Abstract
Prostaglandins are some of the main mediators which control parturition, and their production by intrauterine tissues can be up‐regulated by pro‐inflammatory cytokines. Anti‐inflammatory cytokines may oppose these effects, and in this study we have investigated how two such cytokines affected fetal membrane function. Interleukin‐10 (IL‐10) inhibited the output of prostaglandin E2 (PGE2) from intact fetal membranes under basal and lipopolysaccharide (LPS)‐stimulated conditions, and there was a parallel decrease in the expression of mRNA for COX‐2. IL‐10 also inhibited the production of interleukin‐1β (IL‐1β) and the expression of mRNA for IL‐1β, indicating that this cytokine has a broad anti‐inflammatory effect. Transforming growth factor‐β1 (TGF‐β1), which is generally considered to be anti‐inflammatory had opposite effects on PGE2 production, in that it increased the output of PGE2 for up to 8 hr. TGF‐β1 increased levels of type‐2 cyclo‐oxygenase (COX‐2) and cytosolic phospholipase A2 (cPLA2) protein, and also activated the cPLA2 enzyme present; the profile of effects is similar to that of the pro‐inflammatory cytokine IL‐1β, and was not expected. Combinations of TGF‐β1 with IL‐1β also increased PGE2 output and caused appropriate changes in prostaglandin pathway enzymes, whereas TGF‐β1 and IL‐1α had more limited effects. Further studies are needed to establish the physiological significance of these findings, but TGF‐β1 does not seem to act as an inhibitory cytokine in intact fetal membranes at term.
Introduction
Prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) are produced by fetal membranes and other intrauterine tissues, 1,2 and their levels are elevated in the amniotic fluid at term, 3 as well as in labour. 4,5 This increase in prostaglandin levels is thought to be a critical step in human parturition, 6,7 and fetal membranes obtained from term human pregnancies may show marked increases in PGE2 output, and in the expression of the type‐2 cyclo‐oxygenase (COX‐2) before labour. 8 A number of factors, including the pro‐inflammatory cytokines interleukin (IL)‐1, IL‐6 and tumour necrosis factor‐α (TNF‐α), 9–11 cause similar changes in COX‐2 expression and prostaglandin output when added to fetal membranes in vitro. Human labour at all gestational ages thus has strong similarities with a general inflammatory response; from this is has been suggested that anti‐inflammatory factors will oppose these changes and thus sustain pregnancy. A number of cytokines which possess anti‐inflammatory activity have been identified, but in this study we concentrate on two of them, namely IL‐10 and transforming growth factor‐β1 (TGF‐β1), for the reasons outlined below.
IL‐10 is present in amniotic fluid throughout pregnancy, 12,13 and can be expressed by a number of tissues. 14,15 In general, increased levels of IL‐10 are linked to the continuation of pregnancy, 16,17 which is consistent with the anti‐inflammatory T helper 2 (Th2) profile of this cytokine. 18,19 Higher levels have been found during labour; 13 this has been attributed to increased production in response to the pro‐inflammatory changes of labour, 20 rather than evidence of a requirement of IL‐10 for labour. It is clear that inflammatory factors can induce IL‐10 production from such tissues in vitro. 20 IL‐10 generally down‐regulates the production of prostaglandins and cytokines in other systems, 21–23 although this is not a universal finding. 24 There seem to be no reports on the effects of IL‐10 on fetal membrane prostaglandin output, but a series of studies has shown that IL‐10 can inhibit the production of other inflammatory cytokines (IL‐6, IL‐8 and TNF‐α) from human fetal membranes. 25–27 We therefore studied the effects of IL‐10 on the release of IL‐1β and PGE2 from intact human fetal membranes incubated with bacterial endotoxin.
Likewise, TGF‐β1 is present in intrauterine tissues; it has been localized immunocytochemically to the decidua, 28 and expression of its mRNA has been detected in the decidua, placenta and fetal membranes. 29 It is produced by a number of cell types including activated macrophages, 30 and can antagonize the biological activities of IL‐1. 31 TGF‐β1 generally inhibits the production of prostaglandins from intrauterine tissues, including amnion 32,33 and decidual 34 cells stimulated with inflammatory factors, but it potentiates epidermal growth factor (EGF)‐stimulated PGE2 production from amnion cells. 35 As the functions of cells in intact decidua or placenta differs from those of isolated cells, 36–38 we have assessed the effects of TGF‐β1 on prostaglandin output from intact fetal membranes. The main regulatory point controlling prostaglandin production is considered to be the expression of the inducible enzyme COX‐2. 9,39–43 In particular, it is clear that high levels of mRNA for COX‐2 are linked to high levels of PGE2 output from fetal membranes, 8 and this is independent of whether the activation of the membranes is spontaneous (in vivo) or caused by experimental stimulation (in vitro). 8,44 We will therefore investigate the role of COX‐2 in our findings. The substrate for COX‐2 (arachidonic acid) is liberated from cell membrane stores by the action of phospholipase A2 enzymes, and the cytosolic form (cPLA2) has been implicated in parturition. 45 In most, but not all, tissues pre‐existing cPLA2 is activated (by phosphorylation), 46,47 and Ca2+‐dependent translocation to the endoplasmic reticulum (ER) and nuclear membranes 48,49 rather than there being increased enzyme synthesis. We intended initially to study only COX enzyme expression, but it became apparent that the effects of TGF‐β1 on PGE2 output so resembled those of IL‐1β 44 that further studies on cPLA2 were necessary.
Materials and methods
The methods used in this study are essentially those described in our recent papers. 8,44 The main features are given here.
Tissue collection and culture
Fetal membranes were collected from uncomplicated pregnancies at term (38–40 weeks gestation) after elective Caesarean section in the absence of labour or infection. Patients were not suffering from pre‐eclampsia and had not taken anti‐inflammatory drugs for 2 weeks prior to delivery. Ethics committee approval was obtained to use tissues which would normally be discarded. Tissue was washed with phosphate‐buffered saline containing 10% penicillin, streptomycin and l‐glutamine (Sigma, Poole, UK). 1·5 cm discs of intact tissue were cut using a sharpened punch and cultured in Medium 199 supplemented with ITS (insulin, transferrin, selenium and linoleic acid all at 0·63 mg/ml, and bovine serum albumin at 0·13 mg/ml (Sigma)) in multiwell tissue culture plates for 24 hr at 37° in an atmosphere of 5% CO2 : 95% air. The medium was also supplemented with 1 mm aspirin, as we have shown that fetal membranes may be preactivated and spontaneously release high levels of PGE2. 8 After this time the medium was changed, the tissue washed with medium containing no aspirin, and incubated with IL‐10 (alone or with lipopolysaccharide; LPS), or TGF‐β1 (alone or with IL‐1α or IL‐1β) for various time periods. All cytokines were added at 1 ng/ml, and LPS at 10 ng/ml ∼ these concentrations were chosen on the basis of previous experience or pilot studies. Samples from different membranes were used for the IL‐10 and TGF‐β1 studies. The tissues were snap frozen in liquid nitrogen and stored at –80°. The supernatants were then removed and frozen at –20° until analysis for PGE2 levels by enzyme‐linked immunosorbent assay (ELISA) (Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK). The coefficients of variation were 7·5–8·1% (within assay) and 10·6% (between assay). Levels of PGE2 and IL‐1β were determined in at least five separate replicate experiments of each culture condition.
LPS was used as a general inflammatory stimulus which stimulated the production of both IL‐1β and PGE2, making it possible to assess the effects of IL‐10 on two different aspects of fetal membrane function which may be linked to labour. The surprising effects of TGF‐β1 on PGE2 output resembled those of IL‐1α and IL‐1β reported previously, 44 so we used co‐incubations to assess further the similarities and difference in the effects of these cytokines.
RNA extraction and reverse transcription–polymerase chain reaction (RT–PCR)
Fetal membrane discs were selected for further analysis on the basis of their output of PGE2 or IL‐1β in response to the stimuli and other cytokines used. Discs were taken from three separate replicate experiments. Total RNA was extracted and stored at –80° until use. 50 RNA samples (1 µg) were used as the template for the production of cDNAs and stored at –80° until PCR amplification. 44 The primers used have been reported previously: COX‐1 and COX‐2; 51 cPLA2 and sPLA2; 44 glyceraldehyde phosphate dehydrogenase (GAPDH). 52 PCR was performed according to the manufacturer’s method (Bioline). Primer annealing temperatures were 58° for GAPDH and COX‐2; 55° for sPLA2 and cPLA2. Cycle profiles were performed as described previously to ensure that the exponential phase was used to amplify the products. 51 The cycle numbers chosen are indicated in the figure legends. Aliquots of the PCR products were separated by agarose gel electrophoresis and visualized under UV light.
To quantitate the PCR, 5 µl of each PCR reaction was dotted onto Hybond nylon filters. The filters denatured, neutralized and washed and the DNA was fixed to the filters by UV crosslinking. Filters were first prehybridized for 1–2 hr at 65° followed by hybridization overnight with the appropriate 32P dicytosine triphosphate (dCTP)‐labelled cDNA probe at 65°. Excess probe was removed by washing in buffers of increasing stringency to 0·1 × sodium saline citrate (SSC). The levels of cDNA were then determined by β‐counting of the filter sections. The expression of each product was then expressed as a ratio relative to the expression of GAPDH. The ratios were then represented as a fold increase or decrease in mRNA expression.
Immunoblot analysis for protein phosphorylation of cPLA2
Fetal membrane discs were homogenized in buffer (containing 1 mm sodium orthovanadate and the protease inhibitors pepstatin A (1 µm), phenylmethylsulphonyl fluoride (PMSF) (0·1 mm), and E‐64 (trans‐epoxysuccinyl l‐leucylamido‐(4‐guanidino)butane) (10 µm)). Total lysate was centrifuged for 10 min at 13000 g to pellet the cell debris. Protein levels in the supernatant were determined by the method of Bradford 53 using bovine serum albumin (BSA) as the standard and 200 µg of each sample was loaded on a 12% sodium dodecyl sulphate (SDS)–polyacrylamide gel. The stacking gel used an acrylamide : bis‐acrylamide ratio of 30 : 0·5 and 67 mm imidazole to improve protein band resolution, 54 and hence demonstrate a phosphorylation‐stimulated shift in cPLA2 mobility. 47,55 Purified cPLA2 was also included on each gel as a positive control (Genetics Institute, Cambridge, MA). The proteins were electrophoretically transferred to a nitrocellulose filter, which was blocked in phosphate‐buffered saline (PBS) containing 0·1% Tween and 5% non‐fat milk. The filter was incubated with a rabbit polyclonal immunoglobulin G (IgG) antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), followed by an anti‐rabbit IgG antibody labelled with peroxidase (Sigma). Immunolocalized proteins were detected using a chemiluminescent detection system (ECL, Amersham, Pharmacia Biotech). The filters were also incubated with a β‐actin goat polyclonal antibody to verify equal loading of the samples. The presence of COX‐1 and COX‐2 protein was assessed on the same filters after they were stripped, washed, blocked as before and incubated with either a COX‐1 or COX‐2 goat polyclonal antibody (Santa Cruz). Human platelets were used as a positive control for COX‐1, and for COX‐2 lymphocytes stimulated with LPS were used.
Immunoblot detection of cPLA2 translocation from the cytosol to the membrane
Fetal membrane discs were homogenized in 0·5 ml translocation buffer and the total lysate was centrifuged to remove cell debris. Cytosol (supernatant) and membrane (pellet) fractions were generated by ultracentrifugation at 100 000 g for 1 hr at 4°. 100 µg of protein from the cytosol fractions and the whole membrane fraction were loaded on a 12% SDS–polyacrylamide gel. The proteins were electroblotted and cPLA2 and β‐actin were immunodetected as described above.
Analysis of immunoblots
The autoradiographs of each blot were scanned by a UMAX Mirage D‐16L scanner (UMAX Technologies Inc., Fremont, CA) and the integrated density of each band determined using the software package Whole Band Analyser (Bioimage Services Ann Arbor, MI). The integrated density for cPLA2 or COX‐2 was then expressed relative to the density of β‐actin for each sample.
Statistical analysis
The results are shown as mean± SEM. For statistical analysis, the results were log‐transformed and investigated by anova with post hoc analysis by Fisher’s exact test. Differences of P < 0·05 were considered to be significant.
Results
LPS increased the output of PGE2 from human fetal membranes three‐ to fourfold (Fig. 1a). Co‐incubation with IL‐10 diminished the effect of LPS, and IL‐10 also decreased basal PGE2 output (Fig. 1a). All fetal membrane samples expressed mRNA for COX‐2 (Fig. 1b). Quantification of mRNA levels showed that IL‐10 decreased both LPS‐stimulated and basal COX‐2 levels (Fig. 1c).
Figure 1.
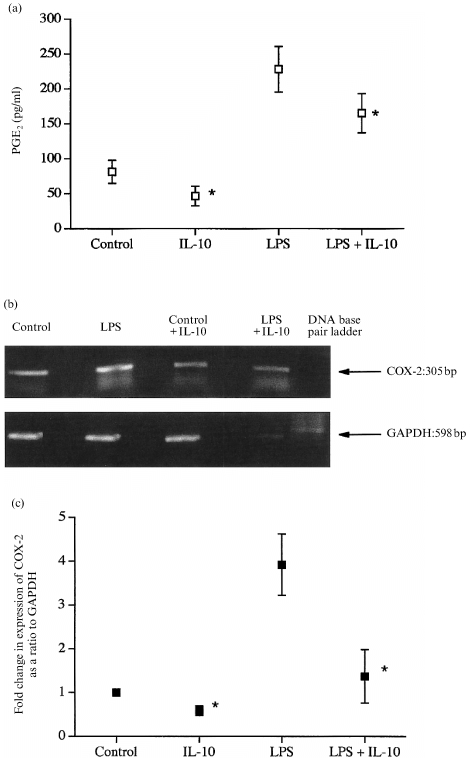
(a) The effects of IL‐10 and LPS on the production of PGE2 from human fetal membranes. All data are means± SEM (n = 5). (b) The presence of mRNA for COX‐2 in typical samples. (c) Quantification of the changes in levels of mRNA for COX‐2 in samples after 8 hr of culture. All data are means± SEM (n = 3). * P < 0·05 versus corresponding samples not containing IL‐10.
LPS also increased the output of IL‐1β from the intact fetal membranes (Fig. 2a). IL‐10 substantially inhibited this increased IL‐1β output (Fig. 2a), whereas alone it had little effect on basal IL‐1β production (Fig. 2a). Fetal membranes expressed the mRNA for IL‐1b (Fig. 2b), and quantification showed that IL‐10 completely inhibited the LPS‐stimulated increased in mRNA for IL‐1β (Fig. 2c).
Figure 2.
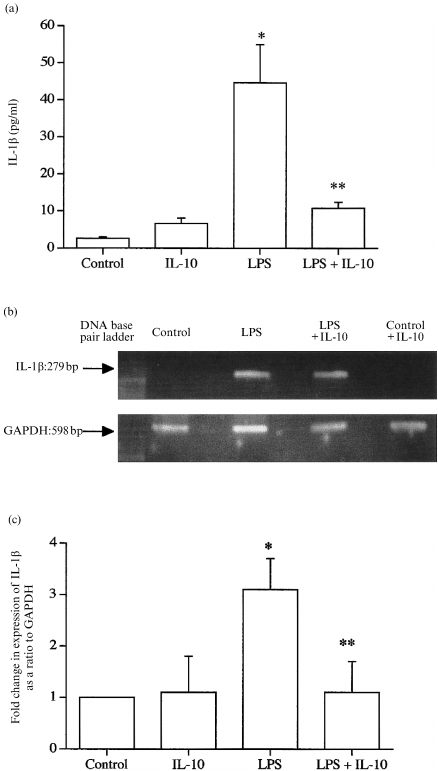
(a) The effects of IL‐10 and LPS on the production of IL‐1β from human fetal membranes. All data are means± SEM (n = 6). (b) The presence of mRNA for COX‐2 in typical samples. (c) Quantification of the changes in levels of mRNA for COX‐2 in samples after 8 hr of culture. All data are means± SEM (n = 3). * P < 0·05 versus control; ** P < 0·05 versus LPS alone.
TGF‐β1 increased the production of PGE2 from fetal membranes after both 4 hr of culture (Fig. 3a) and after 8 hr; control, 41·0 ± 16·6 pg PGE2/ml, TGF‐β1, 110·6 ± 19·5 pg PGE2/ml (triplicate results from a single experiment, typical of three separate experiments). The combination of TGF‐β1 with IL‐1β had similar effects (Fig. 3a), but TGF‐β1 with IL‐1α did not significantly increase PGE2 output. The expression of mRNA for COX‐2 in these samples was variable, so that there were no statistically significant changes (Fig. 3b). We therefore studied levels of COX‐2 protein by Western blotting, and found that the protein was present in all samples (Fig. 4a). TGF‐β1 alone, or in combination with IL‐1β, increased COX‐2 protein levels (Fig. 4b). The combination of TGF‐β1 and IL‐1α was without significant effect, although the mean level of mRNA was similar to the other data (Fig. 4b).
Figure 3.
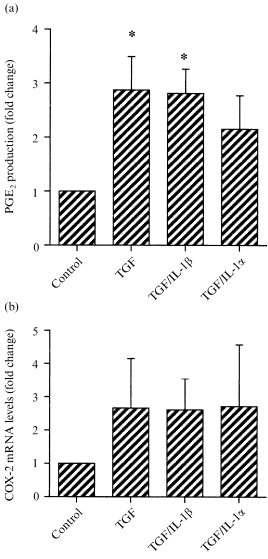
The effects of TGF‐β1 alone, or in combination with IL‐1β or IL‐1α on (a) the output of PGE2 from intact fetal membranes during 4 hr of culture or (b) the expression of mRNA for COX‐2. All cytokines were present at 1 ng/ml. PGE2 output was compared to control from triplicate determinations from five separate replicate experiments (means± SEM). Basal PGE2 output was 20–80 pg PGE2/ml/4 hr, with a maximum stimulated output of 50–200 pg/ml/4 hr. COX‐2 mRNA levels were assessed in fetal membranes from four separate experiments (means± SEM). * P < 0·05 versus control.
Figure 4.
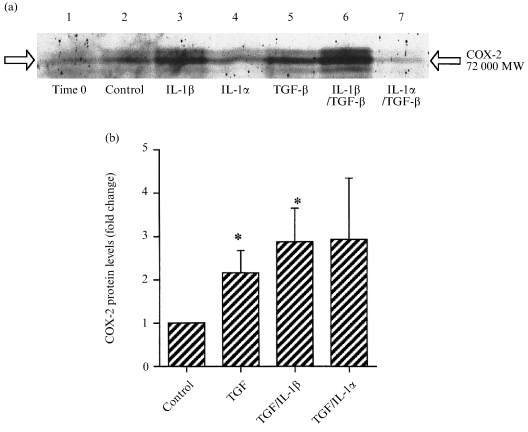
Studies on the presence of COX‐2 protein in intact fetal membranes under the culture conditions shown. (a) Immunoblot for COX‐2 protein from different membranes. (b) Quantification of changes in COX‐2 protein normalized to β‐actin after 4 hr stimulation with the cytokines shown (means± SEM n = 3). * P < 0·05 versus control.
To further explore the mechanism of action of TGF‐β1 on human fetal membranes, we investigated the effects of this cytokine on cPLA2. Levels of mRNA were unaffected (Fig. 5a), but protein concentration was increased twofold by TGF‐β1 (Fig. 5b). This increase was abrogated by IL‐1α or IL‐1β (Fig. 5b).
Figure 5.
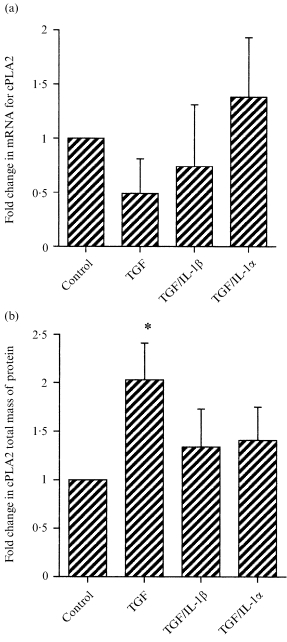
The effects of TGF‐β1 alone, or in combination with IL‐1β or IL‐1α on (a) levels of mRNA for cPLA2 or (b) levels of cPLA2 protein. In (a) the expression of mRNA was normalized to GAPDH prior to expression relative to control tissue samples. In (b) cPLA2 levels were normalized to β‐actin levels and expressed as a fold change (means ± SEM n = 4). * P < 0·05 versus control.
Post‐translational changes involved in the activation of cPLA2, namely phosphorylation and translocation from the cytosol to the membrane, were also investigated. The phosphorylated and dephosphorylated forms of cPLA2 could be resolved with polyacrylamide gel electrophoresis (PAGE) (Fig. 6a). Treatment with TGF‐β1 increased the level of phosphorylated cPLA2 compared to control (Fig. 6b). Co‐incubation with IL‐1β had no additional effect, and IL‐1α inhibited the changes in P‐cPLA2 caused by TGF‐β1 (Fig. 6b).
Figure 6.
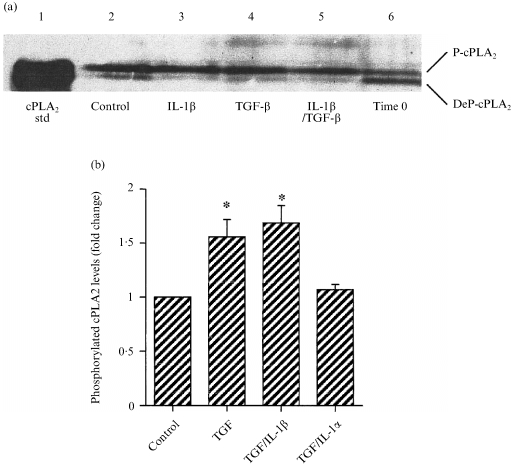
(a) The separation of phosphorylated (P‐cPLA2) and dephosphorylated (DeP‐cPLA2) forms of cPLA2 by PAGE, followed by immunoblotting. Time 0 samples were cut from the tissues and frozen immediately at –70°. (b) Quantification of the levels of P‐cPLA2 from replicate experiments, after 2 hr incubation with the cytokines shown. Data were corrected for β‐actin levels and expressed as a fold change (means± SEM n = 4). * P < 0·05 versus control.
Cytosolic and membrane fractions of intact fetal membranes were prepared as described in the Methods section. Phosphorylated and de‐phosphorylated forms of cPLA2 could be visualized in both fractions (Fig. 7a). Quantification of the data showed that TGF‐β1 increased the translocation of cPLA2 to the membrane fraction (Fig. 7b). The inclusion of IL‐1β or IL‐1α did not alter the effect of TGF‐β1.
Figure 7.
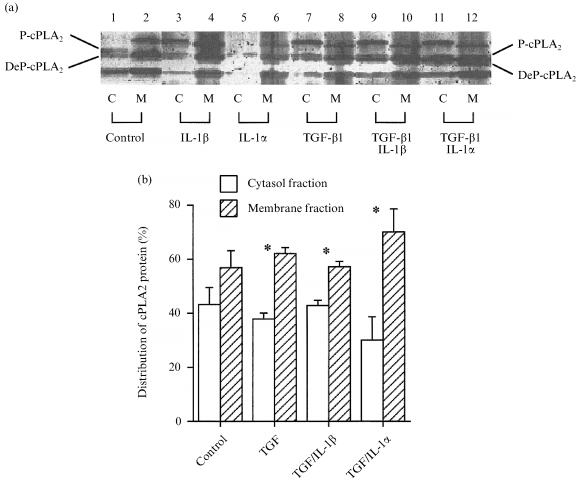
(a) The separation of P‐cPLA2 and DeP‐cPLA2 from cytosolic (C) and membrane (M) fractions of intact fetal membranes, followed by immunoblotting. (b) Distribution of total cPLA2 after correction for β‐actin levels (means±SEM n = 3). * P < 0·05 for difference between cytosol and membrane fractions for each treatment group.
Discussion
The data obtained with IL‐10 were consistent with the broadly anti‐inflammatory profile of this cytokine established in previous studies on fetal membranes. 32–34 The down‐regulation of IL‐1β protein and mRNA in LPS‐stimulated tissues was complete and clear‐cut (Fig. 2), which was consistent with a direct effect of IL‐10 on the induction process as reported in other cell‐types. 21,22 IL‐10 had no effect on IL‐1β when added alone, but inhibited both basal and LPS‐stimulated PGE2 production and COX‐2 expression (Fig. 1). In other tissues, IL‐10 has variable effects on prostaglandin production; in human neutrophils and monocytes IL‐10 decreased the production of PGE2, which is linked to decreased expression of COX‐2 protein and mRNA, 21,22 whereas in human alveolar cells IL‐10 had little effect on PGE2 production, 23 and in mouse mast cells IL‐10 potentiated the effects of other stimuli. 24 Our data do not discriminate between a direct effect of IL‐10 on PGE2 production from fetal membranes, and one mediated through changes in IL‐1β expression, although the difference in effect of IL‐10 on basal PGE2 and on basal IL‐1β output would indicate that separate mechanisms are involved. Further studies are needed to resolve this question, but from a physiological viewpoint it is clear that IL‐10 is potentially a pro‐pregnancy factor via the inhibition of inflammatory pathways. This is consistent with data from other studies, 21,22,25–27 which show that Th2 cytokines (including IL‐10) are linked to normal pregnancy. 56,57
The data obtained with TGF‐β1 were in marked contrast to most of the previous studies on intrauterine tissues which showed that this cytokine was anti‐inflammatory. 32–34 All those studies were done on isolated cells, and the difference in the data obtained may be linked to this. It has been shown that tissue dissociation increases the production of TGF‐β2 (and many other cytokines) from first trimester human decidua, 37 and it seems reasonable that the responses to cytokines may be altered. Comparison with other studies on intact fetal membrane shows that TGF‐β1 had effects very similar to those of IL‐1α and IL‐1β. 44 PGE2 output was increased two‐ to threefold by all three cytokines, linked to moderate changes in mRNA and protein for COX‐2 – again two‐ to threefold (Figs 3 and 4), with varying degrees of statistical significance. The phosphorylation of cPLA2 was increased 1·5‐fold (Fig. 6 and 44), and cPLA2 was redistributed into the membrane faction (Fig. 7). One major difference was that TGF‐β1 increased the levels of cPLA2 protein (Fig. 5), whereas IL‐1α or IL‐1β were without effect. 44
The combination of TGF‐β1 and IL‐1β generally had similar effects to the individual cytokines (PGE2, COX‐2 mRNA and protein, cPLA2 phosphorylation and redistribution) – the only exception was that IL‐1β diminished the effect of TGF‐β1 on cPLA2 total protein. There was no evidence that TGF‐β1 inhibited the effects of IL‐1β on intact fetal membranes, and it was therefore not exhibiting anti‐inflammatory activity.
IL‐1α generally had more variable effects on fetal membranes than did IL‐1β, 44 and this also applied to the combination of TGF‐β1 with IL‐1α. The only significant change was in the distribution of cPLA2 (Fig. 7b). The combination of cytokines had limited effects on PGE2 production and COX‐2 mRNA and protein (Figs 3 and 4), but the quantity and phosphorylation of cPLA2 were unaffected (Figs 5 and 6). TGF‐β1 did diminish the effects of IL‐1α on PGE2 output (Fig. 3), and on the levels of phosphorylated cPLA2 (Fig. 6), but otherwise it could not be stated that TGF‐β1 inhibited the effects of IL‐1α.
We have shown in this study that TGF‐β1 increases PGE2 production from intact fetal membranes after 4 hr or 8 hr of treatment. Other studies performed on amnion or decidual cells found TGF‐β1 to inhibit PGE2 production in the presence of IL‐1, 32–34 whereas TGF‐β1 potentiated the effects of EGF. 35 However, our study was performed using intact tissue and may therefore be a better representative model of the events that take place in vivo compared to those in isolated cells. 36–38 In particular, enzymatic digestion of decidual tissue activates production of TGF‐β2 and so is likely to also increase the production of TGF‐β1· 37 This may contribute to the different findings obtained from digested and intact tissues.
The mechanisms by which TGF‐β1 increased PGE2 output were compared with those of IL‐1α and IL‐1β. 44 None of the factors used in this study affected the expression of mRNA for cPLA2, although TGF‐β1 increased cPLA2 protein levels, and was the only factor to do so (Fig. 2). There was no shift from non‐phosphorylated cPLA2 to the phosphorylated (active) form in the presence of TGF‐β1, but the increase in protein led to an overall increase in phosphorylated cPLA2 (Fig. 3). This may be contrasted with the interleukins, which caused a shift in phosphorylation without affecting cPLA2 protein levels. 10 All the cytokines caused translocation of cPLA2 from cytosol to cell membranes, which is consistent with increased release of arachidonic acid from membrane phospholipids. This analysis may not reflect total cPLA2 levels, as some membranes will be lost in the initial sample preparation, but should reflect the relative distribution between cytosol and membrane fractions.
The important point is that TGF‐β1 had similar effects on COX‐2 protein and mRNA as did the pro‐inflammatory interleukins, indicating the TGF‐β1 is not functioning as the anti‐inflammatory factor we anticipated on the basis of previous data. 32–34 A TGF‐β response element is present in the human COX‐2 promoter, 58 and a similar response element may mediate the stimulatory effects of TGF‐β1 on COX‐2 expression and PGE2 production in murine osteoblastic cells. 59 These findings provide a precedent for our data. Other studies have indicated that TGF‐β can interact in complex ways with many other cytokines, including IL‐1β, IL‐1 receptor antagonist, 34,60 the IL‐2–IL‐2 receptor pathway 30 and IL‐6. 61 Human fetal membranes are very heterogeneous at the cellular level, and further studies are needed to investigate the possible cross‐talk between different cells and cytokines.
We conclude that cytokines whose functions have been identified in other tissues may not exert similar effects in human fetal membranes, and that detailed studies are required for each cytokine. IL‐10 had the anti‐inflammatory effects expected from previous studies, whereas TGF‐β1 more closely resembled a pro‐inflammatory cytokine.
Acknowledgments
The authors would like to thank Action Research (NLB) and WellBeing (SAA) for supporting this work. We would also like to thank the Genetics Institute Inc, MA, USA for supplying us with a sample of pure cPLA2 protein. We also gratefully acknowledge Dr D. Slater for the cloning of the cDNA probes, and Dr S. Dilworth for the use of his image scanner and the Bioimage Whole Band Analyser software.
References
- 1.Duchesne MJ, Thaler‐dao H, Crastes de Paulet A. Prostaglandin synthesis in human placenta and fetal membranes. Prostaglandins. 1978;15:19. doi: 10.1016/s0090-6980(78)80003-3. [DOI] [PubMed] [Google Scholar]
- 2.Olson DM, Zakar T. Intrauterine tissue prostaglandin synthesis: Regulatory mechanisms. Semin Reprod Endocrinol. 1993;11:234. [Google Scholar]
- 3.Nieder J, Augustin W. Increase of prostaglandin E and F equivalents in amniotic fluid during late pregnancy and rapid PGF elevation after cervical dilation. Prost Leukotr Med. 1983;12:289. doi: 10.1016/0262-1746(83)90007-0. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Emamian M, Wan M, Qunitero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra‐amniotic infection and preterm labor. Am J Obstet Gynecol. 1988;157:1461. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labour at term. Am J Obstet Gynecol. 1994;171:1613. doi: 10.1016/0002-9378(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 6.Green K, Bygdeman M, Toppozada M, Wiqvist N. The role of prostaglandin F2α in human parturition. Am J Obstet Gynecol. 1974;120:25. [PubMed] [Google Scholar]
- 7.Lorenz RP, Botti JJ, Chez RA, Bennett N. Variations of biologic activity of low‐dose prostaglandin E2 on cervical ripening. Obstet Gynecol. 1984;64:123. [PubMed] [Google Scholar]
- 8.Brown NL, Alvi SA, Elder MG, Bennett PR, Sullivan MHF. A spontaneous induction of fetal membrane prostaglandin production preceeds clinical labour. J Endocrinol. 1998;157:R1. doi: 10.1677/joe.0.157r001. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell MD, Edwin SS, Lundin‐schiller S, Silver RM, Smotkin D, Trautman MS. Mechanism of interleukin‐1 beta stimulation of human amnion prostaglandin biosynthesis: mediation via a novel inducible cyclooxygenase. Placenta. 1993;14:615. doi: 10.1016/s0143-4004(05)80379-0. [DOI] [PubMed] [Google Scholar]
- 10.Norwitz ER, Lopez Bernal A, Starkey PM. Tumor necrosis factor‐α selectively stimulates prostaglandin F2α production by macrophages in human term decidua. Am J Obstet Gynecol. 1992;167:815. doi: 10.1016/s0002-9378(11)91595-6. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin‐6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol. 1991;192:189. doi: 10.1016/0014-2999(91)90090-d. [DOI] [PubMed] [Google Scholar]
- 12.Ozornek MH, Bielfeld P, Krussel JS, et al. Interferon gamma and interleukin 10 levels in preimplantation embryo culture media. J Assist Reprod Genet. 1995;12:590. doi: 10.1007/BF02212580. [DOI] [PubMed] [Google Scholar]
- 13.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Amniotic fluid interleukin‐10 (IL‐10) concentrations during pregnancy and with labor. J Reprod Immunol. 1997;33:147. doi: 10.1016/s0165-0378(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 14.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paradowska E, Blach‐olszewska Z, Gejdel E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta. 1997;18:441. doi: 10.1016/s0143-4004(97)80045-8. [DOI] [PubMed] [Google Scholar]
- 16.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by hormone‐controlled TH1‐ and TH2‐type cytokines. Immunol Res. 1996;15:141. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 17.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdan C. Macrophage deactivation by IL‐10. J Exp Med. 1991;174:1549. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawelec G, Rehbein A, Schlotz E, Friccius H, Pohla H. Cytokine modulation of TH1/TH2 phenotype differentation in directly alloresponsive CD4+ human T cells. Transplantation. 1996;62:1095. doi: 10.1097/00007890-199610270-00013. [DOI] [PubMed] [Google Scholar]
- 20.Dudley DJ, Edwin SS, Dangerfield A, Jackson K, Trautman MS. Regulation of decidual cell and chorion cell production of interleukin‐10 by purified bacterial products. Am J Reprod Immunol. 1997;38:246. doi: 10.1111/j.1600-0897.1997.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 21.Niiro H, Otsuka T, Tanabe T, et al. Inhibition by interleukin‐10 of inducible cyclooxygenase expression in lipopolysaccharide‐stimulated monocytes: its underlying mechanism in comparison with interleukin‐4. Blood. 1995;85:3736. [PubMed] [Google Scholar]
- 22.Niiro H, Otsuka T, Izuhara K, et al. Regulation by interleukin‐10 and interleukin‐4 of cyclooxygenase‐2 expression in human neutrophils. Blood. 1997;89:1621. [PubMed] [Google Scholar]
- 23.Endo T, Ogushi F, Kawano T, Sone S. Comparison of the regulations by Th2‐type cytokines of the arachidonic‐acid metabolic pathway in human alveolar macrophages and monocytes. Am J Respir Cell Molec Biol. 1998;19:300. doi: 10.1165/ajrcmb.19.2.2915. [DOI] [PubMed] [Google Scholar]
- 24.Moon TC, Murakami M, Ashraf MD, Kudo I, Chang HW. Regulation of cyclooxygenase‐2 and endogenous cytokine expression by bacterial lipopolysaccharide that acts in synergy with c‐kit ligand and Fc epsilon receptor I crosslinking in cultured mast cells. Cell Immunol. 1998;185:146. doi: 10.1006/cimm.1998.1284. [DOI] [PubMed] [Google Scholar]
- 25.Fortunato SJ, Menon R, Swan KE, Lombardi SJ. Interleukin‐10 inhibition of interleukin‐6 in human amniochorionic membrane: transcriptional regulation. Am J Obstet Gynecol. 1996;175:1057. doi: 10.1016/s0002-9378(96)80053-6. [DOI] [PubMed] [Google Scholar]
- 26.Fortunato SJ, Menon R, Lombardi SJ. Interleukin‐10 and transforming growth factor‐beta inhibit amniochorion tumor necrosis factor‐alpha production by contrasting mechanisms of action: therapeutic implications in prematurity. Am J Obstet Gynecol. 1997;177:803. doi: 10.1016/s0002-9378(97)70272-2. [DOI] [PubMed] [Google Scholar]
- 27.Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin‐10 on interleukin‐8 release by human amniochorion may regulate histologic chorioamnionitis. Am J Obstet Gynecol. 1998;179:794. doi: 10.1016/s0002-9378(98)70085-7. [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localisation of transforming growth factor‐β at the human fetal–maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;6:561. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- 29.Kauma S, Matt D, Strom S, Eierman D, Turner T. Interleukin‐1β, human leukocyte antigen HLA‐DRα, and transforming growth factor‐β expression in endometrium, placenta, and placental membranes. Am J Obstet Gynecol. 1990;163:1430. doi: 10.1016/0002-9378(90)90601-3. [DOI] [PubMed] [Google Scholar]
- 30.Wahl SM, McCartney‐francis N, Mergenhagen SE. Inflammatory and immunomodulatory roles of TGF‐β. Immunol Today. 1989;10:258. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- 31.Dubois CM, Ruscetti FW, Palaszynski EW, Falk LA, Oppenheim JJ, Keller JR. Transforming growth factor β is a potent inhibitor of interleukin 1 (IL‐1) receptor expression: proposed mechanism of inhibition of IL‐1 action. J Exp Med. 1990;172:737. doi: 10.1084/jem.172.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bry K, Hallman M. Transforming growth factor‐beta opposes the stimulatory effect of interlukin‐1 and tumor necrosis factor on amnion cell prostaglandin E2 production: implication for preterm labor. Am J Obstet Gynecol. 1992;167:222. doi: 10.1016/s0002-9378(11)91662-7. [DOI] [PubMed] [Google Scholar]
- 33.Bry K, Lappalainen U, Hallman M. Interleukin‐1 binding and prostaglandin E2 synthesis by amnion cells in culture: regulation by tumor necrosis factor‐alpha, transforming growth factor‐beta and interleukin‐1 receptor antagonist. Biochim Biophys Acta. 1993;1181:31. doi: 10.1016/0925-4439(93)90086-g. [DOI] [PubMed] [Google Scholar]
- 34.Bry K, Lappalainen U. Interleukin‐4 and transforming growth factor‐β1 modulate the production of interleukin‐1 receptor antagonist and of prostaglandin E2 by decidual cells. Am J Obstet Gynecol. 1994;170:1194. doi: 10.1016/s0002-9378(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 35.Kniss DA, Zimmerman PD, Fertel RH, Iams JD. Transforming growth factor‐β potentiates epidermal growth factor‐induced prostaglandin E2 production in amnion cells. Prostaglandins. 1993;45:27. doi: 10.1016/0090-6980(93)90087-n. [DOI] [PubMed] [Google Scholar]
- 36.Kauma SW, Walsh SW, Nester JE, Turner TT. Interleukin‐1 is induced in the human placenta by endotoxin and isolation procedures for trophoblast. J Clin Endocrinol Metab. 1992;75:951. doi: 10.1210/jcem.75.3.1517391. [DOI] [PubMed] [Google Scholar]
- 37.Lonsdale LB, Elder MG, Sullivan MHF. A comparison of cytokine and hormone production by decidual cells and tissue explants. J Endocrinol. 1996;151:309. doi: 10.1677/joe.0.1510309. [DOI] [PubMed] [Google Scholar]
- 38.Qin X, Garibay‐tupas J, Chua PK, Cachola L, Bryant‐greenwood GD. An autocrine/paracrine role of human decidual relaxin. I. Interstitial collagenase (matrix metalloproteinase‐1) and tissue plasminogen activator. Biol Reprod. 1997;56:800. doi: 10.1095/biolreprod56.4.800. [DOI] [PubMed] [Google Scholar]
- 39.Hla T, Farrell M, Kumar A, Bailey JM. Isolation of the cDNA for human prostaglandin H synthase. Prostaglandins. 1986;32:829. doi: 10.1016/0090-6980(86)90093-6. [DOI] [PubMed] [Google Scholar]
- 40.Hla T, Neilson K. Human cyclo‐oxygenase‐2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi Y, Ueda N, Yoshimoto T, et al. Immunoaffinity purification and cDNA cloning of human platelet prostaglandin endoperoxide synthase (cyclo‐oxygenase) Biochem Biophys Res Commun. 1992;182:433. doi: 10.1016/0006-291x(92)91750-k. [DOI] [PubMed] [Google Scholar]
- 42.Albert TJ, Su H‐C, Zimmerman PD, Iams JD, Kniss DA. Interleukin‐1β regulates the inducible cyclooxygenase in amnion‐derived wish cells. Prostaglandins. 1994;48:401. doi: 10.1016/0090-6980(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 43.Angel J, Berenbaum F, Le Denmat C, Nevalainen T, Masliah J, Fournier C. Interleukin‐1‐induced prostaglandin E2 biosynthesis in human synovial cells involves the activation of cytosolic phospholipase A2 and cyclooxygenase‐2. Eur J Biochem. 1994;226:125. doi: 10.1111/j.1432-1033.1994.tb20033.x. [DOI] [PubMed] [Google Scholar]
- 44.Brown NL, Alvi SA, Elder MG, Bennett PR, Sullivan MHF. The regulation of prostaglandin production in intact fetal membranes by interleukin‐1 and its receptor antagonist. J Endocrinol. 1998;159:519. doi: 10.1677/joe.0.1590519. [DOI] [PubMed] [Google Scholar]
- 45.Skannal DG, Brockman DE, Eis ALW, Xue S, Siddiqi TA, Myatt L. Changes in activity of cytosolic phospholipase A2 in human amnion at parturition. Am J Obstet Gynecol. 1997;177:179. doi: 10.1016/s0002-9378(97)70459-9. [DOI] [PubMed] [Google Scholar]
- 46.Lin L‐L, Lin AY, Knopf JL. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci USA. 1992;89:6147. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L‐L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 48.Clark JD, Lin L‐L, Kriz RW, et al. A novel arachidonic acid‐selective cytosolic PLA2 contains a Ca2+‐dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 49.Glover S, Bayburt T, Jonas M, Chi E, Gelb MH. Translocation of the 85‐kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 50.Chomczynski P, Sacchi N. Single‐step method of RNA isolation by guanidinium–thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 51.Slater DM, Berger LC, Newton R, Moore GE, Bennett PR. Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol. 1995;172:77. doi: 10.1016/0002-9378(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 52.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterisation of rat and human glyceraldehyde‐3‐phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucl Acids Res. 1985;13:2485. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 1976;72:248. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 54.Rittenhouse J, Marcus F. Peptide mapping by polyacrylamide gel electrophoresis after cleavage at aspartyl‐prolyl peptide bonds in sodium dodecyl sulfate‐containing buffers. Anal Biochem. 1984;138:442. doi: 10.1016/0003-2697(84)90836-4. [DOI] [PubMed] [Google Scholar]
- 55.Croxtall JD, Choudhury Q, Newman S, Flower RJ. Lipocortin 1 and the control of cPLA2 activity in A549 cells. Glucocorticoids block EGF stimulation of cPLA2 phosphorylation. Biochem Pharmacol. 1996;52:351. doi: 10.1016/0006-2952(95)02442-5. [DOI] [PubMed] [Google Scholar]
- 56.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 57.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T‐helper cytokines by decidual T cells in unexplained recurrent abortions. Nature Med. 1998;4:1020. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 58.Yang X, Hou F, Taylor L, Polgar P. Characterization of human cyclooxygenase 2 gene promoter localization of a TGF‐beta response element. Biochim Biophys Acta. 1997;1350:287. doi: 10.1016/s0167-4781(96)00225-4. [DOI] [PubMed] [Google Scholar]
- 59.Pilbeam C, Rao Y, Voznesensky O, et al. Transforming growth factor‐beta 1 regulation of prostaglandin G/H synthase‐2 expression in osteoblastic MC3T3‐E1 cells. Endocrinology. 1997;138:4672. doi: 10.1210/endo.138.11.5495. [DOI] [PubMed] [Google Scholar]
- 60.Wahl SM, Costa GL, Corcoran M, Wahl LM, Berger AE. Transforming growth factor‐β mediated IL‐1‐dependent induction of IL‐1 receptor antagonist. J Immunol. 1993;150:3553. [PubMed] [Google Scholar]
- 61.Turner M, Chantry D, Feldmann M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2:211. doi: 10.1016/1043-4666(90)90018-o. [DOI] [PubMed] [Google Scholar]


