Barton Haynes for providing mAb 4F2 for our initial studies. This work was supported by Wellcome Trust Project Grant 047288 (to APW) and National Institutes of Health Grant CA 62596 (to BWM) and Kleberg Foundation (to BWM).
References
- 1.Woodside DG, Teague TK, McIntyre BW. Specific inhibition of T lymphocyte coactivation by triggering integrin β1 reveals convergence of β1, β2 and β7 signaling pathways. J Immunol. 1996;157:700. [PubMed] [Google Scholar]
- 2.Burkly LC, Jakubowski A, Newman BM, Rosa MD, Chi‐ross G, Cobb RR. Signaling by VCAM‐1 through VLA‐4 promotes CD3‐dependent T cell proliferation. Eur J Immunol. 1990;21:2871. doi: 10.1002/eji.1830211132. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu YSG, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA‐4 and VLA‐5 with fibronectin or VLA‐6 with laminin. J Immunol. 1990;145:59. [PubMed] [Google Scholar]
- 4.Davis LS, Oppenheimer‐marks N, Bednarczyk JL, McIntyre BW, Lipsky PE. Fibronectin promotes proliferation of naı¨ve and memory T cells by signaling through both the VLA‐4 and VLA‐5 integrin molecules. J Immunol. 1990;145:785. [PubMed] [Google Scholar]
- 5.Matsuyama T, Yamada A, Kay J, et al. Activation of CD4 cells by fibronectin and anti‐CD3 antibody: a synergistic effect mediated by the VLA‐5 fibronectin receptor complex. J Exp Med. 1989;170:1133. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA‐1 ligand ICAM‐1 provides an important costimulatory signal for T cell receptor‐mediated activation of resting T cells. J Immunol. 1990;144:4579. [PubMed] [Google Scholar]
- 7.Teague TK, Lazarovits AI, McIntyre BW. Integrin αβ7 costimulation of human peripheral blood T cell proliferation. Cell Adhesion Commun. 1994;2:539. doi: 10.3109/15419069409014217. [DOI] [PubMed] [Google Scholar]
- 8.Bazzoni G, Hemler ME. Are changes in integrin affinity conformation overemphasised? Trends Biochem Sci. 1998;137:422. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 9.Ohmigoto S, Tabata N, Suga S, et al. Molecular characterization of fusion regulatory protein‐1 (FRP‐1) that induces multinucleated giant cell formation of monocytes and HIV gp120‐mediated cell fusion. FRP‐1 and 4F2/CD98 are identical molecules. J Immunol. 1995;155:3585. [PubMed] [Google Scholar]
- 10.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 11.Kappler JW, Kubo R, Haskins K, et al. The major histocompatibility complex‐restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983;35:295. doi: 10.1016/0092-8674(83)90232-5. [DOI] [PubMed] [Google Scholar]
- 12.Woodside DG, McIntyre BW. Inhibition of CD28/CD3‐mediated costimulation of naı¨ve and memory human T lymphocytes by intracellular incorporation of polyclonal antibodies specific for the activator protein‐1 transcriptional complex. J Immunol. 1998;161:649. [PubMed] [Google Scholar]
- 13.Warren AP, Patel K, McConkey DJ, Palacios R. CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood. 1996;87:3676. [PubMed] [Google Scholar]
- 14.Suomalainen HA. The monoclonal antibodies TROP‐4 and 4F2 detect the same membraane antigen that is expressed at an early stage of lymphocyte activation and is retained on secondary lymphocytes. J Immunol. 1986;137:422. [PubMed] [Google Scholar]
- 15.Schlossman S, Boumsell L, Gilks W, et al. Leukocyte Typing V. Oxford: Oxford University Press; 1995. [Google Scholar]
- 16.Quackenbush E, Clabby M, Gottesdiener KM, et al. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell‐surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc Natl Acad Sci USA. 1987;84:6526. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira S, Digrandi S, Kuhn LC. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987;262:9574. [PubMed] [Google Scholar]
- 18.Kanai Y, Segawa H, Miyamoto K‐I, Uchino H, Takeda E, Endou H. Expression cloning and characterisation of a transporter for large neutral amino acids activated by the heavy chain 19 of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 19.Mastroberardino L, Spindler B, Pfeiffer R, et al. Amino acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura E, Sato M, Yang H, et al. 4F2 CD98 heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999;274:3009. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 21.Nakao M, Kubo K, Hara A, et al. A monoclonal antibody (H227) recognizing a new epitope of 4F2 molecular complex associated with T cell activation. Cellular Immunol. 1993;152:226. doi: 10.1006/cimm.1993.1282. [DOI] [PubMed] [Google Scholar]
- 22.Diaz LA, Friedman AW, He X, Kuick RD, Hanash SM, Fox DA. Monocyte‐dependent regulation of T lymphocyte activation through CD98. Inter Immunol. 1997;9:1221. doi: 10.1093/intimm/9.9.1221. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Komada S, Kusagawa M, et al. Fusion regulation proteins on the cell surface: isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in Newcastle Disease Virus‐infected cell lines of human origin. J Virol. 1992;66:5999. doi: 10.1128/jvi.66.10.5999-6007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabata N, Ito K, Shimokata S, et al. Expression of fusion regulatory proteins on human peripheral blood monocytes. Induction of homotypic aggregation and formation of multinucleated giant cells by anti‐FRP‐1 monoclonal antibodies. J Immunol. 1994;153:3256. [PubMed] [Google Scholar]
- 25.Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Current Opinion Cell Biol. 1996;8:657. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 27.Parsons JT. Integrin‐mediated signalling: regulation by protein tyrosine kinases and small GTP‐binding proteins. Current Opinion Cell Biol. 1996;8:146. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 28.Mannion BA, Berditchevski F, Kraeft S‐K, Chen LB, Hemler ME. Transmembrane‐4 superfamily proteins CD81 (TAPA‐1), CD82, CD63 and CD53 specifically associate with integrin α4β1 (CD49d/CD29) J Immunol. 1996;157:2039. [PubMed] [Google Scholar]


