Abstract
Dendritic cells (DC) are extremely efficient at generating both prophylactic and therapeutic anti‐tumour immunity. We aimed to analyse the respective roles of humoral and cellular immune responses generated in mice vaccinated with bone marrow (BM)‐derived DC in terms of in vivo anti‐leukaemia effect. We used the murine L1210 B lymphocytic leukaemia genetically modified to express on the cell surface of human CD4 (hCD4) (L1210/hCD4) as a model tumour‐associated antigen (TAA). DC cultures were loaded with either purified soluble hCD4 (shCD4) protein or unfractionated L1210/hCD4 extracts and injected as vaccine into mice. The efficacy of these vaccinations was compared with that of vaccination with shCD4 protein emulsified in Freund’s adjuvant (FA). We evaluated the immune responses generated after these vaccinal protocols and the survival rate of vaccinated mice subsequently challenged with a lethal injection of L1210/hCD4 cells. Our results demonstrated that vaccination with shCD4 protein or tumour extract‐loaded DC mainly generated an hCD4 antigen‐specific cell‐mediated cytotoxic immune response that was associated with a specific protection against leukaemia. In contrast, vaccination with the protein emulsified in FA only generated potent humoral immune responses that were not protective against leukaemia. Altogether, our results indicate that the unique property of loaded DC to trigger an anti‐leukaemia protective effect is mainly associated with cellular immune responses.
Introduction
The identification and characterization of a growing number of tumour‐associated antigens (TAA) in many neoplasms 1 has paved the way for new approaches in anti‐tumour immunotherapy. 2–8 Indeed, although TAA do not normally elicit protective anti‐tumour immune responses enabling prevention of tumour growth in immunocompetent hosts, they can be manipulated to trigger or reinforce such responses. One of the most efficient approaches relies on the potent antigen‐presenting capacity of dendritic cells (DC). 9,10 DC are bone marrow (BM)‐derived cells that are the most potent cells for antigen presentation and initiation of T‐cell‐dependent immune responses. 11 The DC network is a specialized system for presenting antigens to naive or quiescent T cells and, consequently, plays a central role in the induction of T‐ as well as B‐cell immunity in vivo. 12
Likewise, DC loaded with a tumour antigen can induce a state of prophylactic, and even therapeutic, anti‐tumour immunity in animal models. 13–18 These experimental results have encouraged the first clinical attempts to exploit DC for cellular immunotherapy against human cancers. 19–22
TAA include recombinant molecules, such as mutated oncogenes or tumour suppresser products, 23–25 as well as oncofetal antigens or other aberrantly expressed molecules such as T‐cell receptor (TCR) and immunoglobulin idiotypes. 26–28 Depending on their nature, TAA can be located in intracellular compartments (cytoplasm or nucleus) or on the surface (membrane) of tumour cells. All TAA, processed as peptides and presented on major histocompatibility complex (MHC) class I molecules, can be recognized by T lymphocytes, and membrane‐expressed TAA can also be recognized by antibodies. To date, there has been no direct comparison of the distinct role of humoral and cellular immunity in terms of in vivo protective anti‐tumour effects. The results obtained so far with the strategy involving TAA‐loaded DC are somewhat controversial. Most data demonstrate the effectiveness of this strategy by loading DC with MHC class I restricted‐TAA, in the form of peptides, to elicit potent antigen‐specific T‐cell‐mediated anti‐tumour immune responses. 29,30 In contrast, other results indicate that in vivo tumour protection can be associated with the induction of a specific humoral immune response. 31
In this study, we evaluated the role of humoral and cellular anti‐tumour immunity against the non‐immunogenic L1210 B lymphocytic leukaemia, expressing on the cell surface a model exogenous TAA, the human CD4 (hCD4) (L1210/hCD4). This antigen is able to induce protection against malignant tumour cell challenge by generating specific immune responses directed against hCD4 displayed on the tumour cells, as previously demonstrated in an anti‐tumour vaccination approach in mice based on DNA immunization. 32 In order to generate specific cellular or humoral immunity, we vaccinated mice with DC loaded with either purified soluble hCD4 (shCD4) protein, or unfractionated L1210/hCD4 extracts, or with shCD4 protein emulsified in Freund’s adjuvant (FA). Our results show that cellular‐ but not humoral‐based anti‐hCD4 immune responses have significant anti‐leukaemia effects.
Materials and methods
Animals
Six‐ to eight‐week‐old pathogen‐free female DBA/2 (H‐2d) mice were purchased from Iffa Credo (L’Arbresle, France). Mice were housed in a temperature‐controlled light‐cycled room. All in vivo experiments were performed in accordance with local ethical guidelines.
Tumour cell lines
The murine L1210 B lymphocytic leukaemia cell line (H‐2d), kindly provided by Pierre Golstein (Marseille, France), has been genetically modified by retroviral‐mediated gene transfer in order to express on the surface the hCD4 molecule. After 72 hr of co‐cultivation with the packaging cell line ΨCRIP/hCD4 (kindly provided by Olivier Schwartz, Paris, France) in the presence of 8 µg/ml of polybrene, the transduced L1210/hCD4 cells were separated by cell sorting (FACStarPlus; Becton Dickinson Co., Mountain View, CA) after staining with fluorescein isothiocyanate (FITC)‐labelled Leu‐3a antibody (Becton Dickinson). As target cells for cytotoxic T‐lymphocyte (CTL) analyses, we used the murine MC26 colon carcinoma cell line (H‐2d) kindly provided by Anne Kinsella (Liverpool, UK). MC26/hCD4 cells were obtained, as described above, by using the supernatant derived from ΨCRIP/hCD4 cell cultures, previously passed through a 0·45‐µm filter. Both hCD4‐expressing cells were subsequently propagated in vitro in culture medium. The L1210 and L1210/hCD4 cell lines were grown and maintained in RPMI‐1640 supplemented with 10% decomplemented fetal calf serum (FCS), 1% l‐glutamine and 1% antibiotics. The MC26 and MC26/hCD4 cell lines were grown in the same conditions but in Dulbecco’s modified Eagle’s minimal essential medium (DMEM).
Generation of BM‐derived DC
BM cells were obtained from DBA/2 mice as described elsewhere. 33 Briefly, they were cultured at 7 × 105 cells/ml in RPMI‐1640 supplemented with 5% FCS, 1% l‐glutamine, 20 µg/ml gentamicin, 50 µm 2‐β mercaptoethanol and 2000 U/ml recombinant murine (rm) granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (Genzyme, Cambridge, MA) in 24‐well plates at 37° in 5% CO2. On days 2, 4 and 6, 90% of the medium was replaced by cytokine‐containing medium. On day 7, cells in suspension were harvested and pooled with loosely adherent aggregates of growing DC, dislodged by vigorous pipetting. Cells were then counted, washed in phosphate‐buffered saline (PBS), characterized by flow cytometry and used for animal vaccination.
Loading of BM‐derived DC
With soluble antigen in the form of protein
. On day 5, cultured cells were loaded by addition of 10 µg/ml of the purified shCD4 protein, 34 and on day 7 cells were characterized and used as described above.
With unfractionated tumour extracts
Unfractionated tumour extracts were obtained by sonication of L1210/hCD4 cells followed by several freeze–thaw cycles. BM cells were loaded at day 4 of the culture by addition of tumour extracts using a ratio of BM‐derived DC and tumour cell equivalents (TCE) of 1 : 10 or 1 : 100. At day 6, the medium was renewed and fresh tumour extracts were added similarly until day 7. Cells were then collected, characterized and used as described above.
Flow cytometric analysis
Approximately 5 × 105–1 × 106 cells were suspended in PBS/3% FCS/0·02% sodium azide, centrifuged at 250 g for 5 min, resuspended in 100 µl of buffer, and then single or double labelled with saturating concentrations of monoclonal antibody (mAb).
Unlabelled M5/114 mAb (rat IgG; ATCC TIB120; ATCC, Rockville, MD) used against MHC class II molecules was revealed with a FITC‐conjugated F(ab′)2 goat anti‐rat IgG (Caltag Laboratories, Burlingame, CA). CD11c expression was analysed with unlabelled N418 mAb (hamster IgG; ATCC HB224) and revealed by a phycoerythrin (PE)‐conjugated F(ab′)2 goat anti‐hamster IgG (Caltag Laboratories).
Cells were fixed in 1% paraformaldehyde, and analyses were performed on a FACScan (Becton Dickinson).
In vivo mouse studies
Evaluation of tumour cell immunogenicity
The immunogenicity of unmodified L1210 and L1210/hCD4 was tested by pre‐immunization of mice with lethally irradiated tumour cells to protect against a subsequent challenge with live tumour cells. Groups of five DBA/2 mice were immunized once or twice, at weekly intervals, with a tumourigenic dose (105 cells/100 µl PBS/mouse) of lethally irradiated (25 Gray) L1210 or L1210/hCD4 cells, injected subcutaneously (s.c.). Two weeks after the last immunization, live L1210 or L1210/hCD4 cells were administered intravenously (i.v.) into the retro‐orbital sinus at 105 cells/100 µl PBS/mouse, and the survival of the mice was followed.
Vaccination with loaded and unloaded DC
Loaded DC harvested on day 7 of BM cultures, were irradiated (25 Gray) and injected i.v. into the retro‐orbital sinus of a group of DBA/2 mice (1–2 × 105 cells/100 µl PBS/mouse). Controls were mice identically injected with unloaded DC. Each group consisted of seven animals.
Vaccination with shCD4 protein emulsified in FA
A group of seven DBA/2 mice was vaccinated with the soluble purified hCD4 protein emulsified in FA. The first vaccination was accomplished intraperitoneally (i.p.) with 30 µg of the protein emulsified in complete Freund’s adjuvant (CFA). Two weeks later, the same amount of protein, emulsified in incomplete Freund’s adjuvant (IFA), was administered intramuscularly (i.m.) into the quadriceps muscle.
Anti‐leukaemia protection effect
Fourteen days post‐vaccination, with irradiated loaded or unloaded DC, or with the purified shCD4 protein emulsified in FA, DBA/2 mice were challenged with 105 L1210/hCD4 cells in 100 µl PBS, injected i.v. into the retro‐orbital sinus. Survival was recorded until > day 120.
In vitro assays
In vitro cytotoxicity assay
Two DBA/2 mice per group were killed 14 days post‐vaccination just before tumour challenge, to test CTL reactivities using standard procedures 35 with some modifications. Briefly, splenocytes were incubated in vitro for 3 days with irradiated L1210/hCD4 cells in the presence of 25 U/ml rm interleukin‐2 (rmIL‐2; Genzyme, Cambridge, MA). After restimulation, MC26/hCD4 or unmodified MC26 cells were labelled with 100 µCi/ml Na251CrO4 (Amersham, Les Ulis, France) for 30–60 min and incubated with the stimulated splenocytes for 4 hr at 37°. CTL activity was finally evaluated at an effector to target (E:T) ratio ranging from 100 : 1 to 1 : 1. Supernatant samples were harvested and counted in triplicate in a Wallac 1450 MicroBeta liquid scintillation counter (Turku, Finland). Percentage specific 51Cr release was determined by subtracting spontaneous release from experimental group release and dividing by maximum release minus spontaneous release. Maximum release was determined by treatment of target cells with 1% Triton X‐100 containing medium.
Determination of antibody‐mediated immune responses (enzyme‐linked immunosorbent assay; ELISA)
Blood was harvested at weekly intervals after vaccination and sera were serially diluted (from 1 : 10 to 1 : 10000) to detect the presence of anti‐hCD4 antibodies, as described previously. 36 Briefly, 96‐well microtitration plates (Dynatech Laboratories Inc., Chantilly, VA) were coated overnight at 4° with the soluble purified hCD4 protein at 30 ng/well in 50 µl of 0·05 m sodium carbonate buffer, pH 9·6. Wells were then saturated with 100 µl of PBS containing 8% non‐fat dry milk (Biorad, Hercules, CA) for 1 hr at room temperature (RT). Different dilutions of sera at 50 µl/well were added for 2 hr at RT. Monoclonal antibody Leu‐3a (Becton Dickinson) was used as positive control of the coating. Negative controls were done without serum and with serum of naive mice. Bound antibodies were revealed by the addition of a 1 : 1000 dilution of peroxidase‐conjugated rabbit immunoglobulin to mouse immunoglobulin (Dako Trappes, France) for 2 hr at RT.
All reagents were diluted in PBS containing 0·5% dry milk, 0·05% Tween‐20 (Biorad). At each step, extensive washes were done with PBS, 0·05% Tween. Each point was performed in duplicate. Non‐specific background levels were obtained with uncoated wells saturated under the same conditions. The enzymatic visualization was done by adding to each well 100 µl of the ready‐to‐use substrate ABTS solution (Boehringer Mannheim GmbH, Mannheim, Germany) for 30 min in the dark. Optical density (OD) were determined at 405 nm with a reference wavelength of 492 nm. Results were expressed as the mean of OD 405/492 on coated wells minus OD 405/492 on uncoated saturated wells.
Statistical analyses
Results were determined as mean±SE. Survival data and other observed differences were compared using the Fisher exact test and the unpaired Student’s t‐test, respectively, to calculate the statistical significance, determined at the < 0·05 level.
Results
L1210 and hCD4‐expressing L1210 cells are not immunogenic in vivo
The in vitro growth kinetics of L1210 cells and hCD4 derivatives were similar. Both cell lines expressed MHC class I but not class II molecules, as determined by flow cytometric analysis (data not shown).
To test the potential immunogenicity of L1210 and L1210/hCD4 cells, we analysed the effects of a previous immunization with one or two doses of lethally irradiated tumour cells on the outcome of a subsequent challenge with live tumour cells. Lethally irradiated L1210/hCD4 cell injection did not prevent or delay the survival of mice subsequently challenged with live L1210/hCD4 cells, compared with non‐immunized control mice (Fig. 1). This is in agreement with the absence of detectable antibody‐ and cellular‐mediated immune responses after such immunizations (data not shown).
Figure 1.
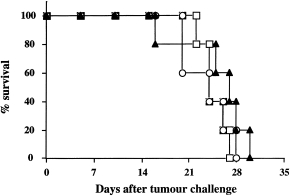
Evaluation of immunogenicity of L1210/hCD4 tumour cells. Groups of five DBA/2 mice were immunized with one or two doses of lethally irradiated L1210/hCD4 cells (circles and squares, respectively). Two weeks post‐last immunization, mice were challenged with a lethal dose of live L1210/hCD4 cells. Mean survival was compared with non‐immunized control mice challenged with L1210/hCD4 cells (triangles). This is one representative out of three experiments.
Vaccination with DC loaded with shCD4 protein induces cell‐mediated cytotoxic immune responses with a protective anti‐leukaemia effect
Unloaded and shCD4‐loaded DC were obtained after 7 days of culture of BM cells in the presence of GM‐CSF and analysed by flow cytometry. This resulted in 70–85% DC, characterized by the co‐expression of MHC class II molecules and the CD11c marker. These cells expressed B7‐1 and B7‐2 costimulatory molecules, as well as DEC‐205, 33D1 and F4/80 myeloid DC markers. 33
Mice vaccinated with DC loaded with shCD4 protein generated an hCD4 antigen‐specific cell‐mediated cytotoxic immune response but a weak humoral immune response (Fig. 2a,b). After challenge with L1210/hCD4 cells, the mean survival of mice vaccinated with shCD4‐loaded DC was significantly prolonged to > 120 days, compared with control mice vaccinated with unloaded DC that succumbed to the tumour with a mean survival of 28 days (P = 0·02) (Fig. 2c).
Figure 2.
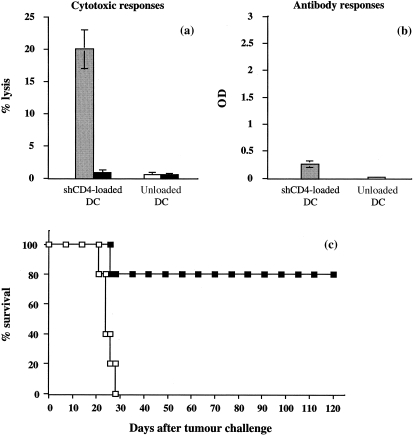
Evaluation of the immune responses generated by vaccination with DC loaded with shCD4 protein, and effects of vaccination on the survival of mice challenged with L1210/hCD4 tumour cells. (a) Cytotoxic T‐lymphocyte‐mediated immune responses, tested against the MC26/hCD4 target cells, were determined in DBA/2 mice vaccinated with DC loaded with shCD4 protein (shaded bars) or with unloaded DC (white bars). Cytotoxic activity was measured by 51Cr release assay. This is one representative out of three experiments, showing the lysis values obtained for a 50 : 1 E : T ratio. Controls, tested against the unmodified MC26 target cells (solid bars), showed absence of lysis. (b) Antibody‐mediated immune responses were determined in mice vaccinated as described in (a). Mouse sera, diluted at 1 : 10 to 1 : 10000, were tested for the presence of anti‐hCD4 antibodies by ELISA. This is one representative out of three experiments, showing the OD values obtained for sera diluted at 1 : 10. (c) After vaccination of DBA/2 mice with DC loaded with shCD4 protein (black squares) or with unloaded DC (white squares), mice (n = 5, per group) were challenged with 105 L1210/hCD4 cells. The mean survival of mice vaccinated with shCD4‐loaded DC was significantly prolonged to > 120 days, compared with the other group of mice (P = 0·02, Fisher exact test). This is one representative out of three experiments.
Condition of loading DC with unfractionated L1210/hCD4 extracts is critical for inducing a protective anti‐leukaemia effect
When mice were vaccinated with DC loaded with unfractionated L1210/hCD4 extracts, the conditions of DC loading appeared to be critical for inducing specific cell‐mediated cytotoxic immune responses (Fig. 3a,b) and for the in vivo anti‐leukaemia effect (Fig. 3c). Indeed, loading BM‐derived DC with a ratio of 1 : 10 DC : TCE induced specific anti‐hCD4 cell‐mediated cytotoxic immune responses and weak humoral immune responses and protected between 40% and 60% of the animals for up to 120 days after tumour challenge (P = 0·22 for the experiment shown and P = 0·003 when the results of three independent experiments were pooled). In contrast, loading DC with a ratio of 1 : 100 DC : TCE induced neither cellular nor humoral anti‐hCD4 immune responses and did not protect mice against tumour challenge (mean survival 42 days). Control mice, vaccinated with unloaded DC, displayed neither cellular nor humoral anti‐hCD4 immune responses and died after tumour challenge (mean survival 30 days).
Figure 3.
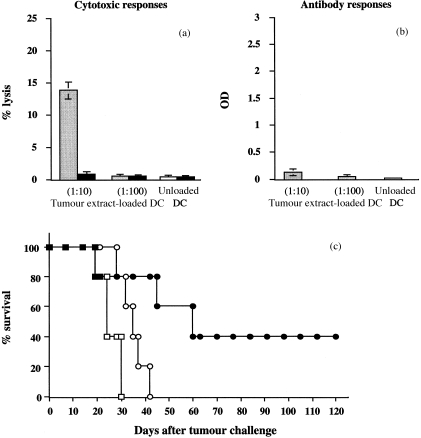
Evaluation of the immune responses generated by vaccination with DC loaded with different amounts of unfractionated L1210/hCD4 extracts, and effects of vaccination on the survival of mice challenged with L1210/hCD4 tumour cells. (a) Cytotoxic T‐lymphocyte‐mediated immune responses, tested against the MC26/hCD4 target cells, were determined in DBA/2 mice vaccinated with DC loaded with unfractionated L1210/hCD4 extracts, at a ratio DC : TCE of 1 : 10 (dark shaded bars) or 1 : 100 (light shaded bars) or with unloaded DC (white bars). Cytotoxic activity was measured by 51Cr release assay. This is one representative out of three experiments, showing the lysis values obtained for a 50 : 1 E : T ratio. Controls, tested against the unmodified MC26 target cells (solid bars), showed absence of lysis. (b) Antibody‐mediated immune responses were determined in mice vaccinated as described in (a). Mouse sera, diluted at 1 : 10 to 1 : 10 000, were tested for the presence of anti‐hCD4 antibodies by ELISA. This is one representative out of three experiments, showing the OD values obtained for sera diluted at 1 : 10. (c) After vaccination of DBA/2 mice with DC loaded with unfractionated L1210/hCD4 extracts, at a ratio DC:TCE of 1 : 10 (black circles) or 1 : 100 (white circles), mice (n = 5, per group) were challenged with 105 L1210/hCD4 cells. The mean survival of mice vaccinated with L1210/hCD4 extract‐loaded DC at a ratio DC : TCE of 1 : 10 was prolonged to > 120 days, compared with the other group (P = 0·22; when the results of three independent experiments were pooled, P = 0·003). The control group consisted of mice (n = 5) vaccinated with unloaded DC (squares). This is one representative out of three experiments.
Immunophenotypical analysis showed that the conditions used for loading BM‐derived cells with tumour extracts induced changes in the proportion of distinct MHC class II‐expressing cell populations (MHC‐IIlo and MHC‐IIhi, respectively). 33 Indeed, after loading with a ratio of 1 : 10 DC : TCE, the MHC‐IIhi cell percentage increased slightly from 24% to 46%, while the percentage of the MHC‐IIlo population decreased from 45% to 40%, compared with the unloaded culture. In contrast, with a ratio of 1 : 100, the MHC‐IIhi cell percentage decreased dramatically from 24% to 3%, while the percentage of the MHC‐IIlo population increased from 45% to 70%, compared with the unloaded culture (Fig. 4).
Figure 4.
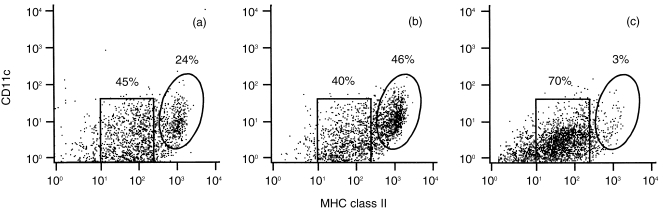
FACS analysis of BM‐derived DC cultured in GM‐CSF, loaded with different amounts of unfractionated L1210/hCD4 extracts. Unloaded DC (a) and DC loaded with unfractionated L1210/hCD4 extracts, at a ratio DC : TCE of 1 : 10 (b) or 1 : 100 (c), were stained with antibodies for MHC class II and CD11c for flow cytometry analysis. Loading DC with different amounts of tumour extracts resulted in striking differences in the proportion of the two MHC class II‐expressing cell populations, differing on their level of expression of MHC class II molecules: low and high levels of expression, MHC‐IIlo (square area) and MHC‐IIhi (ellipse area), respectively. 33
Vaccination with shCD4 protein emulsified in FA induces a potent antibody‐mediated immune response without a protective anti‐leukaemia effect
We next vaccinated DBA/2 mice i.p. with shCD4 protein emulsified in CFA and, 2 weeks later, i.m. with IFA. Fourteen days thereafter, some mice were killed, sera were collected and spleens harvested for antibody detection and standard cytotoxicity assays, as described above. Such vaccination did not induce specific CTL responses but induced strong antibody responses (Fig. 5a,b), compared with vaccination using hCD4‐loaded DC (Figs 2 and 3).
Figure 5.
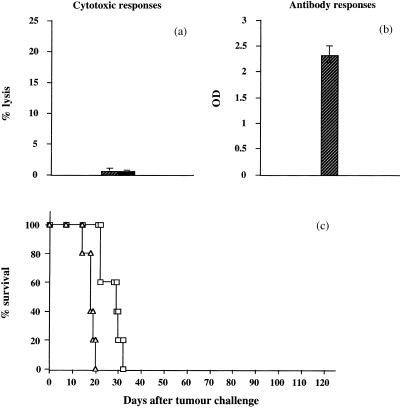
Evaluation of the immune responses generated by vaccination with shCD4 protein emulsified in FA, and effects of vaccination on the survival of mice challenged with L1210/hCD4 tumour cells. (a) Cytotoxic T‐lymphocyte‐mediated immune responses, tested against the MC26/hCD4 target cells, were determined in DBA/2 mice vaccinated with shCD4 protein emulsified in FA (hatched bars). Cytotoxic activity was measured by 51Cr release assay. This is one representative out of three experiments, showing the lysis values obtained for a 50 : 1 E : T ratio. Controls, tested against the unmodified MC26 target cells (solid bars), showed absence of lysis. (b) Antibody‐mediated immune responses were determined in mice vaccinated as described in (a). Mouse sera, diluted at 1 : 10 to 1 : 10000, were tested for the presence of anti‐hCD4 antibodies by ELISA. This is one representative out of three experiments, showing the OD values obtained for sera diluted at 1 : 10. (c) After vaccination of DBA/2 mice with shCD4 protein emulsified in FA (triangles), mice (n = 5) were challenged with 105 L1210/hCD4 cells. The control group consisted of non‐vaccinated mice (n = 5) challenged with L1210/hCD4 cells (squares). Animals from these groups died with a mean survival of 3 and 5 weeks, respectively. This is one representative out of three experiments.
Interestingly, despite this potent antibody‐mediated immunity, the animals were not protected against challenge with L1210/hCD4 cells, as well as control non‐vaccinated animals (Fig. 5c). Mice vaccinated with shCD4 protein emulsified in FA and non‐vaccinated mice succumbed with a mean survival of 3 and 5 weeks, respectively.
Discussion
Numerous TAA are membrane expressed, and hence could be the target of either cytotoxic T cells or immunoglobulins. Because DC play a central role in the induction of both T‐ and B‐cell immunity, 12 it is important to evaluate the respective contribution of humoral and cellular immune responses in DC‐triggered tumour immunity.
There are clear indications that the generation of cytotoxic T cells solely can be sufficient to trigger efficient anti‐tumour immune responses, as demonstrated in experiments where the TAA is cytoplasmic or when the immunization is performed with small peptides that cannot generate antibody responses. 13,30 On the other hand, other studies in mice have shown that successful protection against a B‐cell lymphoma can be achieved by vaccination with idiotype‐loaded DC. In this case, the in vivo tumour resistance is correlated with the induction of a humoral response specific for the idiotype expressed by the tumour. 31
Based on the concept that both humoral and cellular immunity may be required for a vaccine to prevent cancer establishment and/or progression, we investigated the distinct role of each type of immune response in terms of in vivo protective anti‐leukaemia effects in mice.
As the TAA characterizing the tumour cells used in this study were unknown, we designed a model system in which the TAA was known and available as a recombinant protein. Many other experiments in the field of DC‐based cancer vaccines in mice have been conducted using artificial antigen models, such as the Escherichia coli LacZ 15 or ovalbumin 13,16 proteins. We chose the hCD4 protein, which was used previously in an anti‐tumour vaccination approach based on the direct immunization of mice with an hCD4‐expressing plasmid DNA. 32 The hCD4 protein is a prototypic member of the immunoglobulin supergene family and a molecule central to the immune system. DNA immunization was able to induce protection from tumour cell challenge through the generation of specific cellular and humoral immune responses directed against the hCD4 displayed on the tumour cells. These data indicate that the hCD4 protein is specifically recognized by T‐ and B‐cell immunity generated in vaccinated animals, demonstrating the ability of hCD4‐expressing plasmid DNA inoculation to induce relevant protective immune responses against this model antigen. This suggests that the hCD4 molecule can be used as a model antigen in experimental haematological malignancies, because it mimics the expression of molecules such as immunoglobulin idiotypes and T‐cell receptors, which are usually expressed on the membrane surfaces of malignant cells.
As a tumour model, we used the murine L1210 B lymphocytic leukaemia. This tumour was shown to be non‐immunogenic as immunization with lethally irradiated cells did not protect the host against a subsequent challenge with these cells. The expression of hCD4 on this cell line modified neither its tumourigenicity nor its immunogenicity, compared with parental cells. Indeed, immunization with lethally irradiated L1210/hCD4 cells induced neither cellular nor humoral anti‐hCD4 immune responses, and did not prevent tumour development following a subsequent challenge with L1210/hCD4 cells.
Vaccinations have been accomplished by loading DC cultures with either purified shCD4 protein or unfractionated L1210/hCD4 extracts. We compared the efficacy of these vaccinations with that of vaccination with shCD4 protein emulsified in FA. When DC were loaded with shCD4 protein, the survival rate at 120 days was of 80%.
When using L1210/hCD4 extracts, the conditions of loading appeared to be critical for the in vivo anti‐leukaemia effect and for the generation of the two MHC class II‐expressing cell populations. 33 These populations displayed low and high levels of expression of MHC class II molecules (MHC‐IIlo and MHC‐IIhi, respectively) and showed, after cell sorting, differences in terms of in vivo anti‐leukaemia efficacy (MHC‐IIhi were more efficient than MHC‐IIlo). 33 The results presented here demonstrated that loading DC with a ratio of 1 : 100 DC : TCE inhibited the generation of the MHC‐IIhi population and did not protect mice against tumour challenge. In contrast, loading DC with a ratio of 1 : 10 DC : TCE resulted in an increasing MHC‐IIhi population and protected 40% of animals for up to 120 days after tumour challenge. These data could be associated with the amount of TCE used for loading DC. Indeed, it is supposed that high amounts of TCE contain high amounts of transforming growth factor‐β (TGF‐β), a potent regulator of growth and differentiation. 37 We observed that TGF‐β inhibits the production of the MHC‐IIhi population in our BM cultures (data not shown). These observations are in agreement with previous results in mice and humans, demonstrating that TGF‐β suppress mature DC differentiation. 38,39
Altogether, the efficacy of the vaccinations using DC loaded with either purified shCD4 protein or unfractionated tumour extracts at a ratio of DC : TCE of 1 : 10, was associated with the generation of an hCD4 antigen‐specific cell‐mediated cytotoxic immune response. These responses are antigen specific and thus presumably T‐cell mediated.
In contrast, i.p. vaccination with shCD4 emulsified in CFA, followed by an i.m. boosting with IFA, generated only a non‐protective specific humoral immune response. This effect might be explained by the capacity of B cells to inhibit the induction of T‐cell‐dependent tumour immunity. Indeed, it has been shown that B cells cause a non‐protective humoral immune response by impairing CD4+ T‐cell help for CTL‐mediated tumour immunity, during the priming phase. 40
Altogether, these preclinical data obtained with a model TAA in mouse models agree with pioneering clinical trials already conducted in humans. 19–22 In a study concerning four patients with low‐grade B‐cell lymphoma, immunization was performed with DC loaded with immunoglobulin idiotype and with keyhole limpet haemocyanin (KLH). 20 This study demonstrated the ability of antigen‐loaded DC to stimulate both humoral and cellular immune responses. Indeed, all four patients developed potent humoral immune responses and peripheral blood mononuclear cell (PBMC) proliferative responses against KLH as well as cellular proliferative responses against tumour idiotype protein. However, no specific anti‐idiotype antibody response could be detected. In another study concerning the vaccination of melanoma patients with autologous DC loaded with either peptides or autologous tumour lysates combined with KLH, 21 all 16 patients demonstrated strong delayed‐type hypersensitivity (DTH) reactivity towards KLH. Furthermore, biopsies taken from DTH test sites revealed a dense infiltration of CD45R0 memory T lymphocytes. These observations were associated with clinical responses.
These clinical trials have clearly demonstrated that loading of DC in the presence of adjuvant (KLH) can generate both cellular and humoral immune responses. However, in patients it is not possible to determine the respective role of each type of immune response in the anti‐tumour effect.
Our studies are the first that analyse the respective role of humoral and cellular immunity in DC‐based vaccines in terms of in vivo protective anti‐leukaemia effect. Our results clearly indicate that the unique property of DC loaded with soluble antigenic protein or with unfractionated tumour extracts, to trigger effective anti‐tumour immune responses, is associated with cellular but not humoral immune responses.
Moreover, our data underline the heterogeneity of DC generated in vitro from murine BM. They highlight the need to characterize the type of DC before use for immunotherapy and to determine the effects of exposing DC to TAA in order to induce an efficient in vivo anti‐tumoural immune response.
Altogether, these observations demonstrate the pivotal role of the cellular‐mediated immune responses in preventing leukaemia development, and provide information for further development of DC‐based cancer vaccines.
Acknowledgments
This work was supported in part by the ‘Fondation de France – Fondation contre la Leucémie’. We thank Professor Albert Najman and the ‘Association pour la Recherche sur la Moelle Osseuse’ (ARMO) for helpful support.
Abbreviations
- BM
bone marrow
- CFA
complete Freund’s adjuvant
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- DTH
delayed‐type hypersensitivity
- FA
Freund’s adjuvant
- FCS
fetal calf serum
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- hCD4
human CD4
- IFA
incomplete Freund’s adjuvant
- i.m.
intramuscularly
- i.p.
intraperitoneally
- i.v.
intravenously
- KLH
keyhole limpet haemocyanin
- L1210/hCD4
L1210 B lymphocytic leukaemia cell line expressing the hCD4 molecule
- mAb
monoclonal antibody
- OD
optical density
- PBMC
peripheral blood mononuclear cell
- s.c.
subcutaneously
- shCD4
soluble hCD4
- TAA
tumour‐associated antigen
- TCE
tumour cell equivalents
- TCR
T‐cell receptor
- TGF‐β
transforming growth factor‐β
References
- 1.Boon T, Cerottini JC, Van Den Eynde B, Van Der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Greiner JW, Guadagni F, Noguchi P, et al. Recombinant interferon enhances monoclonal antibody‐targeting of carcinoma lesions in vivo. Science. 1987;235:895. doi: 10.1126/science.3580039. [DOI] [PubMed] [Google Scholar]
- 3.Mulé JJ, Mcintosh JK, Jablons DM, Rosenberg SA. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990;171:629. doi: 10.1084/jem.171.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith KA. Lowest dose interleukin‐2 immunotherapy. Blood. 1993;81:1414. [PubMed] [Google Scholar]
- 5.Fisher B, Packard BS, Read EJ, et al. Tumor localization of adoptively transferred indium‐111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor‐infiltrating lymphocytes. Science. 1986;233:1318. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA‐4. Cell. 1992;71:1093. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt‐wolf GD, Schmidt‐wolf IGH. Cytokines and gene therapy. Immunol Today. 1995;16:173. doi: 10.1016/0167-5699(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM. Dendritic cells and immune‐based therapies. Exp Hematol. 1996;24:859. [PubMed] [Google Scholar]
- 10.Troy AJ, Hart DNJ. Dendritic cells and cancer: progress toward a new cellular therapy. J Hematother. 1997;6:523. doi: 10.1089/scd.1.1997.6.523. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow‐derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L, Mayordomo JI, Tjandrawan T, et al. Therapy of murine tumors with tumor peptide‐pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1‐associated cytokines. J Exp Med. 1996;183:87. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen‐presenting cells in vitro and in vivo. J Exp Med. 1996;184:465. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair SK, Snyder D, Rouse BT, Gilboa E. Regression of tumors in mice vaccinated with professional antigen‐presenting cells pulsed with tumor extracts. Int J Cancer. 1997;70:706. doi: 10.1002/(sici)1097-0215(19970317)70:6<706::aid-ijc13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Ashley DM, Faiola B, Nair S, et al. Bone marrow‐generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherji B, Chakraborty NG, Yamasaki S, et al. Induction of antigen‐specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide‐pulsed autologous antigen presenting cells. Proc Natl Acad Sci USA. 1995;92:8078. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B‐cell lymphoma using autologous antigen‐pulsed dendritic cells. Nat Med. 1996;2:52. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 21.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide‐ or tumor lysate‐pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma: a feasibility study. Blood. 1999;93:2411. [PubMed] [Google Scholar]
- 23.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 24.Gedde‐dahl T, Fossum B, Eriksen JA, Thorsby E, Gaudernack G. T cell clones specific for p21 ras‐derived peptides: characterization of their fine specificity and HLA restriction. Eur J Immunol. 1993;23:754. doi: 10.1002/eji.1830230328. [DOI] [PubMed] [Google Scholar]
- 25.Houbiers JGA, Nijman HW, Van Der Burg SH, et al. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild‐type p53. Eur J Immunol. 1993;23:2072. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- 26.Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B‐cell lymphoma against the surface‐immunoglobulin idiotype expressed by their tumors. New Engl J Med. 1992;327:1209. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 27.Nelson EL, Li X, Hsu FJ, et al. Tumor‐specific, cytotoxic T‐lymphocyte response after idiotype vaccination for B‐cell, non‐Hodgkin’s lymphoma. Blood. 1996;88:580. [PubMed] [Google Scholar]
- 28.Kwak LW, Taub DD, Duffey PL, et al. Transfer of myeloma idiotype‐specific immunity from an actively immunised marrow donor. Lancet. 1995;345:1016. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 29.Porgador A, Gilboa E. Bone marrow‐generated dendritic cells pulsed with a class I‐restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD. Peptide‐pulsed dendritic cells induce antigen‐specific, CTL‐mediated protective tumor immunity. J Exp Med. 1996;183:283. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flamand V, Sornasse T, Thielemans K, et al. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24:605. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Merva M, Dang K, et al. Immunization by direct DNA inoculation induces rejection of tumor cell challenge. Hum Gene Ther. 1995;6:407. doi: 10.1089/hum.1995.6.4-407. [DOI] [PubMed] [Google Scholar]
- 33.Masurier C, Pioche‐durieu C, Colombo BM, et al. Immunophenotypical and functional heterogeneity of dendritic cells generated from murine bone marrow cultured with different cytokine combinations: implications for anti‐tumoural cell therapy. Immunology. 1999;96:569. doi: 10.1046/j.1365-2567.1999.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idziorek T, Klatzmann D. Functional expression of the CD4 protein after cross‐linking to red blood cells with a bifunctional reagent. Biochim Biophys Acta. 1991;1062:39. doi: 10.1016/0005-2736(91)90332-3. [DOI] [PubMed] [Google Scholar]
- 35.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current Protocols in Immunology. John Wiley & Sons Inc.; 1994. [Google Scholar]
- 36.Chams V, Jouault T, Fenouillet E, Gluckman JC, Klatzmann D. Detection of anti‐CD4 autoantibodies in the sera of HIV‐infected patients using recombinant soluble CD4 molecules. AIDS. 1988;2:353. doi: 10.1097/00002030-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Todaro GJ, Larco JE, Fryling C, Johnson PA, Sporn MB. Transforming growth factors (TGFs): properties and possible mechanisms of action. J Supramol Struct Cell Biochem. 1981;15:287. doi: 10.1002/jsscb.1981.380150306. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhang YY, Ogata M, et al. Transforming growth factor‐beta 1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell‐like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208. [PubMed] [Google Scholar]
- 39.Geissmann F, Revy P, Regnault A, et al. TGF‐beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567. [PubMed] [Google Scholar]
- 40.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell‐dependent tumor immunity. Nat Med. 1998;4:627. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]


