Abstract
L-selectin (CD62L) is a cell adhesion molecule which plays a key role in the initiation of leucocyte migration from blood vessels to sites of local inflammation. The aim of this study was to investigate T-lymphocyte expression of CD62L antigen and serum levels of soluble L-selectin (sL-selectin) in subjects with clinical and preclinical type I diabetes to determine whether they could provide surrogate markers for disease activity. CD62L selectin expression on memory T lymphocytes was studied by cytometric analysis in 22 patients with newly diagnosed type I diabetes, 20 first-degree relatives of patients with type I diabetes, 14 patients with Graves’ disease, and 22 healthy controls. sL-selectin levels were measured by enzyme-linked immunosorbent assay (ELISA) in enlarged groups of subjects in these categories, as well as in patients with long-standing type I diabetes, treated Graves’ disease and type II (non-insulin dependent) diabetes. L-selectin levels were also related to islet autoantibodies, human leucocyte antigen (HLA) genotype and L-selectin T668C gene polymorphisms. L-selectin expression on memory T lymphocytes was reduced in newly diagnosed diabetes and islet autoantibody positive siblings compared with controls. sL-selectin levels were significantly raised in newly diagnosed type I diabetes compared with controls, with intermediate levels in family members, both with and without islet autoantibodies, and in long-standing type I diabetes. Levels were also raised in patients with untreated Graves’ disease. Patients with type II diabetes had sL-selectin levels which did not differ from controls. sL-selectin levels correlated with the presence of diabetes-associated HLA alleles in both family members and controls; levels also fell with increasing age in family members. Multiple regression analysis showed that HLA genotype and age were independent determinants of sL-selectin levels. sL-selectin levels are raised at the time of diagnosis of type I diabetes and Graves’ disease and appear to be modulated by disease activity, but levels are determined predominantly by HLA-associated genetic susceptibility and age. sL-selectin may provide a late marker of autoimmune destruction of islets and sequential measurement may be useful in monitoring disease activity and the effect of interventions preceding type I diabetes.
Introduction
In the last few years trials of agents that may prevent the development of insulin-dependent diabetes (type I diabetes) at the stage of asymptomatic autoimmunity have been started.1 Current studies use progression to overt diabetes as the endpoint, requiring large and protracted studies. There is therefore an urgent need to identify surrogate immune markers by which effects of manipulation of the autoimmune process can be rapidly evaluated.
Insulitis – mononuclear cell infiltration of the pancreatic islets – is a hallmark of the development of type I diabetes and results from preceding events including cytokine production, lymphocyte activation and their migration from the peripheral blood. Three families of adhesion molecules: selectins, integrins and immunoglobulin-related molecules mediate lymphocyte adherence to vascular endothelial cells and extravasation.2,3
L-selectin (CD62L), a member of the selectin family, is a cell adhesion molecule expressed on the cell surface of peripheral lymphocytes, monocytes and neutrophils. It plays a key role in the initiation of migration of leucocytes from the vessels into tissues,4 and is involved in lymphocyte migration to different sites of local inflammation, including the pancreatic islets in animal models of type I diabetes.5,6
Previous studies have suggested that L-selectin and other adhesion molecules may be useful additional markers of risk of type I diabetes, independent of antibodies against pancreatic islet autoantigens.7–10 In particular, Lampeter et al. have shown elevated serum levels of sL-selectin in recently diagnosed patients and subjects at risk of insulin-dependent diabetes.7
Our aim was to investigate changes in peripheral blood L-selectin levels in type I diabetes, and to determine whether these might be useful markers of ongoing autoimmunity which could potentially be used to monitor the effects of immunomodulation. To address this issue we sought evidence that levels varied during the course of the autoimmune disease process, and that differences we observed between diabetic patients and controls could not be explained by metabolic derangement. We then studied non-diabetic first-degree relatives of children with type I diabetes and related L-selectin levels to immune and genetic markers of susceptibility. We also investigated the relationship between soluble L-selectin levels and L-selectin T668C gene polymorphism, as this mutation was shown to result in an abnormal conformation of the epidermal growth factor (EGF)-domain of the L-selectin molecule and hypothetically is able to influence its functions and/or peripheral blood levels.14
Materials and methods
Subjects
The study was carried out in the following groups of subjects of Polish origin: (a) 39 patients with newly diagnosed type I diabetes; (b) 44 patients with long-standing diabetes; (c) 27 patients with type II diabetes; (d) 90 first-degree relatives of children with type I diabetes; (e) 29 patients with untreated and treated Graves’ disease; and (f) 78 healthy controls. Subject characteristics are summarized in Table 1.
Table 1.
Characteristics of the studied groups
| Patient group | Number | Sex | Mean age (years) | Mean duration of disease |
|---|---|---|---|---|
| Newly diagnosed type I diabetes | 39 | 14F, 25M | 12 7 ± 6·2 | < 3 months from diagnosis |
| Long-standing type I diabetes | 44 | 26F, 18M | 24·8 ± 10·5 | 8·4 ± 4·7 years, 2–18 years |
| Type II diabetes | 27 | 15F, 12M | 53·2 ± 8·0 | 7·1 ± 4·3 years |
| First degree relatives – siblings | 62 | 21F, 41M | 16·7 ± 7·7 | – |
| First degree relatives - parents | 28 | 12F, 16M | 40·5 ± 9·2 | – |
| Untreated Graves’ disease | 14 | 19F, 10M | 36·8 ± 0. 13 3 | – |
| Treated Graves’ disease (methimazole) | 15 | 7–24 months | ||
| Healthy controls | 54 | 29F, 25M | 20 1 ± 3·4 | – |
| Healthy controls-blood donors | 24 | 2 F, 22 M | 33 0 ± 8·9 | – |
Cytometric analyses were performed on 22 of the patients with newly diagnosed type I diabetes, 20 siblings of individuals with disease, 14 patients with Graves’ disease and 22 healthy controls.
No subjects had experienced acute infection or illness during the 3 weeks prior to the sample collection. Informed consent was obtained. The protocol for the study was approved by the Local Human Subjects Committee at the Medical School Białystok.
Sample collection
Fasting venous samples were collected between 7·30 and 8·30 a.m. for measurement of serum sL-selectin levels, genetic analysis, morphology parameters, HbA1C and CD phenotyping. Sera for measurement of sL-selectin were stored at −80° prior to analysis.
Leucocyte preparation and flow cytometry analysis
Leucocyte preparation from whole blood was performed within 2 hr of collection at room temperature on a Q-Prep EPICS Immunology Workstation (Coulter Corp., Hialeah, FL).
The percentages of lymphocyte subsets: CD4+ CD45RO+ CD62L+, CD4+ CD45RO+ CD62L–, CD4+ CD62L+, CD4+ CD62L–, CD4+ CD45RO+ were measured on a Coulter EPICS XL cytometer using combinations of monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), R-phycoerythrin (PE) or phycoerythrin–Texas Red (ECD) directed against CD4, CD45R0, CD62L (L-selectin) and CD14/CD45 were used as control lymphocyte surface markers (Coulter, Krefeld, Germany). A minimum of 10 000 lymphocytes were analysed for each sample. The percentage of positive cells was determined by setting the lower limit over the non-specific fluorescence (< 2%). The combination of antibodies: Ms-immunoglobulin G (IgG)1–ECD, MsIgG2a–PE and MsIgG2b–FITC (Coulter,Krefeld, Germany) were used to determine unspecific antibody binding. The total number of leucocytes and lymphocytes in the peripheral blood were measured by haematological MAXM counter (Coulter, Krefeld, Germany) and the absolute cell numbers of studied lymphocyte subpopulations were counted.
sL-selectin measurement
Levels of sL-selectin in the serum were determined by enzyme-linked immunosorbent assay (ELISA) (Parameter kit, R & D, UK). The intra-assay coefficient of variation (CV) of the method was 3·5% with interassay CV of 6·6%.
Human leucocyte antigen (HLA) typing
HLA typing and L-selectin polymorphism studies were performed in groups newly diagnosed and long-standing type I diabetes and in first degree relatives of children with type I diabetes. DNA was extracted from ethylenediamine tetra-acetic acid (EDTA) blood using a protein salting-out method.11
HLA typing for the presence of HLA class II genes associated with the highest risk of type 1 diabetes development: DQB1*0302, DQB1*0201, DRB1*03, DRB1*04 alleles and with the strongest protection in the Polish population (allele DQB1*0602) 12 was performed using the polymerase chain reaction–sequence-specific primer (PCR–SSP) ‘phototyping’ method.13
Detection of the T668C L-selectin polymorphism
Alleles of the L-selectin T668C gene polymorphism were determined by single-strand conformation polymorphism (SSCP) on mutation detection enhancement (MDE) polyacrylamide gels (Flowgen, Lichfield, UK). The primer sequences were kindly provided by Dr K. Wenzel, Humboldt University, Berlin, Germany.14 To confirm our results, PCR products of a C homozygote and a T homozygote were sequenced with the same 5′ sense primer used for amplification on an ABI Prism 377 (Applied Biosystems, Warrington, UK)
Autoantibody analysis
Islet cell antibodies (ICA) were performed by indirect immunofluorescence on group O human pancreas, antibodies to glutamic acid decarboxylase (GADA), insulin autoantibodies (IAA) and antibodies to tyrosine phosphatase (IA-2A) by radioimmunoassay.15–17 In the first IDS combined antibody workshop, using thresholds giving 99% specificity, these assays achieved sensitivities of 91% for GADA, 58% for IAA and 74·4% for IA-2 A18 Antibody positivity was defined using a threshold equivalent to the 97·5th centile of 2860 schoolchildren.16 HbA1C was quantified by liquid chromatography (Bio-Rad, Richmond, CA), glucose concentration was measured by an enzymatic method (Cormay, Lublin, Poland).
Data analysis
Results are presented as median (interquartile range). HLA genotypes were categorized as high risk (with two main haplotypes predisposing to type I diabetes: DRB1*04-DQB1*0302 and DRB1*03-DQB1*0201), intermediate risk without protective alleles (either DRB1*04-DQB1*0302 or DRB1*03-DQB1*0201 haplotypes, without DQB1*0602 allele), intermediate risk with protective alleles (either DRB1*04-DQB1*0302 or DRB1*03-DQB1*0201 haplotype, with DQB1*0602), and low risk (neither DRB1*04-DQB1*0302 nor DRB1*03-DQB1*0201). The statistical significance of the differences in lymphocyte subsets and sL-selectin concentration between the studied groups was estimated by the Mann–Whitney U-test or Kruskal–Wallis test as appropriate. The relationship between the T lymphocyte L-selectin expression ratio and serum sL-selectin levels were evaluated by Spearman’s rank correlation. The independent effects of HLA genotype and age on sL-selectin levels were analysed using multiple regression. P-values, corrected for the number of comparisons, were considered significant when < 0·05. Statistical testing was performed using Statistica 5·0 (StatSoft, Tulsa, OK) and Statistics Package for Social Sciences version 9·0.0 (SPSS Inc. Chicago, IL).
Results
CD62L antigen expression
The absolute numbers of total leucocytes, lymphocytes and memory T cells (CD4+ CD45RO+) in the peripheral blood did not differ significantly between the studied groups (data not shown).
In patients with newly diagnosed type I diabetes and in relatives with elevated levels of two or more islet antibodies we observed statistically lower numbers of memory T cells expressing CD62L antigen (median 221 cells/ml (interquartile range 139–268 cells/ml) (P < 0·02) and 195 cells/ml (159–241 cells/ml) (P < 0·002), respectively) in comparison to the healthy controls (median 329 cells/ml (interquartile range: 239–339 cells/ml)).
Type I diabetic patients and their at-high-risk-of-diabetes relatives had also significantly increased CD4+ CD45RO+ CD62L–/CD4+ CD45RO+ CD62L+ cell ratios (L-sel–/L-sel+) (respectively: median 0·875 (interquartile range 0·70–1·14) and median 0·887 (0·72–1·10)) versus controls (median 0·574 (interquartile range 0·48–0·75)) (P < 0·02, P < 0·004). The L-sel–/L-sel+ ratio and the number of CD4+ CD45RO+ CD62L– lymphocytes did not differ significantly between patients with Graves’ disease and controls (P = 0·062). There was a significant negative correlation between the levels of sL-selectin in the circulation and the ratio CD62L negative/CD62L positive T-helper lymphocytes in diabetic and prediabetic groups (R = −0·609, P < 0·01). (Fig. 1)
Figure 1.
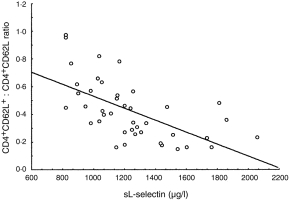
Relationship between the ratio of L-selectin positive and negative helper T lymphocytes (CD4+ CD62L+/CD4+ CD62L–) and soluble L-selectin levels in peripheral blood of diabetic subjects and antibody positive family members (R = −0·609, P < 0·001).
sL-selectin levels
The individual results and median of sL-selectin levels in the peripheral blood are presented in Fig. 2.
Figure 2.
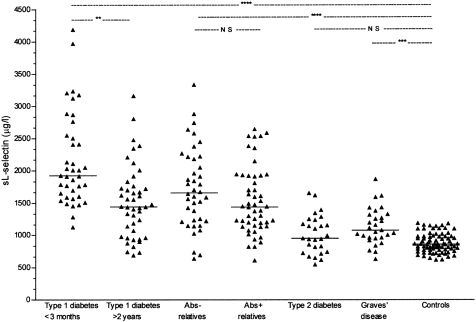
The individual and median (—) sL-selectin levels in the peripheral blood of patients with newly diagnosed (n = 39) and long-term (n = 44) type I diabetes mellitus, first degree relatives of patients with type I diabetes with (Abs+) (n = 50) and without islet antibodies against pancreatic beta-cells (Abs–) (n = 40), patients with type II diabetes mellitus (n = 27), Graves’ disease (n = 29) and healthy controls (n = 78). **Pcor < 0·003, ***Pcor < 0·0002, ****Pcor < 0·00001, Pcor=P × 7.
Clinically overt autoimmune disease
Increased levels of sL-selectin were found in patients with both newly diagnosed diabetes and untreated Graves’ disease compared with controls. Further analysis in the subgroup of the patients with long-term type I diabetes reveals a negative correlation between L-selectin levels and the duration of disease (R = −0·447, t = −3·21, P < 0·003) (Fig. 3). Patients with a diabetes disease duration of more than 10 years, and those with treated Graves’ disease had sL-selectin levels equivalent to those in normal controls (Fig. 3).
Figure 3.
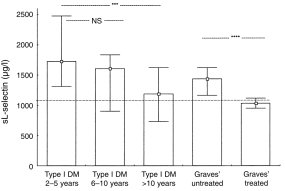
Comparison of sL-selectin levels (median, interquartile range) in the peripheral blood of type I diabetic subjects (> 2 years. from diagnosis) in association with the disease duration and in patients with untreated (n = 14) and methamizole treated (n = 15) Graves’ disease. The dotted line represents mean+ 2SD of the sL-selectin levels in healthy controls. ***P < 0·005,****P < 0·0001.
First degree relatives and controls
sL-selectin levels were significantly higher in unaffected first degree relatives of patients with type I diabetes than in controls (Fig. 2). There was no significant difference in sL-selectin levels according to islet autoantibody status. The median sL-selectin level in antibody negative relatives was 1671 mg/l (1246–2199) compared with 1435 mg/l (1145–1915) in those with one or more antibody markers above the 97·5th centile of schoolchildren (P = 0·08), and 1572 mg/l (1410–2163) in those with two or more antibody markers above this threshold (P = 0·96).
sL-selectin levels in patients with type II diabetes (Fig. 2) did not differ significantly from normal controls. There was no correlation between L-selectin levels and HbA1C or blood glucose concentrations (data not shown).
sL-selectin levels were related to HLA class II determined susceptibility to type I diabetes (P = 0·001) (Fig. 4a). Levels were highest in family members with the high risk DRB1*04-DQB1*0302/DRB1*03-DQB1*0201 genotype and lowest in those with neither haplotype. Intermediate levels were seen in family members with either DRB1*04-DQB1*0302 or DRB1*03-DQB1*0201 and, within this group, a protective DQB1*0602 allele was associated with lower levels. A similar relationship was seen in controls (P = 0·004) (Fig. 4b). Soluble L-selectin concentrations were significantly lower in the subgroups of first degree relatives with the T668C mutation of the L-selectin gene in comparison to the levels in the subjects without this mutation (1230 mg/l (1063–1614) versus 1840 mg/l (1424–1945), P = 0·0006). A similar difference was seen between healthy controls with and without this mutation (805 mg/l (735–910) versus 915 mg/l (825–1020), P = 0·0049). sL-selectin levels did not however, differ according to L-selectin gene polymorphisms in patients with type I diabetes (1605 (1343–1887) versus 1710 (1410–2365), P = 0·28).
Figure 4.
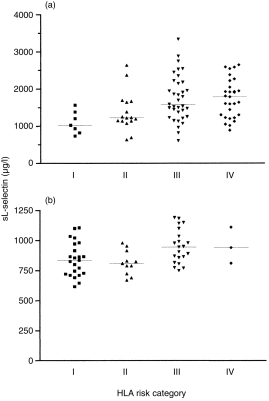
Correlation of sL-selectin levels and HLA class II risk category (a) unaffected relatives of patients with type I diabetes and (b) healthy controls. sL-selectin levels increase progressively with increasing HLA determined genetic susceptibility. Category I contains relatives with no diabetes associated haplotypes, Categories II and III have at least one susceptibility haplotype with or without the protective DQB1*0602 allele, and Category IV contains relatives at highest risk who are heterozygous for DRB1*03-DQB1*0201/DRB1*04-DQB1*0302.
sL-selectin levels were inversely correlated with age in family members (r2 = 0·27, P < 0·0001) but not in controls (P = 0·7) (Fig. 5). Multiple regression analysis showed that, in family members, age and HLA defined risk category were independent determinants of sL-selectin levels with significance P < 0·0001 and P = 0·001, respectively.
Figure 5.
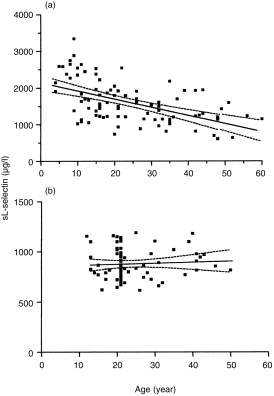
Correlation of sL-selectin levels and age in (a) unaffected relatives of patients with type I diabetes and (b) healthy controls.
Discussion
Our aim was to investigate changes in peripheral blood L-selectin levels in type I diabetes, and to determine whether these might be useful markers of ongoing autoimmunity which could potentially be used to monitor the effects of immunomodulation. We found that L-selectin expression on memory T lymphocytes (CD4+ CD45RO+) cells is decreased in newly diagnosed type I diabetes. We then went on to determine whether this change, which may reflect alterations in the balance of T helper 1 (Th1)/Th2 cytokines,19 might be useful as a surrogate marker to monitor ongoing autoimmunity. Because there is an inverse correlation between CD62L expressing memory cells and serum sL-selectin levels (Fig. 1), the remainder of our evaluation was based on serum sL-selectin levels.
A basic requirement for any marker to be used as a surrogate for monitoring immune inventions is that it should be relatively simple to measure. sL-selectin assay fulfils this since the assay is relatively straightforward and suitable for high throughput. In addition changes in the marker should reflect the disease process and not result from metabolic disturbance. Normal sL-selectin levels in patients with type II diabetes indicated that the changes could not be attributed to hyperglycaemia.
Our main findings were that sL-selectin levels were raised in clinically overt autoimmune disease – in type I diabetes and, to a lesser extent, in Graves’ disease – and that they fell with increasing disease duration and treatment. We also found, however, that levels were higher in relatives of patients with type I diabetes than in controls, and were equivalent to those found in long-standing type I diabetes (Fig. 2). This supports the hypothesis that changes in sL-selectin do reflect the degree of autoimmune activation, but suggests there is also a baseline elevation in relatives, irrespective of disease activity.
There was no clear association between levels of sL-selectin and islet autoantibodies in unaffected first-degree relatives of children with type I diabetes. This finding, which has also been reported by other investigators,7 is unexpected. Elevated levels of antibodies are generally assumed to reflect ongoing autoimmunity and are associated with high risk of progression to diabetes.1 One possible explanation is that autoantibody levels rise as soon as the autoimmune process is activated providing an early marker of disease. In contrast, sL-selectin levels could associate more closely with destructive insulitis occurring later in the disease process. Such a marker might also be expected to reach a peak immediately prior to the clinical onset of diabetes, and to normalize, as we have observed, with reduction of immune activity in long-standing diabetes or treated Graves’ disease.
The baseline elevation of sL-selectin levels in relatives irrespective of autoantibody status is likely to be genetically determined. Our findings in both controls and unaffected relatives suggest that sL-selectin levels are related to HLA class II genotype, and that they are highest in individuals with the DRB1*03-DQB1*0201/DRB1*04-DQB1*0302 genotype which is most closely associated with diabetes. A higher prevalence of diabetes susceptibility genes might therefore contribute to higher sL-selectin levels in unaffected family members both with and without elevated levels of islet autoantibodies. Our preliminary findings suggest that sL-selectin levels may also be modulated by polymorphisms of the L-selectin gene. In this population, however, we observed that the T668C mutation which was associated with lower levels of sL-selectin was also significantly associated with DQ alleles protective for diabetes in the unaffected relatives, though not in patients with diabetes or controls. The role of the sL-selectin gene therefore needs further investigation.
Interpretation is further complicated by our observation that there was a significant decrease in sL-selectin levels with increasing age in unaffected first degree relatives, and multiple regression analysis suggests that genotype and age are independent predictors of sL-selectin levels in this group. Our results in healthy controls are more difficult to interpret since the groups were not of equivalent age. Few studies have addressed the subject of cell adhesion molecules and age, but it has been shown that L-selectin positive memory cells are Th2-like and L-selectin negative cells are Th1-like,19 and some studies have reported that the ratio of Th1/Th2 cells decreases in old age in humans and animals.20–23
We have therefore confirmed that sL-selectin levels are elevated in type I diabetes and Graves’ disease. We found that although they appear to be modulated by disease activity, levels are determined predominantly by HLA-determined genetic susceptibility and age. This implies that sL-selectin levels are unlikely to be superior to autoantibody markers in identifying family members at high risk of progression to diabetes. Sequential measurement may however, be useful in monitoring disease activity, and studies showing longitudinal changes in levels of soluble adhesion molecules in response to treatment in ophthalmic Graves’ disease support the possible use of sL-selectin as a measure of the effect of treatment in the later stages of the pathogenesis of type I diabetes.
Acknowledgments
We are grateful to Professor Edwin Gale from University of Bristol, UK for critically reading this manuscript. P.J.B. is funded by the Juvenile Diabetes Foundation.
Glossary
Abbreviations
- L-sel–/L-sel+ ratio
L-selectin negative cells/L-selectin positive cell ratio
- Th
T helper
- SSCP
single-strand conformational polymorphism
- MDE
mutation detection enhancement
- HLA
human leucocyte antigen
References
- 1.Honeyman M, Wasserfall C, Nerup J, Rossini A. Prediction and prevention of IDDM. Diabetologia. 1997;40:B58. doi: 10.1007/BF03168188. [DOI] [PubMed] [Google Scholar]
- 2.Frenette PS, Wagner DD. Adhesion molecules-part II: Blood vessels and blood cells. N Engl J Med. 1996;335:45. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- 3.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068. [PubMed] [Google Scholar]
- 4.Nicholson MW, Barclay AN, Singer MS, Rosen SD, van der Merwe PA. Affinity and kinetic of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- 5.Feveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969. [PubMed] [Google Scholar]
- 6.Tanaka T, Kitagawa Y, Obayashi H, et al. Prediction of diabetes in BB/WOR rats by measurements of soluble L-selectin. Diabetologia. 1995;38 Suppl 1:a81(abstract). [Google Scholar]
- 7.Lampeter ER, Kishimoto TK, Rothlein R, et al. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992;41:1668. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- 8.Martin S, Lampeter EF. A physiological role for circulating adhesion molecules? Immunol Today. 1994;15:141(Letter). doi: 10.1016/0167-5699(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 9.Martin S, Rothe H, Tschope D, Schwippert B, Kolb H. Decreased expression of adhesion molecules on monocytes in recent onset IDDM. Immunology. 1991;73:123. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Michie SA, Mebius RE, Tisch R, Weissman I, McDevitt HO. The role of cell adhesion molecules in the development of IDDM. Diabetes. 1996;45:705. doi: 10.2337/diab.45.6.705. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen J, Samarut J, Holtta E. A nontoxic and versatile protein salting-out method for isolation of DNA. Biotechniques. 1994;17:316. [PubMed] [Google Scholar]
- 12.Krokowski M, Bodalski J, Bratek A, Boitard CH, Caillat-zucman S. HLA class II-associated predisposition to insulin-dependent diabetes mellitus in a Polish population. Human Immunol. 1998;59:451. doi: 10.1016/s0198-8859(98)00036-6. [DOI] [PubMed] [Google Scholar]
- 13.Bunce M, O’neill CM, Barnardo MC. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel K, Ernst M, Rohde K, Baumann G, Speer A. DNA polymorphisms in adhesion molecule genes – a new factor for early atherosclerosis. Hum Genet. 1996;97:15. doi: 10.1007/BF00218826. [DOI] [PubMed] [Google Scholar]
- 15.Bingley PJ, Bonifacio E, Shattock M, et al. Can islet autoantibodies predict IDDM in the general populations. Diabetes Care. 1993;16:45. doi: 10.2337/diacare.16.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Bingley P, Bonifacio E, Wiliams AJK, Genovese S, Botazzo GF, Gale EAM. Prediction of IDDM in the general population. Strategies based on combinations of autoantibody markers. Diabetes. 1997;46:1701. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 17.Williams AJK, Bingley P, Bonifacio E, Palmer JP, Gale EAM. A novel microassay for insulin autoantibodies. J Autoimmunity. 1997;10:473. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 18.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: combinatorial islet autoantibody workshop. Diabetes. 1998;47:1857. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 19.Kanegane H, Kasahara Y, Niida Y, et al. Expression ofL-selectin (CD62L) discriminates Th1- and TH2-like cytokine-producing memory CD4+ T cells. Immunology. 1996;87:186. doi: 10.1046/j.1365-2567.1996.446530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 21.Rea IM, Stewart M, Campbell P, Alexander HD, Croxkard AD, Morris TC. Changes in lymphocyte subsets, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42:69. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- 22.Pioli C, Pucci S, Barile S, Frasca D, Doria G. Role of mRNA stability in the different patterns of cytokine production by CD4+ cells from young and old mice. Immunology. 1998;94:380. doi: 10.1046/j.1365-2567.1998.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirai A, Conover J, Klinmann DM. Increased activation and altered ratio of interferon-gamma: interleukin-4 secreting cells in MRL-lpr/lpr mice. Autoimmunity. 1995;21:107. doi: 10.3109/08916939508993357. [DOI] [PubMed] [Google Scholar]


