Abstract
Interaction of CD95 (Apo‐1/Fas) and its ligand (CD95L) plays an important role in the regulation of the immune response, since CD95+ lymphocytes may be killed after engagement of the CD95 receptor. Studying the CD95/CD95L system in 40 cases of breast cancer, the malignant cells expressed CD95L, but lost CD95 expression, when compared with non‐malignant mammary tissue. Jurkat T cells incubated on breast cancer sections underwent CD95L‐specific apoptosis. The rate of apoptosis correlated with the CD95L mRNA levels of the tissue samples. In four breast cancer cell lines, CD95L expression was increased by interferon‐γ (IFN‐γ), which resulted in higher levels of CD95L‐specific apoptosis in co‐cultured Jurkat T cells. Since IFN‐γ is mainly secreted by activated T cells, up‐regulation of CD95L in breast cancer cells in response to IFN‐γ may thus counterselect activated tumour‐infiltrating T cells and favour the immune escape of breast cancer. As demonstrated by inhibition of matrix metalloproteinases, CD95L expressed on breast cancer cells can also be shed from the cell membrane into the culture supernatant. Supernatants derived from cultured breast cancer cells induced apoptosis in Jurkat T cells via CD95L. In breast cancer patients, depletion of CD4+ and CD8+ peripheral blood lymphocytes was significantly correlated with CD95L expression in the tumours. This might be suggestive for a relationship between CD95L expression by breast cancer and systemic immunosuppression.
Introduction
CD95 (Apo‐1/Fas) ‐bearing cells are sensitive towards apoptosis, which is mediated by CD95 ligand (CD95L) cross‐linking. 1,2 Transmembrane CD95 (CD95tm) transduces the death signal upon engagement by CD95L. 1,2 A soluble isoform of CD95 is generated by alternative splicing. 3 Soluble CD95 (CD95sol) may rescue CD95+ target cells from apoptosis by neutralization of CD95L preventing the apoptotic signal being initiated. 3,4 CD95L is expressed on the surface of effector cells, but may be cleaved from the cell membrane by specific matrix metalloproteinases (MMPs). 5–8 CD95L processed into a soluble form may cause systemic tissue damage, 5–7 although it is a less potent inducer of apoptosis than its membrane‐bound counterpart. 8 Disturbance of this system and imbalance of its constituents has been implicated in autoimmune disease 9,10 and neoplastic development. 5,11–18
The expression of CD95 has previously been studied in breast cancer cell lines. 11,12 Breast cancer cells lacking expression of CD95 were resistant towards CD95L‐induced apoptosis. 11 Furthermore, studying breast cancer tissue sections, we recently identified breast cancer cells as a source of CD95L expression. 13 However, the functional significance of CD95L expression in breast cancer has not yet been studied.
The present study shows that CD95L, either expressed on the membrane of breast cancer cells or after cleavage by MMPs, killed CD95+ Jurkat T cells. Interferon‐γ (IFN‐γ), mainly secreted by activated T cells, increased CD95L expression in breast cancer cells, which resulted in higher levels of CD95L‐specific apoptosis in co‐cultured Jurkat T cells. Lymphocyte killing by breast cancer may be of clinical interest, since peripheral blood CD4+ and CD8+ lymphocytes in breast cancer‐bearing patients were depleted in close correlation with CD95L expression by the tumours.
Materials and methods
Tissue samples and cell lines
The current study focused on 40 cases of breast cancer and eight cases of benign breast tissues. All breast cancers were primary tumours, among them seven well‐differentiated (low grade; GI), 19 moderately differentiated (intermediate grade; GII) and 14 poorly differentiated (high grade; GIII) carcinomas. The staging of invasive breast cancer ranged from T1 to T4. The present collection comprised 32 invasive ductal carcinomas, five invasive lobular carcinomas, one medullar and two tubular carcinomas. To minimize the possible interference of tumour‐infiltrating lymphocytes (TIL) in CD95L expression of the tissue samples, only tissue samples lacking obvious necrosis or lymphocytic infiltration were selected for reverse transcription–polymerase chain reaction (RT‐PCR) analysis. Applying this criterion, about one‐third of the initially available tissue samples was excluded whereas absence of significant T‐cell infiltration was further confirmed in 48 samples, which were selected for further analysis.
Jurkat T lymphocytes and breast cancer cell lines BT‐20, MCF‐7, SKBR‐3 and T47D were obtained from the American Type Culture Collection (Rockville, MD). Xenografts of the MCF‐7 cell line were carried out as previously described. 19 BT‐20, MCF‐7 and T47D cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). SKBR‐3 cells were cultured in Dulbecco McCoy 5A medium containing 10% FBS. Jurkat T lymphocytes were cultured in RPMI‐1640 medium (with 10% FBS) and stimulated with 2·4 µg phytohaemagglutinin (PHA)/ml. For co‐culture experiments breast cancer cells and Jurkat T cells were co‐cultured in RPMI‐1640.
Reagents and antibodies
A polyclonal rabbit anti‐human CD95L immunoglobulin G1 (IgG1), a monoclonal mouse anti‐human CD3 IgG1 and a monoclonal mouse anti‐human MUC‐1 IgG1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human recombinant CD95L protein corresponding to soluble CD95L (amino acids 103–261) and a CD95:Fc (immunoglobulin) fusion molecule were from Alexis (San Diego, CA). An agonistic anti‐CD95 antibody (CH‐11) which induces apoptosis in CD95+ cells was from Immunotech (Marseille, France). Ac‐YVAD‐cmk, an inhibitor of the interleukin‐1β converting enzyme (ICE), was purchased from Bachem (Basel, Switzerland). BB‐3103, an inhibitor of a variety of MMPs, was a kind gift from British Biotech Pharmaceuticals Ltd (Oxford, UK).
RPMI‐1640 medium and FBS were purchased from Biochrom (Berlin, Germany), PHA and human recombinant IFN‐γ from Seromed (Berlin, Germany) and all other chemicals from Sigma (Deisenhofen, Germany). Oligonucleotides were synthesized by Birsner and Grob (Freiburg, Germany).
Quantitative competitive RT‐PCR
Total RNA from snap‐frozen tissue sections and cultured breast cancer cells was isolated using a total RNA extraction kit (Qiagen, Hilden, Germany) and reverse transcribed using a first‐strand cDNA synthesis kit (Boehringer Mannheim, Mannheim, Germany). As shown in Fig. 1, the cDNA to be assayed was co‐amplified with known amounts of an internal DNA standard (‘Δ4’), which was, apart from a deletion of four nucleotides, identical with the corresponding fragment of the assayed cDNA. For quantification of the transcripts for CD95L, CD95tm, CD95sol, CD3δ chain and hypoxanthine‐guanine phosphoribosyltransferase (HPRT), respectively, a constant amount of cDNA, corresponding to 50 ng reverse transcribed total RNA, was mixed with 108, 107, 106, 105, 104, 103, or 0 copies of the respective standard (Δ4) and then amplified using CD3δ, CD95, CD95L and HPRT‐specific primers as previously described. 13 The read‐out of the amplification involved one additional fluorescent dye‐labelled oligonucleotide, which allows discrimination between wild‐type (wt) and standard (Δ4’) DNA species (Fig. 1). PCR amplification products were specifically labelled in run‐off reactions, loaded on an acrylamide gel and analysed by an automated sequencer (ABI 373A, Applied Biosystems, Foster City, CA). The fluorescent profiles (Fig. 1) were recorded and the profile areas were analysed using the software immunoscope, 20–22 which was kindly provided by Dr C. Pannetier (Unité de Biologie Moléculaire du Gène, INSERM U277, Institut Pasteur, Paris, France).
Figure 1.
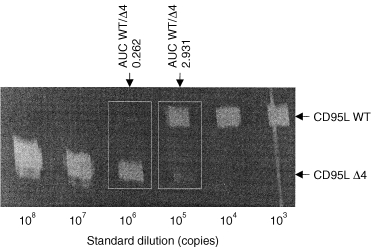
Quantification of CD95 ligand and receptor isoform mRNA levels by quantitative competitive RT‐PCR. The cDNA to be assayed (WT) was coamplified with known amounts of an internal DNA standard (Δ4), which was, apart from a deletion of four nucleotides, identical to the corresponding fragment of the assayed cDNA. For quantification of transcripts for CD95L, a constant amount of cDNA, corresponding to 50 ng reverse transcribed total RNA, was mixed with 108, 107, 106, 105, 104, 103, or 0 copies of the CD95L standard (CD95L Δ4) and then amplified to saturation. The read‐out of the amplification involved one additional fluorescent dye‐labelled oligonucleotide, which allows discrimination between CD95L wild‐type (CD95L WT) and standard (CD95L Δ4) DNA species. PCR amplification products were specifically labelled in run‐off reactions, loaded on an acrylamide gel and analysed by an automated sequencer. The fluorescent profiles were recorded and the profile areas were analysed. For co‐amplifications with 106 and 105 copies of the CD95L standard, respectively, the peak area ratios for CD95L wild‐type (CD95L WT) and standard (CD95L Δ4) were calculated. The number of CD95L WT copies in the cDNA sample was calculated as the mean of WT : Δ4 peak area ratios at two standard dilutions (e.g. for the sample shown here: (0·343 × 106 + 3·461 × 105)/2, i.e. 344 550 copies).
Immunocytochemical procedures
Breast cancer cells grown on cover slides were stained with mouse anti‐human MUC‐1 IgG1 and rabbit anti‐human CD95L IgG1 antibodies as previously described, 13,23 Goat anti‐mouse IgG1 [fluorescein isothiocyanate (FITC) ‐labelled] and goat anti‐rabbit IgG1 (CY3‐labelled) were used as secondary antibodies. Matching mouse anti‐human primary antibodies with goat anti‐rabbit secondary antibody and rabbit anti‐human primary antibody with goat anti‐mouse secondary antibody, no cross‐reactivity between the secondary antibodies was found. Non‐specific binding of primary antibodies was excluded by isotype controls.
Cells on cover slides were fixed with methanol (– 20°) for 10 min. After three washes with phosphate‐buffered saline (PBS), the cover slides were treated with blocking serum for 30 min and washed three times with PBS. The cells were incubated for 1 hr with primary antibodies at a concentration of 0·5 µg/ml. After 1 hr of repeated washing steps, the cells were incubated with the secondary antibodies for 1 hr. After removal of the secondary antibodies, the cover slides were washed for 1 hr with PBS, air‐dried and covered with mounting medium. Staining was visualized by means of fluorescence microscopy.
Detection of CD95L‐specific apoptosis in lymphocytes incubated with breast cancer cells
In one set of experiments, Jurkat T lymphocytes were co‐cultured with four breast cancer cell lines. BT‐20, MCF‐7, SKBR3 and T47D breast cancer cells were seeded on 12‐well plates at a density of 106 cells per well. Breast cancer cells were either kept under control conditions or stimulated with 100 U/ml human recombinant IFN‐γ for 24 hr. For a further 24 hr of culture 106 Jurkat cells were added, keeping an effector to target cell (E : T) ratio of one. Half of the co‐cultures were supplemented with the CD95:Fc (immunoglobulin) fusion molecule (100 µg/ml) to prevent engagement of the CD95 receptor by its ligand on CD95+ Jurkat cells.
In another set of experiments, CD95+ Jurkat T lymphocytes were incubated as described by Strand et al. 5 on 5‐µm cryosections from mammary tissue of different grading. Briefly, cryosections from four benign mammary tissues and 12 breast cancer samples were prepared. For each tissue, two directly adjacent serial sections were prepared and transferred onto silanized glass slides. Jurkat T lymphocytes were incubated with 2·4 µg/ml PHA for 24 hr and 106 stimulated Jurkat cells were seeded on each cryosection in 100 µl RPMI‐1640 medium. For each mammary tissue, one Jurkat cell culture was supplemented with the CD95:Fc (immunoglobulin) fusion molecule (100 µg/ml). The ability of CD95:Fc (immunoglobulin) to prevent CD95L‐mediated apoptosis was verified in preliminary experiments using human recombinant soluble CD95L as an inducer of apoptosis in PHA‐stimulated Jurkat T lymphocytes. After 24 hr, apoptosis in Jurkat T lymphocytes was determined by the TdT‐mediated fluorescein‐dUTP nick end labelling (TUNEL) method as described by Gavrieli et al. 24
Enzyme‐linked immunosorbent assay for soluble CD95 ligand
For quantitative assessment of soluble CD95L levels in culture supernatants from MCF‐7 breast cancer cell cultures an enzyme‐linked immunosorbent assay (ELISA) for soluble CD95L from Immunotech (Marseille, France) was used. For each sample 10 µl culture fluid was collected and the ELISA was performed following the manufacturer’s protocol. Each determination was carried out in duplicate. Contents of soluble CD95L were calculated using an automated ELISA‐reader (Anthos, Cologne, Germany) at a wavelength of 450 nm, based on the results of a standard dilution curve.
Fluorescence‐activated cell sorter analysis of CD4+ and CD8+ T‐lymphocyte subsets
Analysis of T‐lymphocyte subsets was performed by fluorescence‐activated cell sorter (FACS) from Ortho Inc. (Neckargemünd, Germany). Briefly, 100 µl heparinized blood was incubated with a fluorescent antibody mixture purchased from Ortho Inc. following the manufacturer’s instructions, for 30 min at 4°. The cells were then permeabilized and incubated for 20 min at room temperature.
Statistical analysis
Data are expressed as means ± SEM (n = number of independent experiments). Statistical analysis was performed using the Student’s t‐test; P < 0·05 was considered to be statistically significant.
Results
CD95L and receptor expression in breast cancer
In the present study, breast cancer tissues (n = 40) and non‐malignant mammary tissues (n = 8) were analysed for CD95L, CD95tm, and CD95sol isoforms and HPRT expression at the mRNA level. CD95L and CD95 isoform mRNA levels were measured by quantitative competitive RT‐PCR (Fig. 1) and expressed as copies/HPRT copy. In breast cancer cells, CD95L mRNA levels were about 150‐fold higher when compared to non‐malignant tissue (65·9 ± 12·1 CD95L mRNA copies/HPRT copy, n = 40; and 0·4 ± 0·1 CD95L mRNA copies/HPRT copy, n = 8). On the other hand, CD95tm mRNA levels in breast cancer were diminished by about 70% in comparison to non‐malignant tissue (53·3 ± 6·6 CD95tm mRNA copies/HPRT copy, n = 40; and 159·3 ± 12·8 CD95tm mRNA copies/HPRT copy, n = 8). The levels of mRNA for the soluble CD95 splice variant were not significantly different between malignant and benign mammary tissues (50·2 ± 9·3 CD95sol mRNA copies/HPRT copy, n = 40; and 51·6 ± 7·2 CD95sol mRNA copies/HPRT copy, n = 8).
Samples for RT‐PCR analysis were from whole tissue sections. Since CD95L expression was previously attributed to T lymphocytes 2,3 and tissue samples from breast cancer are often highly infiltrated, only tissue samples lacking lymphocytic infiltration were selected for this study. Based on this criterion, about one‐third of the initally available samples was excluded from analysis. The absence of relevant amounts of TIL was controlled by quantification of CD3δ chain transcripts in the tissue samples and immunofluorescence using an anti‐CD3‐specific antibody (not shown).
CD95L and receptor expression in breast cancer cell lines and xenografted breast cancer cells
As shown in Table 1, in four breast cancer cell lines high CD95L and comparatively low CD95tm mRNA levels were found. In MCF‐7 cells, mRNA levels for both CD95L and CD95tm significantly increased in response to treatment with 100 U/ml IFN‐γ. When MCF‐7 cells were incubated in the presence of an agonistic anti‐CD95 antibody, which induces apoptosis in CD95+ cells, only 6 ± 2% (n = 3) of the breast cancer cells became apoptotic. If, however, MCF‐7 cells were pretreated with 100 U/ml IFN‐γ, 34 ± 9% (n = 3) of the breast cancer cells underwent apoptosis in the presence of an agonistic anti‐CD95 antibody, indicating that CD95tm mRNA levels were predictive for sensitivity towards CD95‐dependent apoptosis.
Table 1.
CD95L and receptor mRNA levels in breast cancer cell lines
| mRNA levels | CD95L | CD95tm | CD95sol |
|---|---|---|---|
| BT‐20 | 21·7 ± 5·2* | 15·3 ± 3·4 | 9·3 ± 2·2 |
| SKBR‐3 | 63·1 ± 4·8 | 24·2 ± 6·7 | 15·5 ± 4·2 |
| T47D | 52·3 ± 2·2 | 8·0 ± 2·7* | 10·3 ± 4·1 |
| MCF‐7 | 51·0 ± 8·6 | 44·3 ± 9·6 | 26·7 ± 6·1 |
| MCF‐7 + IFN‐γ | 291·1 ± 58·2* | 163·2 ± 40·5* | 34·1 ± 8·2 |
| Xeno MCF‐7 | 135·1 ± 32·6* | 33·0 ± 3·3 | 26·4 ± 4·1 |
Messenger RNA levels for CD95 ligand (CD95L), transmembrane (CD95tm) and soluble (CD95sol) isoforms of CD95 receptor were determined in BT‐20, SKBR‐3, T47D and MCF‐7 breast cancer cell lines, which were kept under control conditions. Alternatively, MCF‐7 cells were treated for 24 hr with 100 U/ml IFN‐γ or were xenografted into nude mice as described in the Materials and Methods. Data are given as CD95L, CD95tm, or CD95sol mRNA copies/103HPRT mRNA copies, respectively, and as means ± SEM.
denotes statistically significant differences from other cell lines (P < 0·05).
The effect of IFN‐γ on CD95L expression could also be observed at the protein level for MCF‐7 cells and the other three cell lines (Fig. 2).
Figure 2.
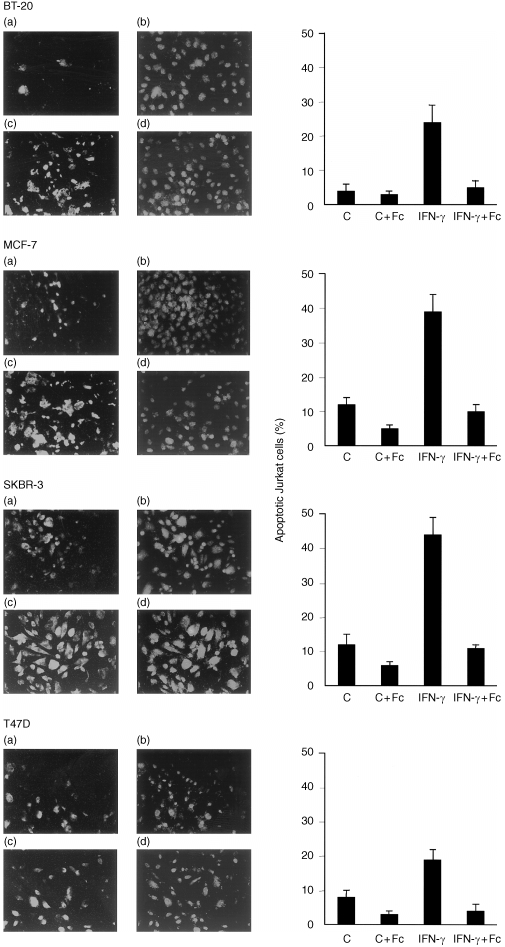
CD95 ligand expression in breast cancer cell lines and induction of apoptosis in co‐cultured Jurkat T lymphocytes. BT‐20, MCF‐7, SKBR‐3 and T47D breast cancer cells were seeded at a density of 106 cells per well and cultured without (a, b) or with100 U/ml IFN‐γ (c, d) and stained for CD95 ligand expression (red fluorescent dye; a, c) and counterstained using an anti‐mucin‐1 (MUC‐1) specific antibody (green fluorescent dye; b, d). Jurkat lymphocytes were co‐cultured with breast cancer cells cells without (C), or with 100 U/ml IFN‐γ (IFN‐γ). Half of the experiments were carried out in the presence of 100 µg/ml of the CD95:Fc (immunoglobulin) fusion molecule (IFN‐γ + Fc). After 24 hr, Jurkat cells were subjected to TUNEL analysis and the percentage of apoptotic cells was determined as described in the Materials and Methods section. Data are given as means ± SEM and are from three independent experiments. Breast cancer cells were fixed and subjected to immunofluorescent staining.
In order to give an estimate of the interference of cell culture artefacts in CD95L and CD95 isoform expression, CD95L and CD95 isoform mRNA levels were determined in MCF‐7 cells which had previously been xenografted into nude mice by Schnürch et al. 19 Xenografted MCF‐7 cells formed solid invasive tumours within 6 weeks. 19 Unexpectedly, CD95L mRNA levels in xenografted MCF‐7 cells were 2·5‐fold higher (Table 1; n = 3; P < 0·05) when compared to non‐transplanted MCF‐7 cells. Since nude mice lack T cells and thus the main source of endogenous IFN‐γ, neither infiltrating T cells nor IFN‐γ were essential for CD95L expression by the breast cancer cells. The levels of mRNA for CD95 isoforms did not significantly differ from those in non‐transplanted MCF‐7 cells (Table 1).
Induction of CD95L‐specific apoptosis in Jurkat T lymphocytes by breast cancer
In order to assess whether CD95L expression in breast cancer was functionally relevant, CD95L‐specific apoptosis of Jurkat T lymphocytes incubated on cryosections from breast cancer tissue or co‐cultured with breast cancer cell lines was examined. About 3% of Jurkat T lymphocytes underwent apoptosis when cultured on non‐malignant tissue (n = 4). This was similar to the rate of apoptosis if CD95L‐mediated apoptosis was blocked by CD95:Fc (immunoglobulin) (Table 2). In comparison, the extent of apoptosis was increased 10‐fold, when Jurkat T lymphocytes were cultured on sections from invasive breast cancer (n = 12). In all cases, induction of apoptosis by co‐culture on breast cancer sections could largely be inhibited by CD95:Fc (immunoglobulin) (Table 2), indicating that Jurkat T‐cell apoptosis induced by breast cancer was mediated to a large part by a CD95L‐dependent pathway. Furthermore, the number of apoptotic Jurkat T lymphocytes correlated with CD95L mRNA levels in the tissues (r = – 0·87, P < 0·01; n = 16; Fig. 3a).
Table 2.
Induction of CD95L‐specific apoptosis in Jurkat cells by co‐culture on breast cancer sections
| Incubation on tissue sections | Apoptotic Jurkat T lymphocytes [%] |
|---|---|
| Control | 3 ± 1 |
| Fibroadenomas | 5 ± 2 |
| Fibroadenomas + CD95:Fc (Ig) | 2 ± 1 |
| Invasive carcinomas | 31 ± 5* |
| Invasive carcinomas + CD95:Fc (Ig) | 11 ± 3* |
Jurkat T lymphocytes were cultured on tissue sections from fibroadenomas (n = 4), breast cancer (n = 12),or in the absence of mammary tissue (control; n = 3) as described in the Materials and Methods. After stimulation with 2·4 µg/ml PHA 106Jurkat cells were cultured in RPMI‐1640 medium. The cell suspensions covered 5‐µm tissue sections of about 4cm2.For each tumour two directly adjacent serial cryosections were prepared. Half of the sections were covered with Jurkat cell suspensions which were supplemented with 100 µg/ml CD95:Fc (immunoglobulin)[+ CD95:Fc (Ig)]Jurkat T lymphocytes were cultured for 24 hr and subjected to TUNEL analysis. Apoptotic nuclei were counted by means of fluorescence microscopy and given as percentage values. Data are given as means ± SEM and are from four (fibroadenomas), 12 (invasive carcinomas) or three (control) experiments, respectively.
Significantly different from control (P < 0·05).
Figure 3.
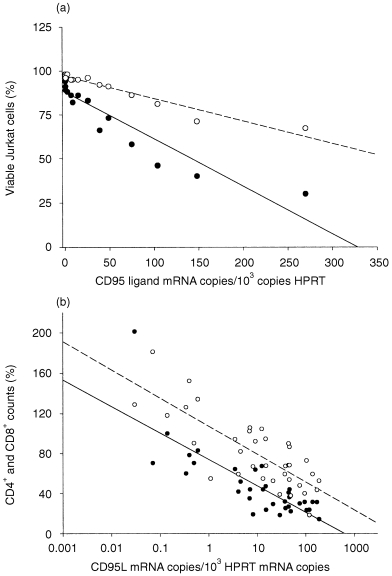
Correlation between CD95 ligand mRNA levels, killing of Jurkat T lymphocytes and depletion of CD4+ and CD8+ peripheral blood lymphocytes in breast cancer patients (a) Jurkat T lymphocytes were cultured on breast cancer tissues as described in the Materials and Methods section. After stimulation with 2·4 µg/ml PHA 106 Jurkat cells were cultured in RPMI‐1640 medium. The cell suspensions covered 5‐µm tissue sections of about 4 cm2. For each tumour two directly adjacent serial cryosections were prepared. The sections were covered with Jurkat cell suspensions which were supplemented with medium without (• solid regression line; r = – 0·87, P < 0·01) or with 100 µg/ml CD95:Fc (immunoglobulin), which specifically blocks CD95L‐mediated apoptosis (○ dashed regression line; r = – 0·84, P < 0·01). Jurkat T lymphocytes were cultured for 24 hr and subjected to TUNEL analysis. TUNEL+ cells having sustained DNA double‐strand breaks were excluded and the remaining TUNEL– cells (Viable cells) were counted by means of fluorescence microscopy and given as percentage values. (b) The number of CD4+ and CD8+ T lymphocytes was determined by FACS analysis. Both CD4+ (○ dashed regression line; r = – 0·79; P < 0·01) and CD8+ (• solid regression line; r = – 0·81; P < 0·01) peripheral blood lymphocyte counts were inversely related to the CD95L mRNA levels. Means of CD4+ (0·88 × 106/ml, n = 50) and CD8+ (0·64 × 106/ml, n = 45) lymphocyte counts in peripheral blood from healthy volonteers were set to 100%.
To verify the correlation between CD95L expression and lymphocyte killing under cell culture conditions, CD95L overexpression in four breast cancer cell lines was induced by the addition of 100 U/ml IFN‐γ (Fig. 2). Comparison was made between the increased CD95L expression and the corresponding levels of co‐culture‐induced Jurkat cell killing. Co‐cultured with four IFN‐γ‐treated breast cancer cell lines, the fraction of apoptotic Jurkat T lymphocytes increased between threefold and sixfold in comparison to co‐culture with untreated breast cancer cell lines (Fig. 2). A large fraction of co‐cultured Jurkat T lymphocytes could be rescued from breast cancer cell‐induced apoptosis when engagement of the CD95 receptor on Jurkat cells was prevented by addition of 100 µg/ml CD95:Fc (immunoglobulin) (Fig. 2). Induction of apoptosis in Jurkat T cells was thus largely due to CD95L‐mediated cytotoxicity. The increase in CD95L‐specific killing of Jurkat cells by breast cancer cells upon IFN‐γ treatment was consistent with increased CD95L mRNA levels (MCF‐7 cells; Table 1) and higher CD95L protein expression (all four breast cancer cell lines; Fig. 2) in breast cancer cells. Interestingly, sensitivity of Jurkat T lymphocytes towards CD95L‐mediated apoptosis in co‐culture experiments required prestimulation of the Jurkat T cells with PHA. Non‐stimulated Jurkat T cells did not exhibit significant apoptosis in co‐culture with breast cancer cell lines (not shown).
Involvement of CD95 ligand shedding in lymphocyte killing by breast cancer cells
In order to determine, whether CD95L after shedding from the cell membrane in breast cancer cells retained its capacity to induce apoptosis in lymphocytes, the effect of soluble CD95L in MCF‐7 cell culture supernatants was examined. MCF‐7 cells were either pretreated with 100 U/ml IFN‐γ or kept under control conditions for 24 hr. Thereafter, the medium was changed and MCF‐7 cells were incubated for further 24 hr in the presence or absence of 2 µmol/l of the MMP inhibitor BB3103. As previously described, 4,22,23 BB3103 inhibits a variety of MMPs which are responsible for CD95L shedding from the cell membrane. Jurkat T lymphocytes were incubated with supernatants derived from MCF‐7 cells and subjected to TUNEL analysis to determine the fraction of apoptotic cells. Supernatants derived from MCF‐7 cells that were either pretreated with IFN‐γ or kept under control conditions, induced apoptosis in Jurkat T lymphocytes eightfold and threefold above the rate of spontaneous apoptosis in Jurkat T cells, respectively (Table 3). In line with these findings, the content of soluble CD95L in the culture supernatants was increased by IFN‐γ as assessed by ELISA (Table 3). The effect of supernatants derived from cultured MCF‐7 cells was sensitive to addition of CD95:Fc (immunoglobulin) and thus largely mediated by CD95L (Table 3). When the shedding inhibitor BB3103 was added to MCF‐7 cell cultures, the effect of supernatants derived from MCF‐7 cells on lymphocyte apoptosis was also drastically diminished (Table 3). As determined by ELISA, CD95L was indeed not detectable in the culture fluid, when MMPs were inhibited by BB3103 (Table 3). MMPs were thus important for cleavage of CD95L from MCF‐7 cells and CD95L induced apoptosis in Jurkat T cells also after processing into a soluble form.
Table 3.
Involvement of CD95L shedding in lymphocyte killing by breast cancer cells
| Apoptotic Jurkat T lymphocytes [%] | |||
|---|---|---|---|
| Content of soluble CD95L [ng/ml] | – CD95:Fc (Ig) | + CD95:Fc (Ig) | |
| Supernatants from MCF‐7 cells | |||
| Control | n.d. | 3 ± 1 | n.d. |
| MCF‐7 | 0·4 ± 0·2 | 10 ± 2* | 3 ± 1 |
| MCF‐7 + BB | < 0·05 | 3 ± 2 | 3 ± 1 |
| MCF‐7 + IFN‐γ | 1·8 ± 0·4 | 26 ± 4* | 9 ± 2* |
| Co‐culture with MCF‐7 cells | |||
| Control | 4 ± 2 | 3 ± 1 | |
| MCF‐7 | 12 ± 2* | 5 ± 1 | |
| MCF‐7 + BB | 27 ± 5* | 9 ± 3* | |
| MCF‐7 + IFN‐γ | 39 ± 5* | 10 ± 2* | |
| MCF‐7 + IFN‐γ + BB | 54 ± 6* | 9 ± 3* | |
Jurkat T lymphocytes were incubated in supernatants derived from MCF‐7 cell cultures (MCF‐7) or in normal medium (Control). Breast cancer cells were treated without, with 2 µmol/l of the CD95L shedding inhibitor BB3103 ( + BB) and/or with 100 U/ml IFN‐γ ( + IFN‐γ) for 24 hr. After incubation in supernatants from MCF‐7 cells or medium, Jurkat cells were subjected to TUNEL analysis. The content of soluble CD95L in the supernatants was measured by ELISA (ng/ml). In another set of experiments, Jurkat lymphocytes were co‐cultured with MCF‐7 cells without (MCF‐7), or with 100 U/ml IFN‐γ (MCF‐7 + IFN‐γ) and/or with 2 µmol/l of the CD95L shedding inhibitor BB3103 (MCF‐7 + BB). All experiments were carried out in the presence (+) or absence (–) of 100 µg/ml of the CD95:Fc (Ig) fusion molecule, which specifically blocks CD95L mediated apoptosis. Data are given as means ± SEM and are from three independent experiments.
denotes significant differences from control (P < 0·05); n.d., not determined.
The level of CD95L‐specific apoptosis in Jurkat T lymphocytes increased if Jurkat T cells were co‐cultured with BB3103‐pretreated MCF‐7 cells compared to co‐culture with MCF‐7 cells without addition of BB3103 (Table 3). This was consistent with the accumulation of CD95L protein in the cytoplasm and on the membrane of MCF‐7 cells in the presence of BB3103 as we have previously shown in embryonal carcinoma cells. 23
Correlation between CD95L mRNA levels and depletion of CD4+ and CD8+ peripheral blood lymphocytes in breast cancer patients
Given the observation, that CD95L processed into a soluble form induced apoptosis in CD95+ target cells, it appears conceivable that soluble CD95L could have systemic detrimental effects on CD95+ cells, e.g. activated T cells.
Peripheral blood CD4+ and CD8+ T‐lymphocyte subsets were quantified in 40 breast cancer patients, five adenoma patients and three mastopathy III patients. FACS counts were performed 1 day prior to mammary surgery and for cancer patients also prior to cytostatic drug treatment. Both CD4+ and CD8+ blood lymphocyte subsets were markedly reduced in patients displaying high levels of CD95L mRNA (Fig. 3b). There was a significant correlation between CD95L mRNA levels in the tumours and the reduction of CD4+ (r = – 0·79, P < 0·01) and CD8+ (r = – 0·81, P < 0·01) T lymphocytes, although without a clear preference for depletion of eitherT‐cell subset.
Discussion
Invasive breast cancer expressed high levels of CD95L with potential detrimental effects on the host organism. CD95+ Jurkat lymphocytes were killed either by direct contact with breast cancer cells (Fig. 2,Fig. 3a) or by incubation in culture supernatants derived from breast cancer cells (Table 3). This indicates that CD95L expressed by breast cancer cells is an active inducer of apoptosis in its membrane‐bound form or after cleavage by MMPs. In both situations about 70% of Jurkat T cells could be rescued by addition of CD95:Fc (immunoglobulin), confirming that lymphocyte killing was indeed largely mediated by CD95L. Furthermore, inhibition of MMPs abrogated the cytotoxic effect of culture supernatants on Jurkat T lymphocytes (Table 3), demonstrating the role of MMPs in CD95L processing.
CD95 expression was largely lost in breast cancer, probably resulting in resistance towards CD95L‐mediated apoptosis. The assumption of a relationship between CD95 expression and sensitivity towards CD95L‐mediated apoptosis is supported by the observation, that MCF‐7 cells regain sensitivity towards CD95L‐induced apoptosis after up‐regulation of CD95 expression by IFN‐γ.
MCF‐7 breast cancer cells xenografted into nude mice formed a solid and invasive tumour and CD95L mRNA levels in the xenotumours were significantly increased compared to CD95L mRNA expression in MCF‐7 cells kept under cell culture conditions. The meaning for this is unclear. However, one possible, albeit speculative, explanation could be that CD95L‐expressing tumour cells up‐regulate CD95L expression by direct contact with the antigenic host organism, which was missing under cell culture conditions. Be this as it may, neither infiltrating T cells nor IFN‐γ were essential for CD95L expression by the breast cancer cells, since nude mice lack T cells and thus the main source of endogenous IFN‐γ. Nevertheless, the capacity of IFN‐γ to enhance CD95L expression was demonstrated for four breast cancer cell lines (Fig. 2).
Advanced breast cancer often shows features of systemic disease, 25,26 among them tumour cachexia, paraneoplastic syndromes and in particular immunosuppression. 25,26 Using several experimental approaches, breast cancer cells induce CD95L‐specific apoptosis in Jurkat T lymphocytes. Induction of apoptosis in Jurkat T cells did not require direct contact with CD95L‐expressing breast cancer cells, since supernatants containing CD95L from breast cancer cell cultures in a soluble form similarly induced CD95L‐specific apoptosis in Jurkat T lymphocytes. This was in line with findings in various non‐Hodgkin lymphomas, which caused systemic tissue damage by shedding of CD95L from malignant cells into the serum. 6 Recent studies, however, demonstrate, that soluble CD95L is a less potent inducer of apoptosis compared to its membrane‐bound counterpart. 8
CD95L mRNA levels in breast cancer samples were closely correlated with induction of CD95L‐specific apoptosis in Jurkat T lymphocytes which were incubated on the corresponding tissue sections (Fig. 3a). Similarly, CD95L mRNA expression in breast cancer was closely correlated with depletion of CD4+ and CD8+ peripheral blood lymphocytes of breast cancer patients (Fig. 3b). Although other agents besides CD95L associated with the severity of the tumour disease unquestionably contribute to the reduction of CD4+ and CD8+ lymphocytes, it appears conceivable that peripheral blood lymphocytes are prone to damage by soluble CD95L that was shed from tumour cells as described for other malignancies. 5–7,23,27 For example, in ovarian carcinoma the link between tumour‐associated CD95L expression and the reduction of distinct T‐cell subsets was demonstrated in vitro. 28 Interestingly, recent data by Rabinovich et al. demonstrate, that CD95L expression in ovarian carcinomas causes a marked reduction of CD3ε and CD3ζ T‐cell co‐receptor‐expressing TIL. 28 Specific elimination of CD3ε+ and CD3ζ+ T cells by CD95L‐expressing tumour cells implies, that tumour‐associated CD95L expression may specifically interfere with tumour antigen recognition by activated TIL.
Given that IFN‐γ mainly originates from activated T cells, we show accordingly that up‐regulation of CD95L in breast cancer cells in response to IFN‐γ may thus counterselect activated tumour‐infiltrating T cells and favour a microenvironment of T‐cell anergy and immune escape of breast cancer cells (Fig. 4).
Figure 4.
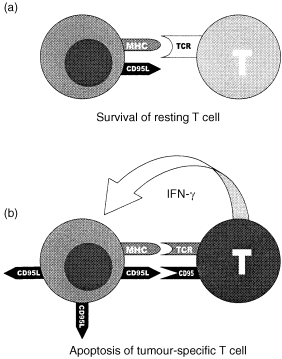
Competence to die: a model for CD95 ligand mediated counterselection of tumour‐reactive T cells in breast cancer. Resting T cells do not recognize tumour antigen, express only little CD95 and were thus not sensitive to CD95L expressed by breast cancer cells (a). Tumour‐specific T cells were activated upon recognition of tumour antigen by interaction between T‐cell receptor (TCR) and major histocompatibility complex (MHC). T‐cell activation is followed by co‐expression of CD95 and IFN‐γ. Increased CD95 expression renders T cells more susceptible to CD95L‐mediated apoptosis. IFN‐γ enhances CD95L expression in breast cancer cells (b). Taken together, activated tumour‐specific T cells were selectively killed by CD95L‐expressing breast cancer cells whereas resting T cells were spared.
Studying CD95 and CD95L expression along the hepatic sinusoids in rats, we recently demonstrated, 4 that CD95L expressed by antigen‐presenting rat liver macrophages (Kupffer cells) in response to IFN‐γ is critical for the generation of an immunoprivileged site in rat liver in vivo. 4 IFN‐γ‐primed Kupffer cells, although acting as antigen‐presenting cells in the liver, highly overexpressed CD95L, resulting in rapid elimination of activated T cells via CD95L. 4 Tumour‐associated immunoprivilege in breast cancer could be generated in a similar way (Fig. 4). Recognition of tumour antigen by a T cell of a distinct T‐cell receptor specificity may lead to activation and clonal expansion of the tumour‐reactive T cell. 29 On the other hand, activated T cells co‐express IFN‐γ and CD95tm and are thus more susceptible to CD95L‐mediated apoptosis 2,4 than resting T cells. In the present study, expression of CD95L in breast cancer cells was significantly increased by, although not dependent on, IFN‐γ. In addition, IFN‐γ‐induced up‐regulation of CD95L expression resulted in increased levels of CD95L‐specific lymphocyte killing. We propose, that CD95L overexpression in breast cancer in response to IFN‐γ released by activated T cells may thus counterselect tumour‐specific T cells (Fig. 4) and spare resting T cells, which are less sensitive towards CD95L‐mediated apoptosis. Selective elimination of activated T cells by CD95L‐expressing breast cancer is further supported by the observation that PHA‐stimulated Jurkat T cells were apoptosis‐sensitive in co‐culture with breast cancer cells, whereas non‐stimulated Jurkat T cells were not. However, more detailed studies are required to firm up this hypothesis. For instance, selective killing of activated (i.e. antigen‐triggered) T cells should have significant impact on the repertoire of T‐cell receptor specificities in breast cancer patients which is a subject of ongoing work.
Acknowledgments
The authors are grateful to Prof. Dr Philippe Kourilsky for encouraging discussions, Dr Christophe Pannetier for providing the software immunoscope and Helga Landmann‐Crijns for excellent technical assistance. Own work was supported by the Deutsche Forschungsgemeinschaft (DFG Be 1215/6‐3).
Abbreviations
- CD95L
CD95 ligand
- CD95tm
transmembrane CD95
- CD95sol
soluble CD95
- FITC
fluorescein isothiocyanate
- HPRT
hypoxanthine‐guanine phosphoribosyltransferase
- IFN‐γ
interferon‐γ
- MUC‐1
mucin‐1
- PBL
peripheral blood lymphocytes
- PCR
polymerase chain reaction
- PHA
phytohaemagglutinin
- TIL
tumour‐infiltrating lymphocytes
- TUNEL
TdT‐mediated dUTP nick‐end labelling
References
- 1.Watanabe‐fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 2.Leithäuser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of Apo‐1, a new member of the Nerve Growth Factor/ Tumor Necrosis Factor Receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415. [PubMed] [Google Scholar]
- 3.Cheng J, Zhou T, Liu C, et al. Protection from Fas‐mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 4.Müschen M, Warskulat U, Peters‐regehr T, Bode JG, Kubitz R, Häussinger D. Involvement of CD95 (Apo‐1/ Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterology. 1999;116:666. doi: 10.1016/s0016-5085(99)70189-7. [DOI] [PubMed] [Google Scholar]
- 5.Strand S, Hofmann WJ, Hug H, et al. Lymphocytes apoptosis induced by CD95 (Apo‐1/Fas) ligand expressing tumor cells – a mechanism of immune evasion? Nature Med. 1996;2:1361. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nature Med. 1996;2:317. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 7.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase‐mediated release of human Fas ligand. J Exp Med. 1995;182:1777. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nature Med. 1998;4:31. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 9.Müschen M, Warskulat U, Moers C, Simon D, Even J, Häussinger D. Derangement of the CD95‐system in a case of Churg‐Strauss vasculitis. Gastroenterology. 1998;114:1351. doi: 10.1016/s0016-5085(98)70458-5. [DOI] [PubMed] [Google Scholar]
- 10.Müschen M, Warskulat U, Even J, et al. Involvement of soluble CD95 in Churg–Strauss syndrome. Am J Pathol. 1999;155:915. doi: 10.1016/S0002-9440(10)65191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keane MM, Ettenberg A, Lowrey GA, Russel EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996;56:4791. [PubMed] [Google Scholar]
- 12.Cai Z, Stancou R, Körner M, Chouaib S. Impairment of Fas‐antigen expression in Adriamycin‐resistant but not in TNF‐resistant MCF‐7 tumor cells. Int J Cancer. 1996;68:535. doi: 10.1002/(SICI)1097-0215(19961115)68:4<535::AID-IJC21>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Müschen M, Moers C, Warskulat U, et al. CD95 ligand expression in dedifferentiated breast cancer. J Pathol. 1999;189:378. doi: 10.1002/(SICI)1096-9896(199911)189:3<378::AID-PATH439>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.O'connell J, O'sullivan GC, Collins JK, Shanahan F. The Fas Counterattack: Fas‐mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahne M, Rimoldi D, Schröter M, et al. Melanoma cell expression of CD95 (Apo‐1/Fas) ligand: implications for tumor immune escape. Science. 1996;274:1363. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 16.Hug H. Fas‐mediated apoptosis in tumor formation and defense. Biol Chem. 1997;378:1405. [PubMed] [Google Scholar]
- 17.Seino KI, Kayagari N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nature Med. 1997;3:165. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 18.Arai H, Chan SY, Bishop DK, Nabel GJ. Inhibition of the alloantibody response by CD95 ligand. Nature Med. 1997;3:843. doi: 10.1038/nm0897-843. [DOI] [PubMed] [Google Scholar]
- 19.Schnürch HG, Stegmüller M, Vering A, Beckmann MW, Bender HG. Growth inhibition of xenotransplanted carcinomas. Eur J Cancer. 1994;30:491. doi: 10.1016/0959-8049(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 20.Pannetier C, Delassus S, Darche S, Saucier C, Kourilsky P. Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucl Acids Res. 1993;21:577. doi: 10.1093/nar/21.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josien R, Douillard P, Guillot C, et al. A critical role for transforming growth factor‐β (TGF‐β) in donor transfusion‐induced allograft tolerance. J Clin Invest. 1998;102:1920. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müschen M, Warskulat U, Douillard P, Gilbert E, Häussinger D. Regulation of CD95 (Apo‐1/Fas) receptor and ligand expression by lipopolysaccharide and dexamethasone in parenchymal and nonparenchymal rat liver cells. Hepatology. 1998;27:200. doi: 10.1002/hep.510270131. [DOI] [PubMed] [Google Scholar]
- 23.Müschen M, Warskulat U, Schmidt B, Schulz WA, Häussinger D. Regulation of CD95 (Apo‐1/Fas) ligand and receptor expression in human embryonal carcinoma cells by IFNγ and all trans‐retinoic acid. Biol Chem. 1998;379:1083. doi: 10.1515/bchm.1998.379.8-9.1083. [DOI] [PubMed] [Google Scholar]
- 24.Gavrieli Y, Sherman Y, Ben‐sasson SA. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beutler B, Cerami A. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature. 1984;320:584. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 26.Beckmann MW, Niederacher D, Schnürch HG, Gusterson B, Bender HG. Multistep carcinogenesis of breast cancer and tumor heterogeneity. J Mol Med. 1997;75:429. doi: 10.1007/s001090050128. [DOI] [PubMed] [Google Scholar]
- 27.Moers C, Warskulat U, Müschen M, et al. Regulation of CD95 (Apo‐1/ Fas) ligand and receptor expression in squamous cell carcinoma by interferon γ and Cisplatin. Int J Cancer. 1998;80:564. doi: 10.1002/(sici)1097-0215(19990209)80:4<564::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovich H, Reichert TE, Kashii Y, Gastmann BR, Bell MC, Whiteside TL. Lymphocyte apoptosis induced by Fas ligand‐expressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor‐associated lymphocytes. J Clin Invest. 1998;101:2579. doi: 10.1172/JCI1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pannetier C, Even J, Kourilsky P. T‐cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]


