Abstract
CD8+ T lymphocytes producing high levels of interferon‐γ (IFN‐γ) and expressing antigen specific cytotoxic activity are effectively induced after plasmid DNA vaccination and mediate protection against several intracellular micro‐organisms. Recent evidence suggests that the priming of CD8+ T‐cell responses following DNA injection involves antigen presentation mediated by dendritic cells. Here, we show that bacterial DNA and synthetic oligonucleotides containing dinucleotide (CpG) motifs activate cytokine expression in dendritic cells and modulate in vivo CD8+ T‐cell priming and differentiation.
Introduction
It is now well established that immunization with plasmid DNA stimulates specific cellular and humoral immune responses 1,2 and protective immunity against several bacterial, viral and parasitic diseases; 3–5 these remarkable effects seem to be associated with the ability of the plasmid DNA to act as an adjuvant, stimulating cytokine production by professional antigen‐presenting cells and with the specific gene expression and efficient priming of naïve CD4+ and CD8+ T cells. 6 In fact bacterial DNA and synthetic oligonucleotides containing unmethylated dinucleotide (CpG) motifs have been shown to directly stimulate monocytes and B cells in vitro to produce cytokines. 7,8 In addition, in vivo studies using β‐galactosidase as a model antigen demonstrated that the priming and expansion of T helper 1 (Th1) T cells induced by DNA vaccination was optimal when the plasmid backbone contained such immunostimulatory motifs, 9 and CpG‐containing oligonucleotides (CG‐ODN) act as Th1 adjuvants when co‐injected with hen egg lysosome in incomplete Freund’s adjuvant. 10
Recent evidence strongly suggests that the priming of CD8+ T‐cell responses following DNA vaccination involves antigen presentation mediated by dendritic cells; 11 however, very little is known about the direct adjuvant effect of bacterial DNA on dendritic cells and it is not clear if the adjuvant activity, associated with bacterial DNA in vivo, results in more efficient CD8+ T‐cell priming and differentiation. In this study we have analysed the ability of synthetic oligonucleotides (ODN) containing CpG motifs (CG‐ODN) to stimulate interleukin (IL)‐6, IL‐12, interferon (IFN)‐α and IFN‐β gene expression in dendritic cells; in addition, using transgenic mice that express a T‐cell receptor (TCR) specific for a peptide aa 366–374 of the nucleoprotein of the influenza virus A/NT/60/68, 12 we show that specific in vitro priming of CD8+ T cells cultured with CG‐ODN and in vivo co‐injection of oligonucleotides containing these immunostimulatory motifs with specific antigen, favours the differentiation of CD8+ T cells into T cytotoxic 1 (Tc1) cells producing high levels of IFN‐γ and expressing antigen‐specific cytotoxic activity.
Materials and methods
Animals and cell lines
Female C57BL/10, and F5 Rag–/– TCR transgenic mice 12 (generously provided by D. Kioussis, The National Institute for Medical Research, London, UK) were kept in conventional animal facilities at the National Institute for Medical Research and used at 4–8 weeks of age. The dendritic cell line ts‐Dc 13 (generously provided by A. Volkmann and B. Stockinger, The National Institute for Medical Research) was cultured in 6‐ or 24‐well plates (Nunc, Roskilde, Denmark) at 1 × 105 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM) (Flow Laboratories, High Wycombe, UK), supplemented with 10% fetal calf serum (FCS), and 10 mm glutamine and kept at 36·5°. The thymoma EL‐4 was maintained in DMEM medium supplemented with 10% FCS, glutamine and 50 µm 2‐mercaptoethanol (2‐ME).
ODN stimulation and peptide immunization
CG‐ODN (GCATGACGTTGAGCT) and GG‐ODN (GCATGAGGTTGAGCT) phosphorothioated oligonucleotides were synthesized according to previously published sequences 8 and were purified using HPLC by Oswell DNA (Southampton, UK). Dendritic or spleen cells were stimulated with 100 µg/ml ODN. For the in vivo experiments the ODN were used at 0·5 mg/ml. Mice were injected intraperitoneally with 0·5 ml of peptide/saline or peptide/ODN solutions. The 9‐mer peptide [NP(366–374)]: Ala‐Ser‐Asn‐Glu‐Asn‐Met‐Asp‐Ala‐Met was used at a final concentration of 0·5 mg/ml.
Flow cytometry and cell sorting
After blocking Fc receptors using anti‐mouse CD16/CD32 (Pharmingen, Oxford, UK) for 15 min, cells were stained for 20 min in ice with directly conjugated antibodies. Two‐colour flow cytometry was used using the following monoclonal antibodies: phycoerythrin (PE)‐conjugated anti‐CD8+ clone 53‐6.7, fluoroscein isothiocyanate (FITC)‐conjugated anti‐CD4 clone H129.19, FITC‐conjugated anti‐CD44 clone IM7, PE‐conjugated anti‐CD11c clone HL3, FITC‐conjugated anti‐Mac‐1 clone M/70 (all obtained from Pharmingen, Oxford, UK); for the intracellular staining the cells were first stimulated with phorbol 12‐myristate 13‐acetate (PMA) 50 ng/ml (Sigma, Poole, UK), Ionomicin 500 ng/ml (Sigma) and brefeldin‐A10‐µg/ml (Sigma) for 4 hr at 37°. After washing, Fc receptors were blocked and the cells stained for 20 min in ice with FITC‐conjugated anti‐CD8+ monoclonal antibody 53‐6.7, cells were fixed for 20 min at room temperature in 4% formaldehyde resuspended in hypertonic phosphate‐buffered saline (PBS) and, after washing, permeabilized in 0·5% saponin buffer (Sigma). For staining, fixed cells were incubated with PE‐conjugated anti IFN‐γ monoclonal antibody or PE‐conjugated anti‐IL‐4 monoclonal antibody (obtained from Pharmingen) for 30 min. The cells were washed and analysed using a fluorescence‐activated cell sorter (FACScan; Becton Dickinson, Oxford, UK) and FACScan software (WinMDI 2.6, The Scripps Research Institute, La Jolla, CA). To purify dendritic cells from spleens, collagenase and DNase treated spleen cells were washed and stained with FITC‐conjugated anti‐Mac 1 and PE‐conjugated anti‐CD11c monoclonal antibodies and sorted using a FACStar flow cytometer. Cells were sorted into the CD11chigh and Mac‐1low stained population. Bone‐marrow‐derived dendritic cells were generated by culturing 5 × 106 bone marrow cells in 10 ml culture medium containing 10% supernatant from Ag8.653 myeloma cells transfected with mouse granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (generously provided by B. Stockinger, National institute for Medical Research). On day 4, non‐adherent granulocytes were removed; the loosely adherent cells were collected at day 7 and depleted of B220 expressing cells by co‐incubation with monoclonal antibody anti‐B220 (clone RA3‐6B2; Pharmingen) and complement (Cedarlane Lab. Ontario, Canada). The remaining cells were stained with PE‐conjugated anti‐CD11c and a biotinylated anti‐major histocompatibility complex (MHC) class IIB (generously provided by O. Williams. The National Institute for Medical Research); the cells were sorted as previously described into CD11chigh and MHC class IIhigh after staining with streptavidin–FITC.
Cytokine and cytototoxicity assay
Quantitation of cytokines produced by cultured cells was carried out by enzyme‐linked immunosorbent assay (ELISA) using commercially available kits (Genzyme, Cambridge, MA for the determination of total IL‐12 (p40) or Amersham International, Amersham, UK for IL‐6, IL‐4 and IFN‐γ). For the determination of IL‐12 and IL‐6, culture supernatants were collected 12–24 hr after the addition of ODNs. The analyses for IL‐4 and IFN‐γ secreted by spleen cells from TCR transgenic mice, were carried out after 40–48 hr of in vitro culture or 24 hr after re‐stimulation with specific peptide NP 366–374. To determine the induction of cytotoxic T cells, JAM cytotoxicity tests were performed. 14 To test for peptide specific cytotoxic cells, EL‐4 (H‐2b) target cells were incubated with 50 µg/ml of NP aa 366–374 for 30 min at 37°, radio‐labelled using [3H]methyl‐thymidine (Amersham) and used at 100 : 1 effector : target ratios; effector T cells were obtained directly from the spleen of immunized animals depleted of red blood cells by hypo‐osmotic shock and were not stimulated in vitro before adding to the assay.
Detection of cytokine gene expression by reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was isolated from 106 dendritic cells using a standard guanidinium thiocyanate/phenol/chloroform extraction and alcohol precipitation procedure, as previously described. 15 A DNA digestion step was included to avoid any genomic DNA contamination. PCR amplification was carried out using specific amplimer pairs for: IFN‐α (sense 5′‐GAC TCA TCT GCT GCT TGG AAT GCA ACC CTC C‐3′; antisense 5′‐GAC TCA CTC CTT CTC CTC ACT CAG TCT TGC C‐3′giving a 294‐bp DNA product); IFN‐β (sense 5′‐CAG CTC CAG CTC CAA GAA AGG ACG AAC ATT CG‐3′; antisense 5′‐CCA CCA CTC ATT CTG AGG CAT CAA CTG ACA GG‐3′ giving a 509‐bp DNA product); IL‐6 (sense 5′ ‐ATG AAG TTC CTC TCT GCA AGA GAC T‐3′; antisense 5′‐CAC TAG GTT TGC CGA GTA GAT CTC‐3′ giving a 638‐bp DNA product); IL‐12 p40 (sense 5′‐CAG AAG CTA ACC ATC TCC TGG TTT G‐3′; antisense 5′‐TCC GGA GTA ATT TGG TGC TTC ACA‐3′ giving a 394‐bp DNA product). All primers were obtained from Clontech, Palo Alto, CA. The preparation of PCR reaction mixture and pipetting of samples were performed in a category II laminar flow hood. The PCR mixture contained 5 µl of 10 × PCR buffer, 5 µl of 25 mm MgCl2, 1 µl of 10 mm deoxynucleoside triphosphate (dNTP), 0·5 µl of 20 µm primers, 0·5 µl of Taq polymerase 5 U/µl and 5 µl of cDNA. The β‐actin primers used as normalizing controls for the PCR reactions were: sense 5′‐ATG GAT GAC GAT ATC GCT‐3′; antisense 5′‐ATG AGG TAG TCT GTC AGG T‐3′ giving a 540‐bp DNA product. PCR products were visualized by electrophoresis through 1·2% agarose containing ethidium bromide.
Results
CG‐ODN stimulate specific cytokine gene expression in dendritic cells
Initially we studied the ability of synthetic oligonucleotides CG‐ODN or GG‐ODN to stimulate cytokine gene expression using the conditionally immortalized bone‐marrow‐derived dendritic cell line tsDC. These cells when cultured at 36·5° are able to grow in the absence of GM‐CSF and share most of the phenotypic cell surface markers of immature dendritic cells. 13 When tsDC cells were stimulated with oligonucleotides containing CpG motifs (CG‐ODN) specific expression of mRNAs for IFN‐α, IFN‐β, IL‐6 and IL‐12 p40 was observed as detected by RT–PCR; however, expression of these cytokines was not observed in GG‐ODN or saline stimulated tsDC cells (Fig. 1). To confirm these results we investigated further the ability of CG‐ODN or GG‐ODN to stimulate tsDC cells to secrete these cytokines. There was a high level of IL‐6 and IL‐12 (p40) in the supernatants of the tsDC cells stimulated with CG‐ODNs, however, these cytokines were not secreted when the cells were stimulated with GG‐ODN or were treated with saline (Fig. 2a). It was important to determine if the CG‐ODNs or GG‐ODNs would stimulate cytokine secretion in primary dendritic cells isolated from the spleen or bone marrow. Spleens from C57/BL10 mice were treated with collagenase and DNAse; the cells were then sorted by FACS into CD11chigh and Mac‐1low (Fig. 2b) and cultured for 6 hr before they were stimulated with the ODNs. As shown in Fig. 2(c), significant levels of IL‐6 and IL‐12 (p40) were once again present in the supernatants of CG‐ODN‐stimulated splenic dendritic cells, but were absent in the supernatants of GG‐ODN or saline treated cells. In addition CG‐ODN were able to stimulate B220+‐depleted bone marrow‐derived CD11chigh and MHC class IIhigh dendritic cells (Fig. 3a) to produce high levels of these cytokines (Fig. 3b).
Figure 1.
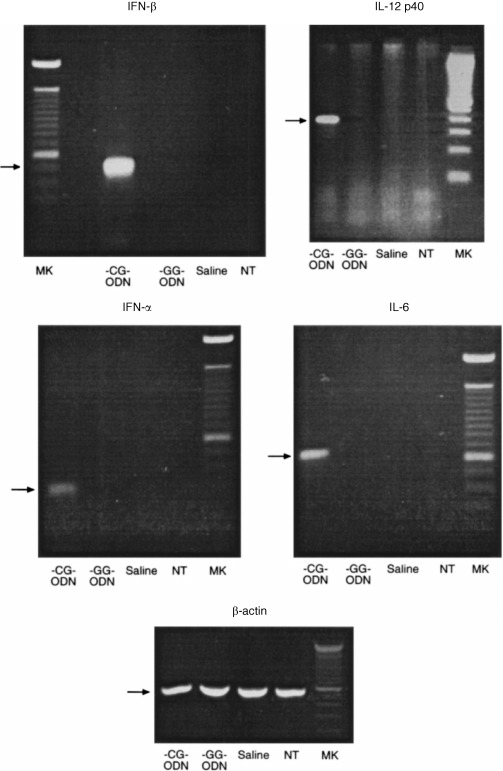
RT‐PCR analysis for the detection of IL‐6, IL‐12(p40), IFN‐α‐, IFN‐β‐ and β‐actin‐specific mRNAs after oligonucleotide stimulation. Total RNA obtained from non‐stimulated or from CG‐ODN, GG‐ODN or saline‐stimulated dendritic cells, was reverse transcribed and amplified with specific primers. ts‐Dc cells (106) were cultured in the presence or absence of ODNs for 24 hr before the RNAs were extracted.
Figure 2.
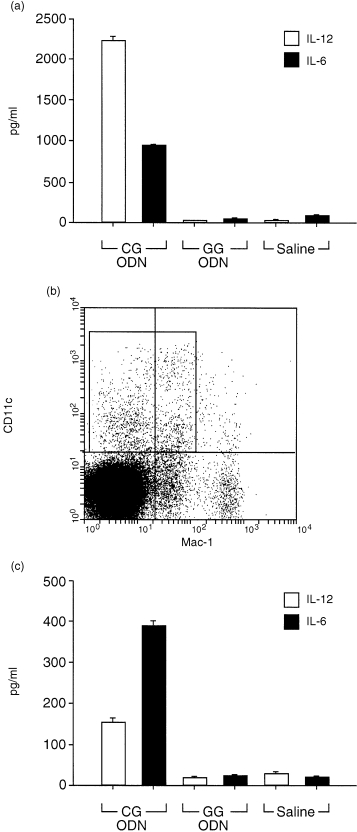
CG‐ODNs stimulate IL‐6 and IL‐12 (p40) secretion by dendritic cells. 106 Ts‐DC cells (a) or 5 × 104 primary dendritic cells from spleens (c) were cultured with CG‐ODNs, GG‐ODNs or saline; the ODNs were used at 100 µg/ml and the supernatants were assayed by ELISA for total IL‐12 (p40) or IL‐6 24 hr later. Error bars show standard deviations; one of two representative experiments is shown. Primary dendritic cells were purified from the spleens of normal C57BL/10 mice by FACS sorting using Mo Abs to CD11c and Mac‐1; the sorted labelled CD11chigh and Mac1low cells are shown in (b).
Figure 3.
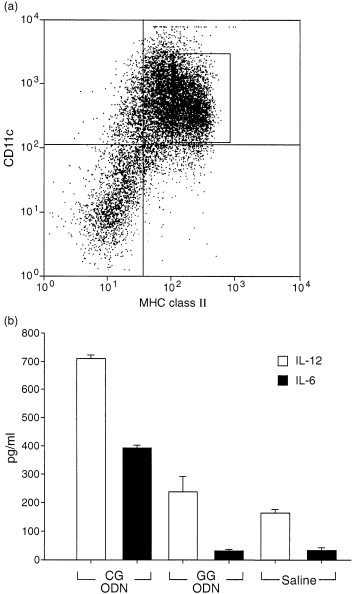
CG‐ODNs stimulate IL‐6 and IL‐12 (p40) secretion by bone‐marrow‐derived dendritic cells. The cells were purified from cultures of GM‐CSF supplemented bone marrow cells by FACS sorting using monoclonal antibodies (mAb) to CD11c and MHC class II; the labelled CD11chigh and MHC class IIhigh cells are shown in (a). Bone‐marrow‐derived DCs (5 × 104) (b) were cultured with CG‐ODNs, GG‐ODNs or saline. The ODNs were used at 100 µg/ml and the supernatants were assayed by ELISA for total IL‐12 (p40) or IL‐6 24 hr later. Error bars show standard deviations. One of two representative experiments is shown.
Cytokine production by F5‐TCR transgenic spleen cells in response to specific antigen stimulation in the presence of CG‐ODN
Previous studies demonstrated that effector CD8+ T cells secrete substantially more IFN‐γ when generated with IL‐12, 16 suggesting a pivotal role of this cytokine in promoting the differentiation of Tc1 cells; because the stimulation of dendritic cells with CG‐ODN induced high IL‐12 production, we hypothesised that specific priming of naı¨ve CD8+ T cells in the presence of CG‐ODN‐stimulated antigen‐presenting cells could favour the differentiation of Tc1 cells. To study the effect of CG‐ODNs in the priming and activation of CD8+ T cells in vitro, we cultured spleen cells from transgenic mice that express a TCR specific for a peptide aa 366–374 of the nucleoprotein of the influenza virus A/NT/60/68, with specific antigen and CG‐ODNs, GG‐ODNs or saline. Transgenic spleen cells cultured in vitro with CG‐ODNs in the absence of specific peptide stimulation, secreted IL‐12 and low levels of IFN‐γ; however, as expected, the level of IFN‐γ secreted in response to specific peptide stimulation was higher and there was a significant increase in the levels of IFN‐γ secreted when the spleen cells were activated with specific peptide in the presence of CG‐ODNs (Fig. 4a, b). The increased level of IFN‐γ observed in the primary cultures was paralleled with a very high level of IFN‐γ secreted by the peptide‐restimulated transgenic T cells. Transgenic spleen cells cultured in vitro with specific antigen for 4–5 days and then restimulated, produced very high levels of IFN‐γ and the presence of CG‐ODN in the primary culture period increased the levels of IFN‐γ secreted in response to peptide specific re‐stimulation (Fig. 4c). It should be noted that these cells did not produce IFN‐γ unless restimulated with specific antigen and that the levels of IL‐4 secreted were lower than 10 pg/ml in all the cultures (not shown). Importantly, blocking IL‐12 during the generation of effectors with CG‐ODN and peptide, diminished substantially the secretion of IFN‐γ after antigen specific re‐stimulation (Fig. 4c).
Figure 4.
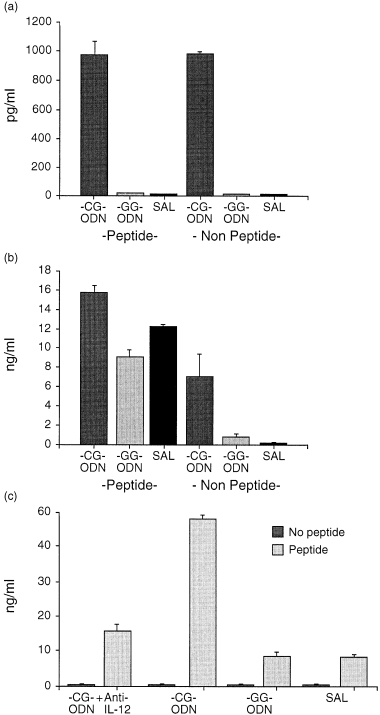
Comparison of cytokine secretion by spleen cells from F5 Rag–/– TCR transgenic mice stimulated in vitro with specific peptide and ODNs. F5 rag–/– spleen cells (5 × 105) were cocultured with 2 × 106 C57/BL10 irradiated spleen cells as APCs and were stimulated with specific peptide NP 366–374, 0·1 µg/ml, in the presence of CG‐ODN, GG‐ODN or saline. The supernatants of the primary cultures were tested for total IL‐12 after 24 hr (a) or IFN‐γ after 48 hr (b). The stimulated effector CD8+ T cells were harvested after 4–5 days, washed, adjusted to 5 × 104 cells/well and restimulated with 106 irradiated spleen cells and specific peptide at a concentration of 0·1 ng/ml. Supernatants generated in the re‐stimulated cultures were harvested after 24 hr and the levels of IFN‐γ detected as determined by ELISA are shown in (c). (Error bars show standard deviations.)
Co‐injection of CG‐ODN with specific antigen promotes TC1 priming
To test in vivo the effect of CG‐ODNs in the priming of Tc1 cells, we co‐injected F5‐TCR transgenic mice with specific peptide and CG‐ODNs, GG‐ODNs or saline and analysed the proportion of IFN‐γ expressing cells directly in the CD8+ cell population using FACS analysis. The injection of specific peptide in these mice induced a change in the proportion of CD8+ and CD44+ cells in the spleens at day 3 in all groups, demonstrating the activation of specific CD8+ T cells after intraperitoneal administration of cognate antigen (Fig. 5). Importantly, the proportion of CD8+ T cells that expressed IFN‐γ was significantly higher in the CG‐ODN/peptide treated mice when compared with the GG‐ODN/peptide or saline/peptide co‐injected mice. We only detected trace amounts of IL‐4 in these cultures and there was no significant difference in the levels of IL‐4 detected as determined by ELISA and FACS analysis between the different groups (data not shown).
Figure 5.
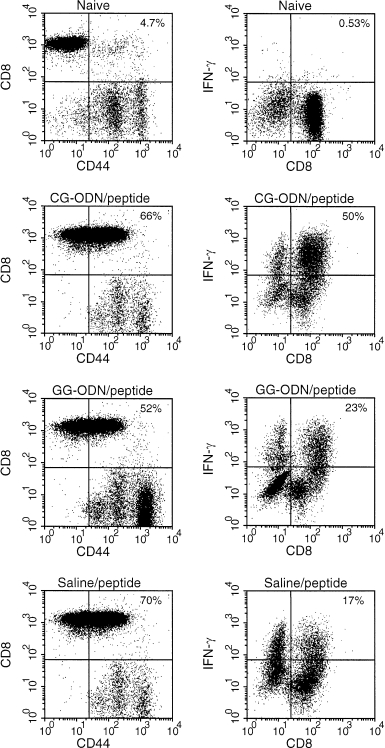
Flow cytometric analysis of spleen cells from immunised F5 Rag–/– TCR transgenic mice. Cells from two to three naı¨ve or CG‐ODN/peptide, GG‐ODN/peptide or saline/peptide‐immunized transgenic mice were prepared from the spleens 3 days after immunization and directly stained with mAbs to assess the expression of CD44 and CD8 (left) or expanded in vitro in IL‐2 supplemented media for 4–6 days and then stimulated with PMA and ionomycin in the presence of protein secretion inhibitor; after surface staining with anti‐CD8+ mAb, cells were fixed, permeabilized and intracellularly accumulated IFN‐γ was detected using a specific PE‐labelled mAb (right). Similar results were obtained from two separate experiments.
Co‐injection of CG‐ODN with specific peptide promotes cytotoxic T lymphocyte (CTL) priming
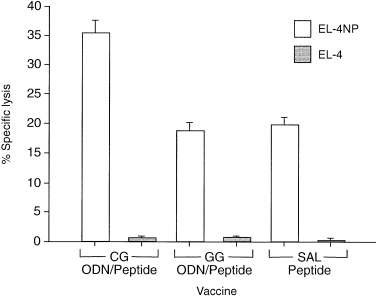
To test if the co‐injection of CG‐ODN/peptide would promote differentiation of precursor CTLs into effector cytotoxic T cells, we analysed the specific cytotoxic activity from the spleen cells after in vivo intraperitoneal injection with ODN/peptide or saline/peptide treatment; we decided not to expand T cells with IL‐2 in vitro in order to have a closer measure of the ODN’s effect in vivo. Our results demonstrated that spleen cells from mice immunised with CG‐ODN/peptide were more efficient in lysing 9‐mer NP 366‐374 pulsed EL‐4 cells compared to spleen cells from mice immunized with GG‐ODN/peptide or saline/peptide (Fig. 6).
Figure 6.
Discussion
We and others have found that immunization of mice with plasmid DNA induces specific protective T‐cell responses against a variety of infectious agents; 3,4 in many cases the protective responses obtained after plasmid DNA vaccination seem to correlate with the specific induction of high levels of CD8+ T cells that produce IFN‐γ and express CTL killing (Tc1 cells). 5,17 However, the mechanisms by which plasmid DNA is able to promote high levels of Tc1 cell priming are not completely understood. Here, we show that bacterial DNA and synthetic ODNs containing immunostimulatory CpG motifs activate cytokine gene expression in dendritic cells. The induction of IFN‐α, IFN‐β, IL‐6 and IL‐12 gene expression parallels the effects of bacterial DNA observed earlier in macrophages; 7 however, because dendritic cells are the most effective antigen‐presenting cells (APCs) for priming naı¨ve T cells and because dendritic cells isolated from the lymph nodes of DNA‐vaccinated mice are able to present specific antigen to CD4+ and CD8+ T cells 11,18,19 it is likely that dendritic cell‐derived costimulatory cytokines are of primary importance in the priming and differentiation of protective CD8+ T cells. Recently, Sparwasser et al. 20 demonstrated activation of bone‐marrow‐derived dendritic cells by CG‐ODNs and plasmid DNA; our data confirm these results and show a direct effect of CG‐ODNs in affecting T‐cell differentiation, using a TCR transgenic mouse model.
The molecular basis for the induction of cytokine gene expression after CG‐ODN stimulation is not known, but studies using B cells have suggested a role for the specific activation of the nuclear factor κB (NF‐κB) transcription factor in CG‐ODN transfected cells. 21 The possibility that, in our experiments, the effects of the CG‐ODNs were caused by contaminating lipopolysaccharide (LPS) is unlikely since the concentration of LPS, as measured by the Limulus amoebocyte assay, was lower than 1 ng LPS/mg DNA, in addition the control GG‐ODNs were synthesized using the same conditions.
It is difficult to study the differentiation of antigen specific CD8+ T cells because of their low frequency. To overcome this problem we used TCR transgenic mice in which the majority of T cells recognize a particular antigen, in this case peptide NP 366–374. We cocultured transgenic spleen cells with specific antigen and CG‐ODNs or GG‐ODNs and showed that the presence of ODNs containing immunostimulatory CpG motifs in the primary cultures favours the differentiation of CD8+ T cells into Tc1 cells that produce high levels of IFN‐γ; similarly, we codelivered, in vivo, specific antigen with CG‐ODNs or GG‐ODNs and showed that the injection of ODNs containing immunostimulatory CpG motifs favours the differentiation of CD8+ T cells in to Tc1 cells that produce IFN‐γ and express antigen‐specific cytotoxic activity. Our results are consistent with the interpretation that the immunostimulatory ODNs promote Tc1 priming and differentiation by inducing a high level of costimulatory activity in professional APCs; as IL‐12 is a well‐characterized promoter of Tc1 differentiation, it is likely that the up‐regulated secretion of this cytokine participates in this process. However, other cytokines such as IL‐6 and IL‐18 could also contribute. The level of costimulatory molecules expressed in the membranes of dendritic cells is also very important in the process of CD8+ T cell priming and differentiation; we are currently studying these effects in our system.
The priming of specific CD8+ T cells following DNA injection has recently been widely studied. 22,23 Sprent et al. 24 showed that the proliferative response of specific CD8+ T cells to cognate peptide and CG‐ODNs was significantly higher and more prolonged than the response to peptide alone. Our results demonstrate that co‐immunization with synthetic ODN containing CpG motifs promotes CD8+ priming and differentiation, and demonstrate the potential for a direct interaction between foreign DNA and dendritic cells, the most potent primary APC. These results could have important implications in vaccine and peptide immunotherapy.
References
- 1.Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 2.Fynan EF, Webster RG, Fuller DH, et al. DNA vaccines: protective immunization by parental, mucosal, and gene‐gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 4.Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 5.Cheider J, Gilbert SC, Blanchard TJ, et al. Enhanced immunogenicity for CD8 T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus ankara. Nat Med. 1998;4:397. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 6.Carson DA, Raz E. Oligonucleotide adjuvants for T helper 1 (th‐1)‐specific vaccination. J Exp Med. 1997;10:1621. doi: 10.1084/jem.186.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman ME, Martin‐orozco S, Goodman MD, et al. Immunostimulatory DNA sequences function as T helper‐1 promoting adjuvants. Nat Med. 1997;3:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 8.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B cell activation. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Roman H, Tighe D, et al. Immunostimulatory DNA sequences are necessary for effective intradermal DNA gene immunization. Science. 1996;273:352. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 10.Chu RS, Targoni OS, Krieg AM, Lehman PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condon C, Watkins SC, Celluzi CM, Thomson K, Falo LD. DNA‐based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 12.Mamalaki C, Norton T, Tanaka Y, et al. Thymic depletion and peripheral activation of class I major histocompatibility complex‐restricted T cells by soluble peptide in T‐cell receptor transgenic mice. Proc Natl Acad Sci USA. 1992;89:11342. doi: 10.1073/pnas.89.23.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkmann A, Neefies J, Stockinger B. A conditionally immortalized dendritic cell line which differentiates in contact with T cells or T cell derived cytokines. Eur J Immunol. 1996;26:2565. doi: 10.1002/eji.1830261105. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger P. The JAM test. J Immunol Methods. 1991;145:185. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 15.Walker BK, Butler R, Colston MJ. The role of th1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1989;148:1885. [PubMed] [Google Scholar]
- 16.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen specific CD8 effector populations: reciprocal action of IL‐4 and IL‐12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA‐vaccinated and Mycobacterium tuberculosis‐infected mice. Infect Immun. 1998;66:169. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II‐restricted viral epitope. J Exp Med. 1997;186:1481. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparwasser T, Koch ES, Vabulas RM, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Yi A‐K, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg A. CpG motifs in bacterial DNA activate leukocytes through the pH‐dependent generation of reactive oxygen species. J Immunol. 1998;160:4755. [PubMed] [Google Scholar]
- 22.Ulmer JB, Deck RR, Dewitt CM, Donnelly JI, Liu MA. Generation of MHC class I‐restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non‐muscle cells. Immunology. 1996;89:59. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J Exp Med. 1998;187:1145. doi: 10.1084/jem.187.7.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


