Abstract
Prodigiosin (PrG) 25-C and concanamycin B (CMB) are immunosuppressants that specifically inhibit the induction of cytotoxic T cells (CTL) without affecting the function of B cells and helper T cells in vivo. Both compounds inhibit acidification of intracellular organelles and induce destruction of cytotoxic granules and degradation of perforin in vitro. Here we show that a single intraperitoneal (i.p.) injection of PrG 25-C, and of CMB, into mice eliminates cytotoxic activity 7 days after alloantigen stimulation (when mature CTL activity has been detected in control mice), with minimal effect on the alloantigen-specific antibody titre in serum. FK506 did not suppress the cytotoxic activity with this administration schedule. Suppression was accompanied by a decrease in the CD8+ population and in perforin expression of spleen cells induced by alloantigen stimulation. The suppression of CTL activity and decrease in CD8+ cell number was detected as early as 7 hr after the injection of compounds. These results suggest that inhibitors of acidification of intracellular organelles suppress CTL activity in vivo by reducing the number of mature CD8+ CTL.
Introduction
Prodigiosin (PrG) 25-C is a family of red pigments produced by micro-organisms, including Streptomyces spp. and Serratia spp.1,2 We have previously determined that these compounds selectively suppress the concanavalin A (Con A)-induced T-cell proliferative response as compared to lipopolysaccharide (LPS)- and phytohaemagglutinin (PHA)-induced responses.3 PrG 25-C suppresses proliferation of T cells with little effect on interleukin-2 (IL-2) production and IL-2 receptor (IL-2R) formation of T cells.4,5 Furthermore, administration of PrG 25-C in vivo suppresses cytotoxic T-lymphocyte (CTL) generation induced by alloantigen, whilst antibody production against T-cell-independent antigen and T-cell-dependent antigen (including alloantigen) is not suppressed.6–8 In addition, PrG 25-C inhibits the efferent phase of delayed hypersensitivity reaction induced by trinitrophenol,7 and the rejection of allogeneic skin and heart transplants.7,8 Recently it has been reported that PrG 25-C suppresses proliferation of T lymphocytes through hypophosphorylation of the retinoblastoma gene product, pRb, and decreased expression of cell cycle regulators, such as cyclin A, cyclin E, cdk2 and cdk4.9 In contrast, FK506 and cyclosporin A, which suppress the nuclear factor of activated T cells (NFAT) activation through the inhibition of calcineurin,10,11 inhibit IL-2 production, antibody production against T-cell-dependent antigens and induction of alloantigen-specific CTL, without affecting proliferation of primed T cells.7,12–17 Thus, PrG 25-C is an immunosuppressant with a distinct mechanism of action as compared to FK506 and cyclosporin A.
Recently, we found that the concanamycin/bafilomycin family of antibiotics, which are specific inhibitors of vacuolar type-proton ATPase (V-ATPase),18,19 also have Con A-selective inhibition of T-cell proliferation.20 As the V-ATPase regulates the acidification of intracellular organelles,21 including the cytotoxic granules of CTL, the possibility exists that concanamycin may inhibit the acidification of cytotoxic granules which results in their disorganization of and degradation of perforin, thus inhibiting the killing machinery of the CTL.22–24 Further studies revealed that PrG 25-C promoted H+/Cl– symport without affecting membrane potential formation or ATP hydrolysis.25,26 As a result, PrG 25-C was considered to uncouple proton translocation through V-ATPase and inhibit the acidification of cytotoxic granules and killing activity of CTL. In addition, concanamycin, like PrG 25-C, selectively suppresses CTL generation in response to alloantigen immunization.27 Taken together, these results suggest that suppression of CTL generation in vivo by PrG 25-C and concanamycin may be caused by the direct inactivation of CTL.
In this work, we compared the efficacy of suppression of CTL activity in different regimens of administration of PrG 25-C and concanamycin B (CMB). Our studies indicate that PrG 25-C and CMB suppress CTL activity by the reduction of mature CD8+ effector T cells induced by alloantigen immunization.
Materials and methods
Immunization with allogeneic antigen and the assay of CTL
Female C57BL/6 (H-2b) mice (6–10 weeks old; Japan Charles River Inc., Atsugi, Japan) were immunized intraperitoneally (i.p.) with P815 mastocytoma cells (H-2d, 2 × 107 cells/mouse). All the experiments using mice and cultured cells were carried out according to the guidelines of Kanagawa-prefecture, Japan. The cytotoxic activity of splenocytes was determined as described previously.6 In brief, spleens were removed, teased to make a single cell suspension and incubated, with 51Cr-labelled target cells (1 × 104 cells/well) for 4 hr at 37° in an atmosphere of 5% CO2, in RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 µg/ml of kanamycin, 8 µg/ml of tylosin tartrate and 50 µ m 2-mercaptoethanol. The isotope released into the culture supernatant was measured using a γ-counter. Maximum and spontaneous isotope release was determined as counts per minute (c.p.m.) in the absence of effector and in the presence of 1% SDS, respectively. Per cent lysis was calculated by using the following formula:
 |
Anti-P815 antibody titre
51Cr-labelled P815 cells (1 × 104 cells/well) were incubated, with serially diluted sera from mice, for 30 min in 200 µl of high glucose medium, as described previously.5 At the end of the culture period, 13 µl of rabbit serum was added as a source of complement and cells were incubated for an additional 30 min. Isotope released into the culture supernatant and per cent lysis was determined as described above.
Analysis of surface phenotype
Splenocytes (1 × 106 cells) were stained with phycoerythrin (PE)-labelled monoclonal antibodies (mAbs), as described previously.27 The percentage of positive cells was determined by flow cytometry (Epics Elite; Coulter, Miami, FL).
Western blotting
Western blotting experiments were performed as described previously.28 In brief, splenocytes (1 × 107 cells) were lysed in 0·1 ml volumes of 2× sodium dodecyl sulphate (SDS) sample buffer containing 100 m m dithiothreitol, and same amounts of each sample were resolved by electrophoresis in denaturing polyacrylamide gels (12% acrylamide). The proteins in the gel were transferred to Immobilon-P membranes (Millipore, Bedford, MA). Blots were blocked in 5% non-fat dry milk in TBS-T (Tris-buffered saline: 20 m m Tris, 0·8% NaCl, 0·1% Tween-20, pH 7·5), probed with a rat anti-mouse perforin mAb (P1-8, 500 ng/ml), and then with a horseradish peroxidase-conjugated anti-rat immunoglobulin G (IgG) antibody (Santa Cruz, Santa Cruz, CA). Signals were detected by using the enhanced chemiluminescence (ECL) system (Amersham, Little Chalfont, Bucks, UK). The intensity of each band was analysed by densitometry (Molecular Imager FX; Bio-Rad Laboratories, Hercules, CA).
Chemicals
PrG 25-C was kindly donated from Chugai Pharmaceutical Co. (Gotenba, Japan) and stored at − 20°. It was dissolved in dimethyl sulphoxide at 10 mg/ml, and further diluted with phosphate-buffered saline (PBS, 0·8% NaCl, pH 7·6) containing 0·2% Tween-80 immediately before injection. CMB was kindly provided by Taisho Pharmaceutical Co. (Tokyo, Japan). It was diluted from the stock solution (10 mg/ml in ethanol, − 20°) in PBS before use. Diluted antibiotics (0·2 ml) were injected i.p. into mice. Anti-mouse perforin antibody, P1-8, was kindly provided by Dr H. Yagita (Juntendo University, Tokyo). Fluorescein isothiocyanate (FITC)-labelled anti-CD4, anti-CD8, anti-Thy-1.2, and anti-B220 antibodies were purchased from Pharmingen. [51Cr]sodium chromate was obtained from Amersham. The other reagents used were of analytical grade and purchased from commercial suppliers.
Results
C57BL/6 (H-2b) mice were immunized with allogeneic mastocytoma cells, P815 (H-2d). This immunization induces active CTL in the spleen 10 days after immunization. These CTL are CD8+ and specific for the H-2d haplotype of major histocompatibility antigen (MHC) class I (Fig. 1 and data not shown). As reported previously,6,7 administration of 0·4 mg/kg of PrG 25-C on days 0, 3, 5, 7 and 9 completely suppressed the CTL activity. To determine the administration time that was most effective in the suppression, we compared the suppressive effect with a single injection of 1 mg/kg of PrG 25-C. As shown in Fig. 1(a), administration of PrG 25-C only once on day 5, or on day 7, was as effective as the multiple treatment schedule. In contrast, single administration of the same dose of PrG 25-C on day 0 had no effect on the induction of CTL activity (Fig. 1b). Single injection of PrG 25-C on day − 1 or on day 1, as well as dual injection on days − 1 and − 3, or on days 1 and 3, failed to inhibit CTL activity on day 10 (data not shown). As significant CTL activity is attained by day 5 or day 7 (data not shown), these results indicate that PrG 25-C directly inactivates activated mature CTL, but does not inhibit induction of CTL in vivo.
Figure 1.
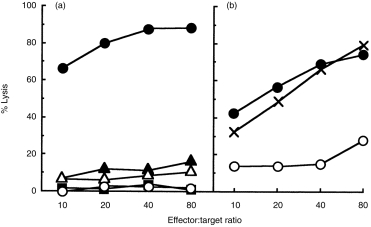
Suppression of cytotoxic T-lymphocyte (CTL) activity by single injection of prodigiosin (PrG) 25-C after maturation of CTL induced by alloantigen stimulation. Mice (three per group) were immunized with P815 on day 0. (a) PrG 25-C was injected on days 0, 3, 5, 7 and 9 (0·4 mg/kg, filled square), on day 5 (1 mg/kg, filled triangle) and on day 7 (1 mg/kg, open triangle). (b) PrG 25-C was injected on day 0 (1 mg/kg, cross). Immunized control, filled circle; naive control, open circle. Mice were killed on day 10 and the CTL activity of the pooled splenocytes was determined. Two representative results of eight experiments are shown.
Antibiotics of the concanamycin family inhibit ATPase activity of V-ATPase,18,19 whereas PrG 25-C uncouples proton transport mediated by V-ATPase.25,26 Both antibiotics inhibit acidification by preventing proton pump activity of intracellular organelles, including cytotoxic granules of CTL.22,25 A single i.p. injection of 0·4 mg/kg of CMB, 7 days after the immunization, similarly to PrG 25-C completely suppressed CTL activity (Fig. 2a). The effect was comparable to multiple administration of 0·4 mg/kg of CMB on days 0, 2, 4, 6 and 8 (data not shown). In contrast, single postoral administration on day 7 of 300 or 600 mg/kg of FK506, which suppresses the early phase of CTL induction by inhibiting IL-2 production,7, 15–17 did not affect CTL activity. We have previously shown that multiple doses of 200 mg/kg of FK506 on days 0, 3, 5 and 8 suppress CTL generation almost completely.4 These results demonstrate that PrG 25-C and CMB, unlike FK506, directly inactivate mature CTLs induced by alloantigen immunization.
Figure 2.
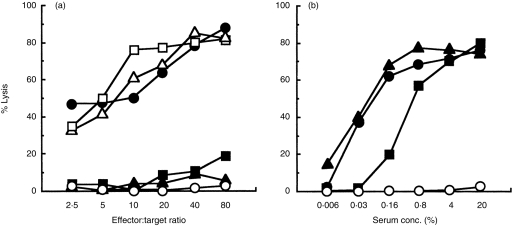
Selective effect of prodigiosin (PrG) 25-C and concanamycin B (CMB) on the function of mature cytotoxic T lymphocytes (CTLs). Mice (three per group) were immunized with P815 on day 0. On day 7, PrG 25-C (1 mg/kg, closed square) or CMB (0·4 mg/kg, closed triangle) was injected intraperitoneally (i.p.), or FK506 (300 mg/kg, open triangle; 600 mg/kg, open square) was administered orally. The CTL activity of pooled spleens (a), and anti-P815 antibody titre in the pooled serum (b) were determined on day 10. Immunized control, closed circle; naive control, open circle. The representative result of four experiments is shown.
The alloantigen immunization also induced antibody against P815 cells. Serum obtained from the mice 10 days after immunization contained a significant level of anti-P815, as determined by using the complement-dependent cytolysis assay (Fig. 2b). A single administration of 0·4 mg/kg of CMB on day 7, which completely suppressed CTL activity, did not affect the antibody production. Although the single injection of 1 mg/kg of PrG 25-C partially decreased the P815 antibody titre of the serum, the suppressive effect of PrG 25-C on CTL activity was much stronger than the suppressive effect on the allospecific antibody production.
To study the inactivation of CTL as a function of time, antibiotics were injected 11 days after alloantigen immunization, when CTL activity reached its maximum level, and cytolytic activity of the splenocytes was determined 7 or 11 hr after administration (Fig. 3). Administration of 1 or 1·5 mg/kg of PrG 25-C significantly suppressed CTL activity as early as 7 hr after administration, and the suppression was more significant 11 hr after administration. Similar suppression was observed with the administration of 0·4 or 0·6 mg/kg of CMB.
Figure 3.
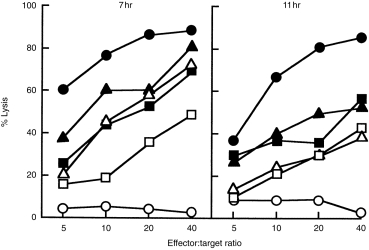
The decrease of cytotoxic T-lymphocyte (CTL) activity of the mice treated with prodigiosin (PrG) 25-C or concanamycin B (CMB) as a function of time. Mice (three per group) were immunized with P815 on day 0, and PrG 25-C (1 mg/kg, closed square; 1·5 mg/kg, open square) or CMB (0·4 mg/kg, closed triangle; 0·6 mg/kg, open triangle) was injected intraperitoneally (i.p.) on day 11. CTL activity of the pooled spleens was determined 7 hr or 11 hr after the injection. Immunized control, closed circle; naive control, open circle. The representative result of three experiments is shown.
Mice were immunized with P815, and treated with antibiotics 11 days after the immunization. The spleen lymphocytes of the mice were analysed by flow cytometry 12 hr after the antibiotic injection (Table 1). Alloantigen immunization markedly increased the total cell number. The increased cell populations were mainly Thy-1+ and CD8+ populations, which mediate CTL activity, and the non-T non-B (Thy-1–, B220–) population. The level of CD4+ T cells and B220+ cells showed only a slight increase. A single administration of PrG 25-C or CMB suppressed the increase of the CD8+ population as early as 12 hr after administration, without marked decrease of CD4+, B220+ or non-T, non-B populations. These results suggest that PrG 25-C and CMB diminish CD8+ CTL activity by the rapid reduction of mature CTL induced by alloantigen stimulation.
Table 1.
Decrease in the number of CD8+ cells (from mice immunized with alloantigen) as a result of prodigiosin (PrG) 25-C and concanamycin B (CMB)
| Average cell number per spleen (× 106 cells) | |||||||
|---|---|---|---|---|---|---|---|
| Immunization treatment | Total | Thy-1+ | CD4+ | CD8+ | B220+ | Thy-1– B220–* | |
| − | – | 34·3 | 11·0 | 6·1 | 4·6 | 20·9 | 2·4 |
| + | – | 84·7 | 34·9 | 10·0 | 25·0 | 33·8 | 16·0 |
| + | PrG 25-C | 62·0 | 19·6 | 9·0 | 10·3 | 28·6 | 13·8 |
| + | CMB | 73·3 | 22·6 | 9·3 | 12·8 | 34·2 | 16·5 |
Note: PrG 25-C (2 mg/kg) or CMB (0·4 mg/kg) was administered intraperitoneally (i.p.) 11 days after the immunization. Mice (three mice per group) were killed 12 hr after the injection, and the number of lymphocytes in the pooled splenocytes was determined by flow cytometry. The representative result of five experiments is shown.
Total cell number (Thy-1+ cell number + B220+ cell number).
Western blotting demonstrated a marked increase of perforin expression in spleen cells from mice immunized with alloantigen, and treatment with PrG 25-C or CMB significantly reduced the expression of perforin (Fig. 4). It should be noted that PrG 25-C reduced the expression of perforin to a level similar to that of the naive control, whereas the expression of CD8 was reduced to ≈50% of the immunized control by the same treatment (Table 1).
Figure 4.
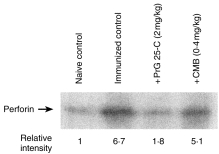
Reduction of perforin expression of splenocytes by the treatment with prodigiosin (PrG) 25-C or concanamycin B (CMB). Western blotting of whole cell extract of splenocytes used in Table 1 was carried out as described in the Materials and methods. The relative intensity of each band was quantified by densitometry.
Discussion
Our results with specific inhibitors of intracellular acidification strongly suggest that acidification is essential for maintaining the integrity of the structure and function of cytotoxic granules of CTL. The inhibitors, PrG 25-C and CMB, induce the disorganization of cytotoxic granules and degradation of perforin, which result in the inactivation of the cytotoxic function of activated mature CTL in vitro.22,23,27 In this work we studied the direct effect of these inhibitors on mature CTL activated by alloantigen stimulation in vivo. Our results demonstrate that a single injection of PrG 25-C, after the maturation of CTL had been completed, was as effective as multiple doses throughout the whole induction period, whereas FK506, which suppresses the early phase of CTL induction7,15–17 did not have suppressive effects with the same administration schedule. The suppression of CTL activity was observed as early as 7 hr after the administration of PrG 25-C, and was accompanied by a reduction in the number of CD8+ cells, which are mediators of cytotoxic activity. Taken together, these results suggest that PrG 25-C suppresses CTL activity in vivo by the rapid decrease of mature CD8+ CTL.
The single administration of CMB on day 11 also inactivated mature CTL and decreased the number of CD8+ cells. CMB inhibits V-ATPase activity specifically,18,19 whereas PrG 25-C uncouples proton pump activity mediated byV-ATPase.25,26 The observation that both inhibitors (which have different mechanisms for the inhibition of granule acidification) suppressed the CTL activity in vivo, argues strongly that inhibiting granule acidification is essential for the suppression of CTL activity by PrG 25-C and CMB in vivo. Although both inhibitors had more potent suppression on CTL activity than on production of antialloantigen antibody, PrG 25-C was less selective than CMB. As PrG 25-C also slightly uncouples the mitochondrial proton pump,24 suppression of acidification mediated through V-ATPase by PrG 25-C is less selective as compared to CMB. It is possible that the suppression of antibody production against alloantigen results from an effect other than by inhibition of the V-ATPase-mediated proton pump. PrG 25-C also suppresses the function of antigen-presenting cells (APC) in alloantigen-stimulated mixed lymphocyte reaction (MLR), which was not observed with CMB.29 Such effects of PrG 25-C, which do not result from the inhibition of acidification of cytotoxic granules, may contribute to the immunosuppressive effect of PrG 25-C.
Consistent with the PrG 25-C- and CMB-induced reduction of cytotoxicity and of the CD8+ population, administration of PrG 25-C or CMB reduced the perforin expression in splenocytes. The effect was more marked with PrG 25-C than with CMB. The expression level of perforin was reduced to the level observed in naive mice, while reduction in the number of CD8+ cells by PrG 25-C was only partial. Thus, it is probable that PrG 25-C, but not CMB, degraded the perforin of CD8+ cells that were still present in the spleen. Although previous work suggested that CMB, but not PrG 25-C, induced degradation of perforin in vitro,23,24 the results of this study demonstrate that PrG 25-C also induced the degradation of perforin in vivo. As degradation of perforin by the inhibitors was dependent on the ability to inhibit the acidification of cytotoxic granules,24 it is expected that the higher dose of CMB could induce the degradation of perforin in vivo. PrG 25-C might inhibit the acidification more effectively in vivo than in vitro.
Although CMB and PrG 25-C inactivate the function of activated CTL in vitro,22–24,27 it is probable that they inhibit CTL activity by inactivation and the subsequent reduction of activated CTL in alloantigen-stimulated mice. The decrease of CTL activity and the reduction of CD8+ cells 11 hr after the injection of antibiotics were closely related to each other (Fig. 4 and Table 1). Although concanamycin A induces disorganization of cytotoxic granules and decreases the intracellular activity of perforin, and induces DNA fragmentation activity, N-α-benzyloxycarbonyl- l-lysine thiobenzyl-esterase activity, which is mainly mediated by granzyme A, is conserved, and the amount of diisopropylfluorophosphoridate-binding proteins (mainly granzyme A) is not changed.22,23 Granzyme family proteases induce apoptosis, initiated by the activation of caspase family proteases, if introduced directly into the cytoplasm of target cells.30–32 Given that cytotoxic granules protect CTL from apoptosis with their own cytotoxic mediators by physical isolation of granzymes in acidic environments, it is possible that disorganization of cytotoxic granules promotes the apoptosis of CTL with their own cytotoxic factors in the granules which are leaked to, and activated in, the cytoplasm. In fact we recently found that concanamycin A induced the apoptosis of activated CD8+ CTL, but not apoptosis of CD4+ CTL,33 which kill tumour cells through a Fas-mediated mechanism.34,35 This result is consistent with the observation that concanamycin A does not affect the Fas-mediated cytotoxic activity of CTL.36
In conclusion, the present study demonstrates that PrG 25-C and CMB decrease the number of mature CTL which have been activated through alloantigen stimulation. This effect is entirely different from the immunosuppressive effect of FK506 and cyclosporin A, which inhibit the early phase of CTL induction following stimulation with alloantigen. Therefore, PrG 25-C and CMB may be clinically useful as immunosuppressants for the later phase of graft rejection, by eliminating mature CTL that have escaped suppression by FK506 and cyclosporin A. PrG 25-C and CMB might also be of use for studying the effect of CTL in various experimental models.
Acknowledgments
We are grateful to Dr H. Yagita (Juntendo University, Tokyo), Chugai Pharmaceutical Co. and Taisho Pharmaceutical Co., for kindly supplying antiperforin antibody (P1-8), PrG 25-C and CMB. We also thank Dr Y. Magae (Forestry and Forest Products Research Institute, Tsukuba, Japan) and Science Editing International for assistance with densitometry and with editing the manuscript. This work was supported in part by a Grant-in-Aid for the Biodesign Research Program from the Institute of Physical and Chemical Research (RIKEN) to K. Nagai and by a research grant from the Ministry of Education, Science and Culture of Japan.
References
- 1.Harashima K, Tsuchida N, Tanaka T, Nagatsu J. Prodigiosin 25-C: isolation and the chemical structure. Agric Biol Chem. 1967;31:481. [Google Scholar]
- 2.Wasserman HH, Rodgers GC, Keith DD. Undecylprodigiosin. Tetrahedron. 1976;32:1851. [Google Scholar]
- 3.Nakamura A, Nagai K, Ando K, Tamura G. Selective suppression by prodigiosin of the mitogenic response of murine splenocytes. J Antibiot. 1986;39:1151. doi: 10.7164/antibiotics.39.1155. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji RF, Yamamoto M, Nakamura A, et al. Selective immunosuppression of prodigiosin 25-C and FK506 in the murine immune system. J Antibiot. 1990;43:1293. doi: 10.7164/antibiotics.43.1293. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka T, Magae J, Nariuchi H, Yamasaki M, Nagai K. Enhancement by concanavalin A of the suppressive effect of prodigiosin 25-C on proliferation of murine splenocytes. J Antibiot. 1992;45:1301. doi: 10.7164/antibiotics.45.1303. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura A, Magae J, Tsuji RF, Yamasaki M, Nagai K. Suppression of cytotoxic T cell induction in vivo by prodigiosin 25-C. Transplantation. 1989;47:1013. doi: 10.1097/00007890-198906000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji RF, Magae J, Yamashita M, Nagai K, Yamasaki M. Immunomodulating properties of prodigiosin 25-C, an antibiotic which preferentially suppresses induction of cytotoxic T cells. J Antibiot. 1992;45:1295. doi: 10.7164/antibiotics.45.1295. [DOI] [PubMed] [Google Scholar]
- 8.Magae J, Miller MM, Nagai K, Shearer GM. Effect of metacycloprodigiosin, an inhibitor of killer T cells, on murine skin and heart transplants. J Antibiot. 1996;49:86. doi: 10.7164/antibiotics.49.86. [DOI] [PubMed] [Google Scholar]
- 9.Songia S, Mortellaro A, Taverna S, et al. Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol. 1997;158:3987. [PubMed] [Google Scholar]
- 10.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked byFK-506 and cyclosporin A. Nature. 1991;352:803. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell. 1991;66:807. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 12.Larsson E-J. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980;124:2828. [PubMed] [Google Scholar]
- 13.Shevach E. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- 14.Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 15.Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987;40:1256. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 16.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797. [PubMed] [Google Scholar]
- 17.Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718. [PubMed] [Google Scholar]
- 18.Bowman EJ, Shiebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo J-T, Shinohara C, Sakai K, Hasumi K, Endo A. Inhibition of the acidification of endosomes and lysosomes by the antibiotic concanamycin B in macrophage J774. Eur J Biochem. 1992;207:383. doi: 10.1111/j.1432-1033.1992.tb17061.x. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka T, Magae J, Kasamo K, et al. Effects of prodigiosin 25-C on cultured cell lines: its similarity to monovalent polyether ionophores and vacuolar type H+-ATPase inhibitors. J Antibiot. 1992;45:1618. doi: 10.7164/antibiotics.45.1618. [DOI] [PubMed] [Google Scholar]
- 21.Mellman I, Fucks R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka T, Takaku K, Magae J, et al. Acidification is essential for maintaining the structure and function of lytic granules of CTL. J Immunol. 1994;153:3938. [PubMed] [Google Scholar]
- 23.Kataoka T, Togashi K, Takayama H, Takaku K, Nagai K. Inactivation and proteolytic degradation of perforin within lytic granules upon neutralization of acidic pH. Immunol. 1997;91:493. doi: 10.1046/j.1365-2567.1997.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togashi K, Kataoka T, Nagai K. Characterization of a series of vacuolar type H+-ATPase inhibitors on CTL-mediated cytotoxicity. Immunol Lett. 1997;55:139. doi: 10.1016/s0165-2478(97)02698-9. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka T, Muroi M, Ohkuma S, et al. Prodigiosin 25-C uncouples vacuolar type H+-ATPase, inhibits vacuolar acidification and affects glycoprotein processing. FEBS Lett. 1995;359:53. doi: 10.1016/0014-5793(94)01446-8. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Konno H, Tanaka Y, et al. Prodigiosins as a new group of H+/Cl– symporters that uncouple proton translocators. J Biol Chem. 1998;273:21455. doi: 10.1074/jbc.273.34.21455. [DOI] [PubMed] [Google Scholar]
- 27.Lee M-H, Kataoka T, Magae J, Nagai K. Prodigiosin 25-C suppression of cytotoxic T cells in vitro and in vivo is similar to that of concanamycin B, a specific inhibitor of vacuolar type H (+)-ATPase. Biosci Biotech Biochem. 1995;59:1417. doi: 10.1271/bbb.59.1417. [DOI] [PubMed] [Google Scholar]
- 28.Magae J, Illenye S, Tejima T, et al. Transcriptional squelching by ectopic expression of E2F-1 and p53 is alleviated by proteasome inhibitors MG-132 and lactacystin. Oncogene. 1997;15:759. doi: 10.1038/sj.onc.1201251. [DOI] [PubMed] [Google Scholar]
- 29.Lee M-H, Yamashita M, Tsuji RF, et al. Suppression of T cell stimulating function of allogeneic antigen presenting cells by prodigiosin 25-C. J Antibiot. 1998;51:92. doi: 10.7164/antibiotics.51.92. [DOI] [PubMed] [Google Scholar]
- 30.Hayes MP, Berrebi GA, Henkart PA. Induction of target cell DNA release by the cytotoxic T lymphocyte granule protease granzyme A. J Exp Med. 1989;170:933. doi: 10.1084/jem.170.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Kam CM, Powers JC, Aebersold R, Greenberg AH. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992;176:1521. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Togashi K, Kataoka T, Nagai K. Concanamycin A, a vacuolar type H+-ATPase inhibitor, induces cell death in activated CD8+ CTL. Cytotechnology. 1997;25:127. doi: 10.1023/A:1007995212658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127. [PubMed] [Google Scholar]
- 35.Ju S-T, Cui H, Panka D, Ettinger R, Marshak-rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kataoka T, Shinohara N, Takayama H, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678. [PubMed] [Google Scholar]


