Abstract
Bacterial cytidine–phosphate–guanosine (CpG-DNA) activates antigen-presenting cells (APC) and drives T helper 1 (Th1)-polarized immune responses in the mouse. Claims have been made that CpG-DNA costimulates murine T cells. We examined the direct and indirect effects of CpG-oligodeoxynucleotides (CpG-ODN) on human T-cell activation. CpG-ODN failed to costimulate purified human T cells activated with α-CD3 or α-T-cell receptor (TCR)αβ antibodies. In contrast, CpG-ODN sequence-specifically caused increased expression of CD69 on CD4 and CD8 T cells when peripheral blood mononuclear cells (PBMC) were stimulated via α-CD3. CpG-ODN and α-CD3 stimulation synergized to induce interferon-γ (IFN-γ) in T cells and natural killer (NK) cells, as shown by intracellular fluorescence-activated cell sorter (FACS) staining. These effects of CpG-ODN on human T cells were caused by the release of IFN type I (IFN-I) and interleukin-12 (IL-12) from PBMC. Enhancement of CD69 expression on α-CD3-triggered T cells could be reproduced in a coculture transwell system of purified T cells and PBMC, was inhibited by neutralizing antibodies to IFN-I and could be mimicked by adding exogenous IFN-I. Furthermore, neutralization of either IFN-I or IL-12 diminished, and in combination abolished, IFN-γ production. These findings show that CpG-ODN potentiate TCR-triggered activation of human T cells in an APC-dependent manner.
Introduction
Bacterial DNA and synthetic cytidine–phosphate–guanosine (CpG) oligodeoxynucleotides (CpG-ODN) derived thereof have attracted attention because they activate cells of the adaptive immune system (lymphocytes) and the innate immune system (antigen-presenting cells [APC]) in a sequence-dependent manner. CpG-ODN are not only mitogenic for B cells,1,2 but activate APC, such as macrophages and dendritic cells, via the stress kinase pathway.3 As a consequence, APC produce cytokines, including interleukin (IL)-12, tumour necrosis factor-α (TNF-α) and IL-6, and up-regulate costimulatory cell-surface molecules.4–6 This explains, at least in part, the powerful T helper 1 (Th1)-polarizing adjuvanticity of CpG-ODN,7–10 which can also be used to trigger protective and curative Th1 reponses in vivo.11,12 Induction of IL-12 from APC is thought to be a major reason for the Th1-biasing effects of CpG-ODN.13 Interestingly, Sprent and coworkers have provided evidence in vivo and in vitro that up-regulation of CD69 on T cells may be induced by interferon type I (IFN-I) from APC activated by CpG-ODN.14 On the other hand, direct APC-independent costimulation of proliferation by CpG-ODN has been demonstrated in our laboratory using purified murine T cells.15
Whilst the immunobiology of CpG-ODN has been studied intensively in the mouse, to date there are only a few studies in the human system. Previous work has shown that CpG-ODN are mitogenic for human B cells, and activate monocytes and dendritic cells to up-regulate surface molecules and produce cytokines such as IL-12.10,16,17 Data on the effects of CpG-ODN on human T cells, however, are scarce, and it is not known whether the effects are direct or mediated via stimulation of APC. We therefore analysed:
(1) whether CpG-ODN provide costimulation for α-CD3-triggered purified human T cells; and
(2) whether CpG-ODN-mediated activation of APC within peripheral blood mononuclear cells (PBMC) influences T-cell activation in terms of IFN-γ production and up-regulation of the T-cell activation marker, CD69.
Materials and methods
Reagents
ODN were used single-stranded and synthesized by TiB MOLBIOL (Berlin, Germany) with a fully phosphorothioate-stabilized backbone, solubilized in sterile endotoxin-free water at a concentration of 250 µ m and stored at − 20° until use. Sequences of the ODN were: 2006, TCGTCGTTTTGT-CGTTTTGTCGTT; PZ2, CTCCTAGTGGGGGTGTCCTAT; 2006K, TGCTGCTTTTGTGCTTTTGTGCTT; AP1, GCTTGATGACTCAGCCGGAA; and Poly A, AAAAAAA-AAAAAAAAAAAAA. ODN were used at a concentration of 2 µ m. Monoclonal antibodies (mAbs) for T-cell stimulation were: mouse anti-human CD3 (clone UCHT1), mouse anti-human CD28 (clone CD28.2) (both from Immunotech, Marseilles, France) and mouse anti-human T-cell receptor (TCR)αβ (clone T10B9.1A-31; Pharmingen, San Diego, CA). Recombinant human IL-2 (rhIL-2) was a gift from Eurocetus (Amsterdam, the Netherlands) and used at concentration of 10 U/ml. rhIL-12 (Pharmingen) was used at 2 ng/ml and recombinant IFN-α (PBL, Brunswick, NJ) at 5000 U/ml. For blocking experiments, polyclonal rabbit anti-human IFN-α and IFN-β antiserum (PBL) and a monoclonal mouse anti-human IL-12 antibody (Pharmingen) were used.
Preparation of PBMC and purified T cells
Peripheral blood from healthy volunteers or blood bank-derived buffy coats was the source of PBMC. After discontinuous gradient centrifugation (Ficoll–Hypaque 1·077 g/l; Biochrom-Seromed, Berlin, Germany), the interphase was harvested and the cells washed four times with Hanks’ balanced salt solution (HBSS). Finally, PBMC were suspended in complete medium (RPMI-1640 containing 10% fetal calf serum [FCS], 10 m m glutamine and 50 µ m 2-mercaptoethanol), which was also the culture medium used in all experiments. T cells were selected negatively by a magnetically activated cell sorting (MACS) column, using the selection kit according to the manufacturer’s protocol (Miltenyi, Bergisch Gladbach, Germany). Purity of T cells was controlled by staining for CD3 and was consistently > 95%. T-cell blasts for analysis of IFN-γ mRNA levels were generated by culture of PBMC in the presence of 1 µg/ml of phytohaemagglutinin (PHA) and IL-2 for 4 days, followed by washing and expansion of the cells in IL-2 (according to reference 18). After 2 to 3 weeks of expansion, T-cell blasts were washed free of IL-2 and used as described in the legend to Fig. 8.
Figure 8.

CpG-oligodeoxynucleotide (ODN)-induced type-I interferon (IFN-I) and interleukin (IL)-12 mediate the increase in IFN-γ production from peripheral blood mononuclear cells (PBMC). (a) PBMC were stimulated and the supernatants assayed for IFN-γ, as described in Figure 3, except that neutralizing antibodies to IFN-α/β and IL-12, as well as the recombinant cytokines (for concentrations see Figure 6, recombinant (r)IL-12: 2 ng/ml), were added where indicated. The mean + SEM values from four experiments are shown. (b) Intracellular IFN-γ staining on T cells (fluorescein isothiocyanate [FITC]-positive, stained with α-CD4 and α-CD8) and non-T cells (FITC-negative). Numbers indicate the percentages of IFN-γ+ T cells and non-T cells, respectively. The results of a representative experiment are shown. (c) Supernatants from PBMC treated with CpG-ODN 2006 alone or in combination with soluble α-CD3 up-regulate mRNA levels for IFN-γ in T-cell blasts. Supernatants of PBMC were harvested after 20 hr of culture and transferred to T-cell blasts (2 × 106 in 1 ml). Four hours later, total RNA from T-cell blasts was prepared and analysed by using semiquantitative TaqMan reverse transcription–polymerase chain reaction (RT–PCR). Mean and SD values are shown from duplicate determinations of a representative experiment out of three.
Cell culture
For stimulation of purified T cells, 96-well culture plates were coated overnight at 4° with anti-CD3 or anti-TCRαβ plus or minus anti-CD28, in a volume of 50 µl/well. After washing with phosphate-buffered saline (PBS) and blocking with complete medium followed by two additional washing steps with PBS, the indicated reagents were added first, followed by addition of purified T cells. Proliferation was assessed by using a standard [3H]thymidine assay in quadruplicate samples. PBMC (5 × 106/well in 24-well plates) were stimulated with soluble anti-CD3 and ODN at the indicated concentrations. In some experiments (e.g. Fig. 7), a transwell culture system was applied to separate purified T cells from PBMC. Purified T cells (106/well) were added in 500-µl volumes to a 24-well plate coated with anti-CD3 (0·8 µg/ml) as described above, followed by positioning of a 0·2-µm Anopore Membrane (Nunc, Roskilde, Denmark) and pipetting of 3 × 106 PBMC (from the same donor), in a volume of 500 µl, onto the membrane.
Figure 7.

Enhancement of CD69 up-regulation on purified, α-CD3-triggered human T cells by CpG-oligodeoxynucleotide (ODN)-induced soluble factors. T cells (106) were stimulated in 24-well plates coated with 0·8 µg/ml of α-CD3 antibody. ODN were added, where indicated, at a concentration of 2 µ m. In 50% of the samples, 3 × 106 peripheral blood mononuclear cells (PBMC) were placed onto a set-in membrane (designated TRANSWELL), while the other wells contained only T cells. After 24 hr, T cells were harvested and stained with fluorescein isothiocyanate (FITC)-labelled anti-CD69 combined with phycoerythrin (PE)-labelled monoclonal antibodies (mAbs) to either CD4 (hatched bars) or CD8 (black bars).
Measurement of CD69 and CD25 expression by flow cytometry
Unless stated otherwise, PBMC or T cells were harvested (after 24 hr of incubation) by centrifugation, washed with PBS containing 2% FCS and incubated for 10 min at room temperature with human IgG (Miltenyi) to saturate non-specific binding of antibodies. For specific staining, antibodies directly labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridininchlorophyll protein (PerCP), or biotinylated antibodies (Pharmingen) were used at optimized concentrations in PBS (containing 2% FCS) in a final volume of 150 µl. After 30 min of incubation at 4°, cells were washed twice with PBS containing 2% FCS. In the case of biotinylated antibodies, this was followed by incubation with streptavidin–fluorochrome conjugates (Pharmingen) for 30 min at 4° and again two washing steps. Finally, the cells were taken up in a 1% paraformaldehyde solution and analysed on a flow cytometer (Coulter EPICS XL, Heidelberg, Germany).
Measurement of cytokines
Enzyme-linked immunosorbent assay
At the indicated time-points, supernatants were harvested from the cultures and cytokine levels determined by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (IFN-γ: Pharmingen; IFN-α: PBL), according to the manufacturers’ instructions.
Detection of intracellular IFN-γ by fluorescence-activated cell sorter (FACS) analysis
PBMC were cultured in 24-well plates, as described above. After 8 hr of incubation, 5 µg/ml of Brefeldin A (Sigma, Deisenhofen, Germany) was added to prevent secretion of cytokines into the culture medium. After an additional 12–16 hr of culture, cells were harvested and fixed in 2% paraformaldehyde solution (Merck, Darmstadt, Germany) for 10 min at room temperature. Cells were rewashed and then stained with PE-conjugated anti-human IFN-γ (Pharmingen) and the indicated antibody to lineage markers in 50 µl of buffer (PBS containing 0·5% saponin, 0·05% sodium azide and 0·5% bovine serum albumin [BSA]) for 10 min at room temperature. After washing with 1 ml of buffer, biotinylated antibodies were detected by incubation with streptavidin–PerCP conjugates, washed and then analysed on a flow cytometer. Staining for the surface molecules CD16, CD19 and CD56 was performed, as described above, before fixation of the cells in paraformaldeyde.
IFN-γ mRNA expression in T-cell blasts
Four hours after the addition of PBMC supernatants, T-cell blasts were harvested, total RNA prepared using a guanidinium-based method (TriFast, Peqlab, Erlangen, Germany) and random-primed cDNA synthesis was carried out. TaqMan reverse transcription–polymerase chain reaction (RT–PCR) for relative quantification of IFN-γ mRNA was performed using the primers and fluorescently labelled TaqMan probes for IFN-γ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) described previously.19 Briefly, threshold cycles (CT) for IFN-γ and GAPDH were determined in separate reactions (each in duplicate) by RT–PCR (5-min initial denaturation at 95°, followed by 35 cycles at 95° for 15 seconds and 60° for 1 min) on a 7700 sequence detection system (Perkin-Elmer, Foster City, CA). ΔCT values for each cDNA sample were calculated and subtracted from the ΔCT of the cDNA from untreated T-cell blasts, which were used as the calibrator to generate ΔΔCT values.19 Calculation of the relative abundance as fold induction relative to untreated T-cell blasts was carried out using the equation:
Results
CpG-ODN fail to costimulate purified α-CD3-triggered human T cells
To examine whether CpG-ODN costimulate human T cells, we set up a culture system where purified human T cells were stimulated with plate-bound α-CD3 or α-TCRαβ mAb in the presence or absence of co-coated α-CD28 mAb. When α-TCRαβ mAb was used as signal 1, T-cell proliferation was strictly dependent on signal 2 provided by α-CD28, while high concentrations of α-CD3 mAb were mitogenic in the absence of signal 2 (Fig. 1a). Addition of CpG-ODN 2006, known to stimulate human B cells and monocytes,16 as well as various non-CpG ODN, to submitogenic doses of either α-CD3 or α-TCRαβ had no costimulatory effect on T-cell proliferation (Fig. 1b).
Figure 1.
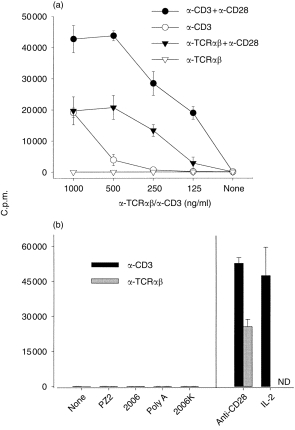
Failure of various oligodeoxynucleotides (ODN) to costimulate purified human T cells. After negative selection of human T cells, 2 × 104 cells were cultured in quadruplicate in 96-well plates coated, as indicated, with monoclonal antibodies (mAbs) to CD3, T-cell receptor (TCR)αβ or CD28. [3H]Thymidine incorporation was determined on day 4 of culture and is expressed as counts per minute (c.p.m.). Data shown represent mean ± SD from representative experiments. (a) Titration of antibodies to CD3 and TCRαβ. (b) The indicated ODN were added at a concentration of 1·25 µ m to wells coated with either α-CD3 (0·2 µg/ml) (black bars) or α-TCRαβ (1 µg/ml) (grey bars). ND, not done.
CpG-ODN enhance CD69 expression on T cells in α-CD3-stimulated PBMC
To investigate indirect effects of CpG-ODN on T cells, T cells within unseparated PBMC were stimulated with graded amounts of soluble α-CD3 antibody. In this system, CpG-ODN-mediated up-regulation of the activation marker CD69 on subsets of PBMC was analysed by FACS. Figure 2(a) depicts histograms from one experiment, while in Fig. 2(b), mean and standard error of the mean (SEM) values from several independent experiments, using different donors, are shown. Addition of CpG-ODN 2006 alone to PBMC had only a small and variable effect on CD69 expression by CD4 or CD8 T cells, while it induced CD69 expression on B cells and, to a lesser extent, also on natural killer (NK) cells. At a mitogenic concentration (100 ng/ml) soluble α-CD3 strongly promoted CD69 expression on a high percentage of CD4+ or CD8+ T cells, while at the submitogenic concentration of 10 ng/ml, α-CD3 was less effective and no effect was seen with a concentration of 1 ng/ml or less (not shown). Addition of CpG-ODN 2006 to submitogenic concentrations of α-CD3 (10 ng/ml) increased the percentage of CD69-expressing CD4+ or CD8+ T cells. Although the absolute values of CD69 expression showed considerable interindividual variation, CpG-ODN 2006 mediated enhanced up-regulation, which was donor-independent and significant for CD4+ and CD8+ T cells. Stimulation of PBMC with α-CD3 induced up-regulation of CD69 on a high percentage of B cells, which was further enhanced by CpG-ODN 2006. While only a minor percentage of NK cells were stimulated to express CD69 by addition of α-CD3 to PBMC, CpG-ODN 2006 and α-CD3 synergized to induce CD69 on greater than 40% of NK cells. In experiments using titrated amounts of ODN 2006, enhancement of CD69 expression on α-CD3-triggered T cells was observed by concentrations of ODN 2006 as low as 0·05 µ m, with the maximal effect in the range of 0·2–2 µ m (results not shown). CpG-ODN 2006 did not significantly trigger IL-2 receptor-α (CD25) expression on T cells or T cells stimulated with submitogenic doses of α-CD3 (Fig. 2c), but activated B cells to express CD25 (results not shown).
Figure 2.

Expression of CD69 and CD25 on T cells, B cells and natural killer (NK) cells. Peripheral blood mononuclear cells (PBMC) (5 × 106) were incubated in 24-well plates with the indicated amounts of soluble α-CD3 monoclonal antibodies (mAbs) in the presence or absence of CpG-oligodeoxynucleotides (ODN) 2006 or the non-CpG-ODN AP1 or 2006K. After 24 hr, cells were harvested and stained with fluorescence-labelled antibodies to lineage markers and CD69 or CD25. (a) CD69 expression on T cells (upper panel, fluorescein isothiocyanate [FITC]-labelled antibodies to CD4 and CD8 were combined to gate on T cells) or B cells (lower panel, gated on CD19 FITC-positive cells). Numbers in the upper right corner of histograms show percentages of gated cells lying in the indicated gate on days 1 and 2 of culture. NS, not stimulated. (b) Mean and SEM values of CD69 expression from independent experiments for T cells (CD4 and CD8 stained separately), B cells (CD19+ cells) and natural killer (NK) cells (CD16+ CD56+ cells). Number of experiments carried out: seven for CD4+, five for CD8+, four for CD19+ and three for CD16+ CD56+. (c) Mean and SEM values of CD25 expression on CD4 and CD8 T cells (n = 3).
CpG-ODN and α-CD3 synergize to promote IFN-γ production from PBMC: stimulation of T cells and NK cells
Th1-polarized immune responses are characterized by high levels of IFN-γ production from T cells. We therefore analysed the ability of CpG-ODN to enhance production of IFN-γ from PBMC stimulated with α-CD3. IFN-γ secretion by PBMC is shown in Fig. 3 as mean and SEM values from seven experiments. At mitogenic doses, soluble α-CD3 mAb induced highly variable amounts of IFN-γ and low to undetectable amounts at submitogenic concentrations. CpG-ODN 2006 alone was also a poor inducer of IFN-γ production. A combination of submitogenic α-CD3 and CpG-ODN 2006, however, was highly effective. To identify the IFN-γ-producing cells, we stained for intracellular IFN-γ in combination with antibodies to surface markers for different cell lineages within PBMC. Figure 4(a) details histograms from a representative experiment showing that:
Figure 3.

CpG-oligodeoxynucleotides (ODN) 2006 and α-CD3 synergize to induce interferon-γ (IFN-γ) production from peripheral blood mononuclear cells (PBMC). PBMC (5 × 106) were stimulated overnight either with ODN 2006 or AP1 (both at 2 µ m) or with α-CD3 (10 ng/ml or 100 ng/ml) or with the indicated combinations. Supernatants were analysed by using enzyme-linked immunosorbent assay (ELISA) (detection limit 40–80 pg/ml). Mean + SEM values are shown from seven experiments (using cells from six different donors).
Figure 4.

Detection of intracellular interferon-γ (IFN-γ) after stimulation of peripheral blood mononuclear cells (PBMC) with α-CD3 and CpG-oligodeoxynucleotides (ODN). PBMC were stimulated as described in the Material and methods, and Brefeldin A was added 8 hr after onset of culture. After an additional 12 hr of incubation, cells were harvested and double stained for surface lineage markers and intracellular IFN-γ, as outlined in the Materials and methods. (a) A representative experiment showing IFN-γ-producing cells in T cells (fluorescein isothiocyanate [FITC])-positive CD8+ cells have a higher intensity than CD4+ cells) or non-T cells (FITC-negative). Numbers indicate the percentage of IFN-γ+ cells within T cells and non-T cells (FITC-positive and FITC-negative, respectively). (b) Mean and SEM of IFN-γ-producing CD4+ and CD8+ T cells and non-T cells from four (CD4+ and CD8+) and two (non-T cells) experiments.
(1) ODN 2006 alone failed to stimulate T cells but activated a minor percentage of non-T cells to produce IFN-γ;
(2) submitogenic doses of α-CD3 mAb induced IFN-γ production only in 2·1% of T cells as compared to 7·1% at mitogenic concentrations; and
(3) the combination of ODN 2006 and submitogenic doses of α-CD3 increased the percentages of IFN-γ-producing cells in both T cells (7·0%) and non-T cells (16·5%).
Separate staining for CD4+ and CD8+ T cells revealed that CD8+ cells produced much higher levels of IFN-γ, while CpG-ODN 2006 increased IFN-γ production from both T-cell subsets (Fig. 4b).
Role of IL-12 and IFN-I in CpG-mediated enhanced IFN-γ production and expression of CD69
Increased T-cell activation in PBMC treated with CpG-ODN could be mediated by soluble factors, for example IL-12 and IFN-I. We have previously demonstrated that CpG-ODN 2006 sequence-specifically induces IL-12 production from PBMC.16 High levels of IFN-I were detected in PBMC supernatants treated with CpG-ODN 2006 (Fig. 5). As shown in Fig. 6, neutralization of IFN-I, but not of IL-12, abrogated the enhanced expression of CD69 by ODN 2006 on CD4+ and CD8+ T cells within PBMC stimulated with ODN 2006 plus submitogenic doses of α-CD3 mAb. Furthermore, addition of exogenous IFN-I to α-CD3-stimulated PBMC caused greatly enhanced CD69 expression on T cells, thus mimicking the effect of ODN 2006. In the absence of α-CD3 antibody, IFN-I stimulated CD69 expression on only a small percentage of T cells, while it was more effective on B cells and NK cells (results not shown), similar to previous observations with ODN 2006 (Fig. 2b). To test whether cell–cell contact was required for enhancement of CD69 expression on T cells, we used a transwell coculture system, where purified T cells were triggered with immobilized α-CD3 antibody in the lower chamber, while PBMC were placed on a set-in membrane. Addition of ODN 2006 to the wells containing PBMC effectively increased the expression of CD69 on purified T cells, but had no stimulatory effect in the absence of PBMC (Fig. 7). These data indicated that CpG-ODN 2006 increased CD69 expression on T cells subjected to TCR ligation by inducing production of IFN-I in APC contained within PBMC.
Figure 5.

CpG-oligodeoxynucleotide (ODN) 2006 induces production of type-I interferon (IFN-I) from peripheral blood mononuclear cells (PBMC). Supernatants of PBMC, stimulated as indicated for 24 hr, were analysed by using an enzyme-linked immunosorbent assay (ELISA) for the presence of IFN-α. ND, not detectable.
Figure 6.

Blockade of type-I interferon (IFN-I) inhibits the enhancement of CD69 up-regulation by CpG-oligodeoxynucleotide (ODN) 2006. Peripheral blood mononuclear cells (PBMC) were stimulated and analysed as described in Figure 2, except that neutralizing antibodies to human IFN-α and IFN-β (500 U/ml of each) and interleukin (IL)-12 (2 µg/ml) were added to the cultures together with the respective stimuli. Recombinant human IFN-α was added at a concentration of 5000 U/ml. Shown are mean and SEM of CD69 expression on CD4+ and CD8+ cells (from six and five experiments, respectively).
Blocking antibodies to either IL-12 or IFN-I greatly reduced the secretion of IFN-γ from PBMC stimulated with α-CD3 plus ODN 2006, neutralization of IFN-I being slightly more effective (Fig. 8a). When both cytokines were neutralized, IFN-γ production was almost completely inhibited. Similar results were obtained when IFN-γ levels were assayed at the single cell level by intracellular FACS staining (Fig. 8b). Of note, exogenous IFN-I and IL-12 both effectively increased IFN-γ production in α-CD3-triggered PBMC, as did CpG-ODN 2006 (Fig. 8a). In this setting, T cells and non-T cells produced IFN-γ (Fig. 8b). Cell-to-cell contact appeared not to be mandatory for CpG-ODN-driven IFN-γ production by T cells, because T-cell blasts up-regulated mRNA expression of IFN-γ upon transfer of supernatants of stimulated PBMC (Fig. 8c).
Discussion
Immunostimulatory CpG-ODN activate murine6,20 and human16,17 APC to express costimulatory molecules and to secrete cytokines, and are mitogenic for murine1 and human16 B cells. APC activation is pivotal for CpG-mediated Th1 polarization,8–10 protection from experimental leishmaniasis11 and cytotoxic T-lymphocyte (CTL) induction7,21 and thus accounts, at least partly, for activation of murine T cells by CpG-ODN in vivo. However, APC-independent costimulation of TCR-triggered murine T cells by CpG-ODN has been reported,15 raising the possibility of additional, direct CpG effects on T cells. As in the mouse, CpG-ODN do not activate resting purified human T cells to produce IFN-γ10 or to proliferate,16 but information is lacking in regard to CpG effects on TCR-triggered human T cells.
With this data in mind, we addressed the question of whether CpG-ODN activate TCR-triggered human T cells. Using purified human T cells, the results obtained failed to provide evidence that CpG-ODN directly trigger or costimulate human T-cell proliferation (Fig. 1). As we observed that G-rich ODN are more potent in costimulating murine T cells (G. B. Lipford et al., manuscript in preparation), such sequences were also tested on human T cells but were not active (unpublished). In contrast, in the presence of APC (i.e. when PBMC were used) CpG-ODN enhanced CD69 expression on TCR-ligated T cells (Fig. 2) and increased the production of IFN-γ from T cells and NK cells (Fig. 3 and Fig. 4). In the absence of α-CD3 mAb, CpG-ODN had no significant effect on T cells within PBMC. As discussed below in detail, enhanced CD69 expression and IFN-γ production occur as a result of IFN-I and IL-12 produced by CpG-ODN-activated APC. We conclude that CpG-ODN do not directly stimulate or costimulate human T cells, while TCR triggering renders T cells responsive to APC-derived cytokines induced by CpG-ODN.
Enhanced CD69 expression (caused by CpG-ODN) on human T cells was also evident in transwell cocultures of purified T cells stimulated with immobilized α-CD3 mAb, provided that APC within the membrane-separated PBMC were activated with CpG-ODN (Fig. 7). IFN-I is the decisive mediator of CpG-ODN-induced enhanced CD69 expression on T cells because its neutralization abolished the effect of CpG-ODN, while recombinant IFN-α was sufficient to enhance CD69 expression in α-CD3-triggered T cells (Fig. 6). Recombinant IFN-α alone induced CD69 expression on B cells and NK cells, but only poorly and variably on T cells (results not shown), similarly to CpG-ODN. In the murine system, Sun et al. have analysed direct induction of CD69 expression by CpG-ODN and described a requirement for APC-derived IFN-I.14 As reported in this work for human PBMC, murine B cells up-regulated CD69 more readily than T cells when splenocytes were incubated with either CpG-ODN or IFN-α. Murine memory cells (especially of the CD8 subset), however, displayed up-regulation of CD69 in response to CpG-ODN or IFN-I in the absence of TCR triggering, which contrasts with our data on human T cells, which acquired sensitivity to these stimuli after TCR ligation.
In accordance with previous studies,10,22,23 we found that IFN-γ production from PBMC induced by CpG-ODN was mainly from NK cells. TCR-triggered T cells within PBMC produced markedly increased amounts of IFN-γ when CpG-ODN were added to the cultures (Fig. 4). Although CD8+ cells produced higher levels of IFN-γ than CD4+ cells, both T-cell lineages were stimulated by CpG-ODN (Fig. 4). APC-derived IFN-I and IL-12 are important mediators of CpG-ODN-induced increased IFN-γ production from T cells, because blocking antibodies to either one greatly diminished, and in combination abolished, IFN-γ production (Fig. 8). In addition, both cytokines mimicked the effect of CpG-ODN when added to PBMC stimulated with α-CD3, and induced up-regulation of mRNA coding for IFN-γ in T-cell blasts, similarly to supernatants of PBMC-stimulated with CpG-ODN (Fig. 8).
We have not addressed the question of whether IFN-γ-producing CD4+ or CD8+ T cells belong to a memory/effector type subset, or contain naive T cells stimulated by CpG-ODN-induced IFN-I and IL-12 to produce IFN-γ. Both IL-12 and IFN-I act as powerful stimuli to promote differentiation of human T cells towards Th1-type immune responses.24–26 Indeed, in ongoing experiments it has been observed that – in the presence of APC – CpG-ODN enhance the differentiation of Th1 cytokine-producing T cells (M. Bauer, unpublished).
In view of the potential therapeutic applications of CpG-ODN as adjuvants and immunomodulators, it remains important to investigate whether effects observed in mouse models can be extrapolated to the human system. The results described here contribute to this process because they demonstrate that CpG-ODN potentiate responses of human T cells by activating APC to produce IFN-I and IL-12.
Acknowledgments
This work was supported by grants from Bundesministerium für Bildung und Forschung and CpG-Immunopharmaceuticals GmbH.
References
- 1.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Yi AK, Krieg AM. Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J Immunol. 1998;161:4493. [PubMed] [Google Scholar]
- 3.Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116. [PubMed] [Google Scholar]
- 5.Lipford GB, Sparwasser T, Bauer M, et al. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 6.Sparwasser T, Koch ES, Vabulas RM, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Lipford GB, Bauer M, Blank C, et al. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 8.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J Exp Med. 1998;187:1145. doi: 10.1084/jem.187.7.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman M, Martin-orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nature Med. 1997;3:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann S, Egeter O, Hausmann S, et al. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627. [PubMed] [Google Scholar]
- 12.Kline JN, Waldschmidt TJ, Businga TR, et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555. [PubMed] [Google Scholar]
- 13.Lipford GB, Heeg K, Wagner H. Bacterial DNA as immune cell activator. Trends Microbiol. 1998;6:496. doi: 10.1016/s0966-842x(98)01408-5. [DOI] [PubMed] [Google Scholar]
- 14.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Bauer M, Heeg K, Wagner H, Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699. doi: 10.1046/j.1365-2567.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sareneva T, Matikanen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J Immunol. 1998;160:6032. [PubMed] [Google Scholar]
- 19.Lang R, Heeg K. Semiquantitative determination of human cytokine mRNA expression using TaqMan RT–PCR. Inflammopharmacology. 1998;6:297. doi: 10.1007/s10787-998-0014-4. [DOI] [PubMed] [Google Scholar]
- 20.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042. [PubMed] [Google Scholar]
- 21.Davis HL, Weeranta R, Waldschmidt TJ, et al. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870. [PubMed] [Google Scholar]
- 22.Yamamoto T, Yamamoto S, Kataoka T, et al. Synthetic oligonucleotides with certain palindromes stimulate interferon production of human peripheral blood lymphocytes in vitro. Jpn J Cancer Res. 1994;85:775. doi: 10.1111/j.1349-7006.1994.tb02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohle B, Jahn-schmid B, Maurer D, Kraft D, Ebner C. Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-gamma production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur J Immunol. 1999;29:2344. doi: 10.1002/(SICI)1521-4141(199907)29:07<2344::AID-IMMU2344>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Rogge L, D’Ambrosio D, Biffi M, et al. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567. [PubMed] [Google Scholar]
- 25.Gerosa F, Paganin C, Peritt D, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183:2559. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]


