Abstract
Relatively little is known of the details of human leucocyte antigen (HLA) expression and thymocyte selection in human thymus. In both humans and mice major histocompatibility complex (MHC) molecules have been described which show a highly restricted thymic expression. Such patterns may offer clues about cellular interactions in thymic selection because transgenic mice with MHC expression targeted to specific thymic sites show altered T-cell receptor (TCR) repertoire selection. We have analysed human thymic HLA class II expression, relating the expression pattern to sites of thymocyte apoptosis. While HLA-DQ is poorly expressed by most peripheral antigen-presenting cells (APC), thymus stains strongly for HLA-DQ as well as for HLA-DR. HLA-DM is abundant in medulla but weakly expressed by cortical cells. Class II expression in Hassall’s corpuscles (HC) is unusual in several respects: we have previously shown them to be encircled by HLA-DO+ epithelial cells and here further demonstrate that HC are negative for HLA-DR and HLA-DP, but often positive for HLA-DQ and HLA-DM. Transcriptional control of HLA class II products at this site is thus unlike cells that have previously been studied. Apoptotic thymocytes are restricted to the cortex and the corticomedullary junction. However, a minority of apoptotic cells are visible in the medulla, these being found in the HLA-DQ positive HC. The apoptotic thymocytes in HC can be CD4+ single positive (SP), CD8+ SP or CD4+CD8+ double-positive (DP). This study thus shows that the HC within human thymic medulla are noteworthy both for their unusual hierarchy of HLA class II expression and because they are the only medullary site of thymocyte apoptosis. We propose that HC are a site at which mature thymocytes receive activation/tolerization signals from peptides reprocessed from apoptotic cells. The differential HLA transcriptional control at this site may indicate that specific T-cell subpopulations are affected.
Introduction
The interactions between major histocompatibility complex (MHC) molecules, T-cell receptor (TCR) and peptide, which lead to positive or negative selection of developing thymocytes, have been the source of extensive investigation in mouse models. Using either in vivo systems or fetal thymic organ culture, negative selection has been analysed in terms of the acquisition of tolerance in TCR transgenic mice or T-cell deletion by endogenous superantigens. The observation that either positive or negative selection can ensue from engagement of the TCR expressed by thymocytes may be attributable to differences in affinity1 and/or qualitative differences in the ligand recognized.2 An anatomical distinction between positive and negative selection was initially suggested by experiments showing that positive selection is dependent on thymic epithelial cells while negative selection requires expression of MHC by dendritic cells, abundant in the medulla.3–5 We, as well as other laboratories, have more recently investigated the anatomy of negative selection directly, visualizing the sites of thymocyte apoptosis by staining for DNA strand breaks with diuridine triphosphate (dUTP), using the terminal deoxynucleotidyl dUTP nick end labelling (TUNEL) method.6–8 Thymocyte apoptosis caused by interaction with cognate peptide and MHC is most commonly localized to the cortex but can sometimes be found at the corticomedullary junction or in the medulla, while negative selection caused by the endogenous superantigens mouse mammary tumour virus (MTV) 8 and 9 is seen only in the medulla.6,7
Relatively little is known about the possible relationship between these observations in the mouse and human thymocyte development. Human thymus differs from mouse thymus in several respects including the larger size of human thymus, the higher proportion of B cells, the absence of any expressed endogenous superantigens and the greater size and abundance of Hassall’s corpuscles (HC). HC are unique structures found within the medulla, of varying size and morphology, consisting of ‘swirls’ of keratinized epithelium and which are present in all mammalian thymi described.9 These structures contain myoid cells, macrophages and granulocytes.10,11 A population of B cells that show evidence of activation exist in the thymus and these are almost exclusively found clustered around HC.12 It was noted 30 years ago that this site appears to be a ‘graveyard’ for dying lymphocytes.13 The epithelial cells that make up the HC themselves differ phenotypically from other medullary epithelial cells in that several monoclonal antibodies (mAb) differentiate between the two.14
While H-2A and H-2E are expressed strongly and equally by thymic antigen-presenting cells (APC) as they are by peripheral APC,15,16 regulation of human leucocyte antigen (HLA) class II products in the human is more variable. Peripheral APC display a hierarchy of expression levels in the order HLA-DR > HLA-DP > HLA-DQ,17–19 yet there are reports of relatively strong expression of HLA-DQ in the thymus.18 HLA-DO, an intracellular class II molecule that interacts with HLA-DM in class II peptide exchange, is expressed weakly by peripheral APC but is strongly expressed by some thymic cells, notably the epithelial cells which ring Hassall’s corpuscles.20
We have therefore undertaken TUNEL analysis of normal human paediatric thymus, investigating the relationship between sites of thymocyte apoptosis and the distribution of HLA class II products.
Materials and methods
Tissues
Thymi were obtained peroperatively from normal human paediatric subjects. Tissue was immediately embedded in optimal cutting temperature (OCT) compound (R. A. Lamb, UK) and snap frozen in isopentane in liquid nitrogen. Blocks were stored at –70° until cut into 5-µm sections with a cryostat.
First-layer antibodies
Biotin-conjugated or fluorescein isothiocyanate (FITC)-conjugated L243, anti-HLA-DRα mAb; biotin-conjugated or FITC-conjugated L2, anti-HLA-DQα mAb; biotin-conjugated B7/21, anti-HLA-DP mAb (Becton Dickinson, Cowley, UK); biotin-conjugated RPA-T4, mouse anti-human CD4 mAb (PharMingen, San Diego, CA); FITC-conjugated RPA-T8, mouse anti-human CD8 mAb (Pharmingen); PS1, mouse anti-human CD3ε mAb (Novocastra, Newcastle Upon Tyne, UK); A575, polyclonal rabbit anti-keratin antibody (Dako Ltd, High Wycombe, UK); FS1, rabbit anti-DMα antiserum21 (a gift from J. Trowsdale, University of Cambridge, UK).
Second-layer reagents
FITC-conjugated human and rabbit-adsorbed F(ab′)2 donkey anti-mouse immunoglobulin G (IgG)(H + L); Texas Red-conjugated human, sheep and rabbit-adsorbed F(ab′)2 donkey anti-mouse IgG(H + L); Texas Red-conjugated human, sheep and mouse-adsorbed F(ab′)2 donkey anti-rabbit IgG(H + L) (all the above from Jackson ImmunoResearch, West Grove, PA); streptavidin–Texas Red (Vector Laboratories, Peterborough, UK); streptavidin–FITC (Pierce and Warriner, Chester, UK); streptavidin–Cy5 (Biological Detection Systems, Pittsburgh, PA); FITC-conjugated Fab fragment of sheep anti-digoxigenin (Boehringer Mannheim, Lewes, UK and Appligene Oncor, Watford, UK).
Immunohistochemistry
Cryosections (5 µm) were air-dried, fixed in ice-cold acetone for 10 min and incubated for 1 hr with first-layer antibodies, as indicated in the figure legends and washed in Tris-buffered saline (TBS; 50 m m Tris, 150 m m NaCl pH 7·6). Sections were then incubated with appropriate second-layer reagents for 30 min. After washing, sections were mounted in Vectashield fluorescence mounting medium (Vector Laboratories) and viewed by epifluorescence microscopy or confocal laser scanning microscopy (Leica, Heidelberg, Germany). Double staining with FITC and Texas Red conjugates was scanned and detected simultaneously, using narrow band pass filters to prevent cross-talk between signals. Triple staining with FITC, Texas Red and Cy5 was scanned and detected with each fluorophore separately, using narrow-band pass filters for FITC and Texas Red and a low-pass filter for Cy5. No cross-talk was ever detected. Because a combination of fluorochrome-conjugated, biotin-conjugated antibody, and unconjugated first-layer antibody from a different species was used in conjunction with multiply adsorbed second-layer antibodies and fluorochrome-conjugated streptavidin, double and triple staining could be performed with no cross-reactivity. In multiple staining experiments, sections were included where all primary antibodies were added except for that probed with a second layer antibody to control for specificity of binding (data not shown).
Terminal deoxynucleotidyl dUTP nick end labelling (TUNEL) staining
In situ TUNEL staining was performed according to the method of Gavrielli with some modifications (Douek et al. 19967). Reagents were either obtained from Boehringer Mannheim (TdT, cacodylate buffer, digoxigenin-conjugated dUTP and anti-digoxigenin antibodies) or were Oncor ApopTag reagents (Oncor). Cryosections were air-dried very briefly and fixed in ice-cold acetone for 10 min and then equilibrated in cacodylate buffer for 5 min at room temperature. Sections were incubated for 1 hr at 37° with 10 U TdT and 4 µ m digoxigenin-conjugated dUTP in 20 µl cacodylate buffer. After washing in phosphate-buffered saline (PBS), sections were incubated with fluorescein-conjugated Fab antibody fragments specific for digoxigenin. Sections were then incubated for 30 min with anti-class II or anti-CD3 mAbs and after washing were incubated for 30 min with the appropriate Texas Red-conjugated second layers. Sections were washed, mounted and immediately visualized by indirect fluorescence microscopy or confocal laser scanning microscopy. No staining of nuclei was ever seen when TdT was not included in the reaction (data not shown).
Results
Expression of HLA class II products in human thymus
We initially investigated expression of HLA-DP, -DQ, -DR and -DM in human thymic sections, expanding on our previous characterization of HLA-DR and -DO localization.20 Representative images from a number of fields taken from several paediatric thymi are shown in Fig. 1. HLA-DR is the most strongly expressed class II molecule in peripheral APC and as can be seen in Fig. 1a and b (green stain), is abundantly expressed in human thymic medulla and somewhat less in cortex. Staining in cortex gives the sparse reticular pattern characteristic of class II expression by cortical epithelial cells, whereas medullary staining is more confluent. Thus, the corticomedullary junction is clearly defined between the two. HLA-DM (shown in Fig. 1(a, b), stained in red), which in peripheral, professional APC would be considered functionally linked to HLA-DR, shows only partial colocalization in the thymus. HLA-DM expression in the cortex is extremely weak. Expression of HLA-DM in the medulla is stronger than in the cortex, although not as abundant as HLA-DR. Relative expression in cortex and medulla was quantified by recording the fluorescence intensity for HLA-DR and HLA-DM in each of five stained fields. The ratio for HLA-DR was 2·2:1 (medulla:cortex) and for HLA-DM, 4·7:1. The exception to the generally stronger medullary expression of HLA-DR over HLA-DM is the pattern of expression in the HC. These bodies are frequently positive for HLA-DM but not for HLA-DR. This can be most clearly seen in the composite image to the right of the figure. Staining is specific as no fluorescent signal could be observed when using rabbit serum as a negative control first layer; an example of this data is shown in Fig. 1(c), central panel.
Figure 1.
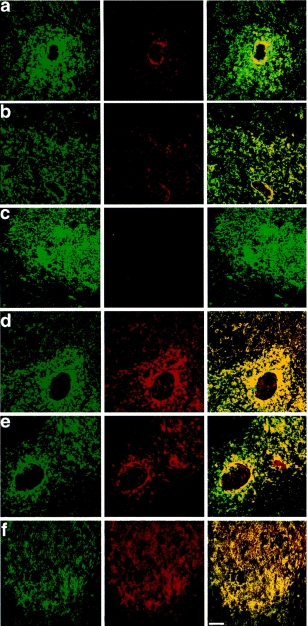
Thymic distribution of HLA-DM, -DR, -DQ, and -DP. (a and b) Left panel, FITC-conjugated L243 (anti-DRα); the central panel shows the same field stained for HLA-DM (red); colocalization of DR and DM expression is shown in the dual image in the right hand panel in which cells staining with both antibodies appear as yellow. (c) As (a) and (b) except that control serum was added to sections in place of the HLA-DM antiserum. (d and e) Left panel shows HLA-DR in green, the central panel shows HLA-DQ in red and the right hand panel shows colocalization of the two images. (f) Left panel shows HLA-DR in green, the central panel shows HLA-DP in red and the right-hand panel shows colocalization of the two images. The white size bar in the right-hand panel denotes 50 µm.
It is well documented that HLA-DQ is poorly expressed by APC in the periphery, an observation which is partly due to low affinity binding to HLA-DQ promoters of some of the trans-cription factors required for HLA class II expression.21–24 However, thymus stains strongly for HLA-DQ (Fig. 1d, e, red stain), with a similar level of expression to HLA-DR (green stain). This result is obtained irrespective of the specific mAbs used for detection of HLA-DR and DQ or the second-layer fluorochrome reagents used to detect them (data not shown). Both L2 (HLA-DQ) and L243 (HLA-DR) stain the medulla with a relatively strong, confluent pattern and cortex more sparsely in the reticular pattern characteristic of cortical epithelium. Quantitative estimates previously reported for the relative expression of HLA-DR versus -DQ are 7:1 in activated peripheral monocytes25 and 60:1 in B lymphoblastoid cell lines.17 A quantitative estimate of HLA-DR versus -DQ expression in human thymus was obtained by comparing the amount of class II heterodimer which could be affinity purified from a unit volume of tissue on either an L2 (DQ) or L243 (DR) affinity column, giving a ratio of 1:1, while the ratio using peripheral blood from the same individual was 3:1 (data not shown). While no Hassall’s corpuscles contain HLA-DR positive cells, they can contain HLA-DQ positive cells. Note the red-only staining of the HC shown in the HLA-DQ/DR double image to the right of Fig. 1(d, e). The epithelial cells surrounding the HC are positive for both mAbs, while the inside of the corpuscle stains for HLA-DQ but not HLA-DR. HLA-DP is expressed with similar distribution and intensity to HLA-DR and is not found in Hassall’s corpuscles (Fig. 1f).
TUNEL staining to localize apoptotic cells in human thymus
We then investigated the relationship between these differential patterns of thymic HLA class II expression and apoptosis as an indicator of negative selection (Fig. 2). The overwhelming majority of apoptotic cells stained by TUNEL are limited to the cortex and the corticomedullary junction (Fig. 2a). As in the mouse, thymic cortex is largely populated by CD4+ CD8+ DP cells and medulla largely by CD4+ and CD8+ SP cells (Fig. 2c). Some apoptotic cells are seen in the medulla, but these are restricted exclusively to the HLA-DQ+ HC (Fig. 2b). The finding that apoptotic thymocytes segregate differentially between cortex and medulla is meaningful because these anatomical sites reflect different stages of thymocyte maturation. This is clearly shown in Fig. 2(c) where the cortex contains small immature CD4 CD8 DP thymocytes whereas the medulla contains larger mature CD4+ and CD8+ SP thymocytes.
Figure 2.
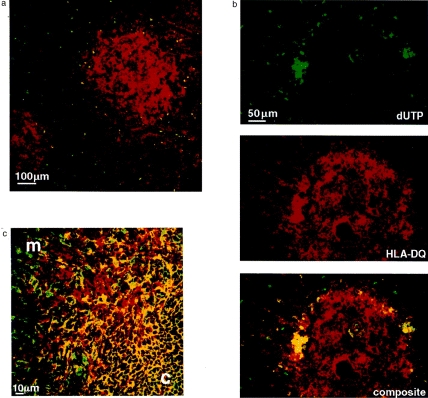
TUNEL staining of thymic cryosections. (a) Double immunofluorescence confocal image of a thymic section with apoptotic nuclei stained by the TUNEL method (in green) and HLA-DR stained with biotin-conjugated L234 (anti-DRα) probed with streptavidin-Texas Red (in red). Cortex is defined by the sparse reticular class II staining and medulla by the more confluent staining. Apoptotic cells are limited to the cortex and corticomedullary junction. (b) Three fluorescence images of the same field showing a thymic section with apoptotic nuclei stained by the TUNEL method (in green), HLA-DQ stained with biotin-conjugated L2 (anti-DQα) probed with streptavidin–Texas Red (in red) and the composite image below. Apoptotic cells are localized predominantly in the cortex and corticomedullary junction. One of two Hassall’s corpuscles in the medulla stains for DQ and also contains apoptotic cells. (c) Double immunofluorescence confocal image of thymic cryosection showing medulla (m) and cortex (c) and the corticomedullary junction between them spanning from bottom left to top right. The section was stained with FITC-conjugated anti-CD8 (in green) and biotin-conjugated anti-CD4 probed with streptavidin–Texas red (in red). CD4+ CD8+ DP cells are seen in the cortex (in yellow).
Because HC contain terminally differentiated epithelium,14 it was possible that the epithelial cells themselves undergo apoptosis and stain by TUNEL. However, in Fig. 3(a) which shows colocalization with CD3, it can be seen that the majority of apoptotic cells are CD3+ and all CD3+ cells in HC are apoptotic. Occasional CD3– apoptotic cells are seen (Fig. 3b), which may be any of the many other cell types described to exist within HC, such as myoepithelial cells, macrophages or granulocytes.10,11,13
Figure 3.
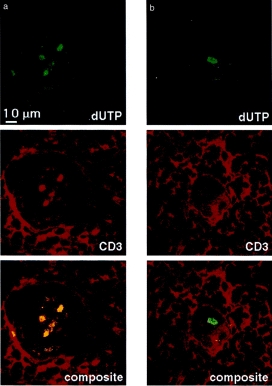
TUNEL staining of Hassall’s corpuscles. (a) Double immunofluorescence confocal image of a Hassall’s corpuscle stained by the TUNEL method (in green) and with anti-CD3 probed with Texas Red-conjugated antimouse immunoglobulin (in red); the composite image is shown below. The three apoptotic cells within the Hassall’s corpuscle are also CD3 positive. (b) A Hassall’s corpuscle from the same thymic cryosection stained in the same way as Fig. 3(a). The apoptotic cell is CD3 negative.
Characterization of apoptotic thymocytes
Because the HC are sites of strong and differential class II expression and of thymocyte apoptosis, it is possible that this might be the site of a late selection event. We therefore investigated the developmental stage of thymocytes found within HC with respect to the expression of the CD4 and CD8 coreceptors. Figure 4 shows fields of HC from representative experiments in which HC epithelial cells are stained with the anti-cytokeratin polyclonal antibody A575 in purple, CD4 in red and CD8 in green, the overlaid images showing as blue. It can be seen that apoptotic thymocytes in HC comprise CD4+ SP, CD8+ SP and CD4+ CD8+ DP cells.
Figure 4.
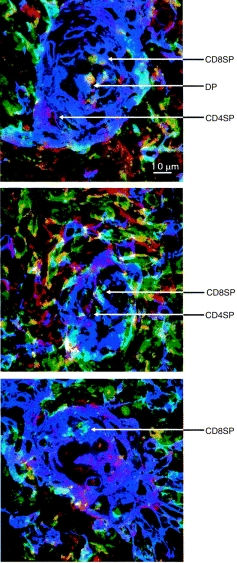
Triple immunofluorescence confocal images of three Hassall’s corpuscles. Thymic cryosections were stained with FITC-conjugated anti-CD8 (in green), biotin-conjugated anti-CD4 probed with streptavidin-Cy5 (in red) and A575 (anti-cytokeratin) probed with anti-rabbit immunoglobulin (in purple). The overlaid fluorochromes show as blue. Large CD4+ SP and CD8+ SP thymocytes can be clearly seen surrounding the Hassall’s corpuscles. Within the Hassall’s corpuscles there are CD4+ SP, CD8+ SP and CD4+ CD8+ DP (in yellow) thymocytes.
Discussion
While much is known about the relationship between MHC expression and negative selection in the mouse, this has not been previously addressed in the human. We here report two observations concerning these events in human thymus: (1) the HC within the thymic medulla are highly unusual with respect to their hierarchy of HLA class II gene expression and (2) these bodies are the only site within human thymic medulla where thymocyte apoptosis can be detected.
In the present report and recent work we have shown that some HC are encircled by HLA-DO+ epithelium and are HLA-DM+, HLA-DQ+, HLA-DR– and HLA-DP–.20 This phenotypic profile is distinct from any other HLA class II+ tissue, which normally follows the hierarchy DR > DP > DQ. No peripheral APC or transformed cell line has been described that expresses HLA-DQ but not HLA-DR. At the level of transcriptional and post-transcriptional control, there are many possible explanations for the low expression of HLA-DQ in classic APC. The TATA box and octamer sequences are missing from DQ promoters, the X-box binding nuclear factor (RF-X) transcription factor binds weakly and another factor, S-box binding nuclear factor (NF-S), binds much more strongly to DQ than to DR promoters.22–24 Assays of HLA transcriptional control are dependent on the cell type analysed, T cells and B cells for example showing subtle differences. It is likely that HLA class II transcriptional control in HC will prove to be radically different from other cell types which have been analysed. Analysis of DRα promoter/transcription factor interactions in thymic epithelial cells shows differences from B cells,26 many of the differences being found around the W/S and V boxes. The fact that HLA-DQ is expressed strongly in the thymus may prove relevant to resolution of the apparent paradox that despite poor expression of this product by peripheral APC, several autoimmune diseases such as type I diabetes and pemphigus vulgaris show strong associations with alleles of HLA-DQ.27
Another noteworthy feature of HLA class II expression in HC is the presence of HLA-DM in the absence of HLA-DR. This is noteworthy because HLA-DM function has in the past been defined in terms of the catalysis of class II-associated invariant chain peptide (CLIP) removal from HLA-DR. The present observations indicate that HLA-DM is likely to have a role in interactions with other class II molecules and is not inextricably linked to HLA-DR. The marginal expression of HLA-DM in thymic cortex might indicate that cortical class II molecules will be occupied by CLIP peptides. It is difficult to interpret the lack of HLA-DM without knowledge of the expression of class II/CLIP complexes at this site. In mouse thymic cortex, class II/CLIP complexes are sparse.28 Nevertheless, the differential expression of HLA-DM between thymic cortex and medulla suggests differences in class II peptide exchange at these locations, with all that implies for differences in thymocyte selection. A related question is why the population of medullary epithelial cells in HC should selectively extinguish expression of HLA-DR. This is reminiscent of findings in mouse thymic medulla where there is a subpopulation that is H-2A– but H2-O+.29
In human thymus, as we and others have previously demonstrated in the mouse, the vast majority of thymocyte negative selection events are anatomically localized to the cortex and at the corticomedullary junction.7,8 Le et al., like the present study, noted apoptosis of human thymocytes at these sites.30 Where negative selection in mouse thymus has been localized to the medulla, this has generally been attributable to deletion by endogenous superantigens, a phenomenon that has not been identified in humans. What then could be the nature of the medullary negative selection events detectable in the HC of human thymus? HC constitute a tuberous network running throughout the medulla. They are dependent for their development and survival on the presence of thymocytes and HC themselves secrete cytokines likely to influence thymocyte development. In particular, HC are strongly positive for transforming growth factor-β (TGF-β) and interleukin-7 (IL-7), a cytokine that is essential for early thymocyte expansion as well as for survival of more mature cells.31,32 The strongly IL-7-positive environment of the HC may be a site of action for some of the thymic effects of IL-7 including differentiation of natural killer (NK)1.1 cells and γδ cells,33,34 although it should be noted that cortical epithelial cells also transcribe IL-7.35 The unusual cytokine microenvironment may itself cause skewed MHC expression as well as exerting a chemotactic effect on medullary thymocytes. CD44 plays an important role in the migration and maturation of thymocytes36 and it has been shown that whereas mAbs specific for all isoforms of CD44 stain both thymic epithelial cells and thymocytes at 8 weeks of embryogenesis, mAbs specific for CD44 splice variants containing membrane-proximal inserts stained only the epithelial cells of HC.37 Because all thymocytes found in HC are apoptotic, the mechanism leading to selective cell death must depend on the factors attracting thymocytes to this specialised site rather than differences in TCR-specific engagement of ligand once there. Although we demonstrate aberrant HLA class II regulation in HC, there is no evidence for a specialized role in late negative selection of CD4+ SP cells with inappropriately high affinity for class II, as CD4+ SP, CD8+ SP and CD4+ CD8+ DP cells are all present. However, we have not yet investigated whether HLA class I expression is anomalous in HC.
This study shows that the HC within human thymic medulla are noteworthy both for their unusual hierarchy of HLA class II expression and because they are the only medullary site of thymocyte apoptosis. The selection event governing such thymocyte death is unlikely to depend on specific ligand recognition by TCR but rather may depend on the chemokine and trafficking molecules controlling which cells home to this site. Why should the epithelium of HC have evolved such novel patterns HLA class II transcriptional control if thymocytes reaching this part of the thymus have already been positively selected? It is becoming apparent that reprocessing and presentation of material from apoptotic cells is an important event in T-cell stimulation and probably also in T-cell tolerization.38 Furthermore, mature thymocytes in the medulla can respond to activation or tolerization signals.39 We favour the hypothesis that the HC are a site at which mature thymocytes receive activation/tolerization signals from peptides reprocessed from apoptotic cells. In this context, it is possible that the differential HLA transcriptional control at this site bears on the specific T-cell subpopulations affected. In light of the earlier speculations that DQ restricted cells may show functional differences from DR restricted cells, it is noteworthy that thymic HC peptide presentation may act preferentially through DQ.40
Acknowledgments
The authors would like to thank Prof. M. Ritter for advice and Drs E. Lightstone and J. Trowsdale for antibodies. D.C.D. was supported by the Wellcome Trust and the Muirhead Trust.
References
- 1.Ashton-rickardt PG, Bandeira A, Delaney JR, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 3.Ready AR, Jenkinson EJ, Kingston R, Owen JJT. Successful transplantation across major histocompatibility barrier of deoxyguanosine-treated embryonic thymus expressing class II antigens. Nature (Lond) 1984;310:231. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- 4.Lo D, Sprent J. Identity of cells that imprint H-2-restricted T cell specificity in the thymus. Nature (Lond) 1986;319:672. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- 5.Van Ewijk W, Ron Y, Monaco J, et al. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988;53:357. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]
- 6.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature (Lond) 1994;372:100. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 7.Douek DC, Corley KTT, Zal T, Mellor A, Dyson PJ, Altmann DM. Negative selection by endogenous antigen and superantigen occurs at multiple thymic sites. Int Immunol. 1996;8:1413. doi: 10.1093/intimm/8.9.1413. [DOI] [PubMed] [Google Scholar]
- 8.Liblau R, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral apoptosis. Proc Natl Acad Sci USA. 1996;93:3031. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey DI, Izon DJ, Tucek CL, Wilson TJ, Boyd RL. The phenotypic heterogeneity of mouse thymic stromal cells. Immunology. 1990;70:66. [PMC free article] [PubMed] [Google Scholar]
- 10.Puchtler H, Meloan SN, Branch BW, Gropp S. Myoepithelial cells in human thymus: staining, polarization and fluorescence microscopic studies. Histochemistry. 1975;45:163. doi: 10.1007/BF00495159. [DOI] [PubMed] [Google Scholar]
- 11.Izon DJ, Boyd RL. The cytoarchitecture of the human thymus detected by monoclonal antibodies. Hum Immunol. 1990;27:16. doi: 10.1016/0198-8859(90)90092-4. [DOI] [PubMed] [Google Scholar]
- 12.Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet. 1987;2:1488. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- 13.Blau JN. A phagocytic function of Hassall’s corpuscles. Nature. 1965;208:564. [Google Scholar]
- 14.Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. Immunol Today. 1993;14:445. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 15.Van Ewijk W, Rouse RV, Weissman IL. Distribution of H-2 microenvironments in the mouse thymus. Immunoelectron microscopic identification of I-A and H-2K bearing cells. J Histochem Cytochem. 1980;28:1089. doi: 10.1177/28.10.6999083. [DOI] [PubMed] [Google Scholar]
- 16.Rouse RV, Van Ewijk W, Jones PP, Weissman IL. Expression of MHC antigens by mouse thymic dendritic cells. J Immunol. 1979;122:2508. [PubMed] [Google Scholar]
- 17.Gorga JC, Horejsi V, Johnson DR, Raghupathy R, Strominger JL. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987;262:16087. [PubMed] [Google Scholar]
- 18.Ishikura H, Ishikawa N, Aizawa M. Differential expression of HLA-class II antigens in the human thymus. Relative paucity of HLA-DQ antigens in the thymic medulla. Transplantation. 1987;44:314. doi: 10.1097/00007890-198708000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Janossy G, Bofill M, Poulter LW, et al. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol. 1986;136:4354. [PubMed] [Google Scholar]
- 20.Douek DC, Altmann DM. HLA-DO is an intracellular class II molecule with distinctive thymic expression. Int Immunol. 1997;9:355. doi: 10.1093/intimm/9.3.355. [DOI] [PubMed] [Google Scholar]
- 21.Sanderson F, Kleijmeer MJ, Kelly A, et al. Accumulation of HLA-DM a regulator of antigen presentation in MHC class II compartments. Science. 1994;266:1566. doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- 22.Miwa K, Doyle C, Strominger JL. Sequence-specific interactions of nuclear factors with conserved sequences of human class II major histocompatibility complex genes. Proc Natl Acad Sci USA. 1987;84:4939. doi: 10.1073/pnas.84.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang SY, Nakanishi M, Peterlin BM. B-cell-specific and interferon-gamma-inducible regulation of the HLA-DR alpha gene. Proc Natl Acad Sci USA. 1988;85:8598. doi: 10.1073/pnas.85.22.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobr M, Reith W, Herrero Sanchez C, Mach B. Two DNA-binding proteins discriminate between the promoters of different members of the major histocompatibility complex class II multigene family. Mol Cell Biol. 1990;10:965. doi: 10.1128/mcb.10.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins PA, Maino VC, Warner NL, Brodsky FM. Activated T cells and monocytes have characteristic patterns of class II antigen expression. J Immunol. 1988;141:1281. [PubMed] [Google Scholar]
- 26.Rigaud G, Lerma Barbaro A, Nicolis M, et al. Induction of CIITA and modification of in vivo HLA-DR promoter occupancy in mormal thymic epithelial cells treated with IFN-γ. J Immunol. 1996;156:4254. [PubMed] [Google Scholar]
- 27.Altmann DM. HLA-DQ associations with autoimmune disease. Autoimmunity. 1992;14:79. doi: 10.3109/08916939309077360. [DOI] [PubMed] [Google Scholar]
- 28.Farr A, Deroos PC, Eastman S, Rudensky AY. Differential expression of CLIP: MHC class II and conventional endogenous peptide: MHC class II complexes by thymic epithelial cells and peripheral antigen presenting cells. Eur J Immunol. 1996;26:3185. doi: 10.1002/eji.1830261252. [DOI] [PubMed] [Google Scholar]
- 29.Surh CD, Gao EK, Kosaka H, et al. Two subsets of epithelial cells in the thymic medulla. J Exp Med. 1992;176:495. doi: 10.1084/jem.176.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le PT, Maecker HT, Cook JE. In situ detection and characterization of apoptotic thymocytes in human thymus. Expression of Bcl-2 does not prevent apoptosis. J Immunol. 1995;154:4371. [PubMed] [Google Scholar]
- 31.Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med. 1995;181:1399. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plum J, De Smedt M, Leclercq G. Exogenous IL-7 promotes the growth of CD3– CD4– CD8– CD44+ CD25+/– precursor cells and blocks the differentiation pathway of TCR-alpha beta cells in fetal thymus organ culture. J Immunol. 1993;150:2706. [PubMed] [Google Scholar]
- 33.Oosterwegel MA, Haks MC, Jeffry U, Murray R, Kruisbeek A. Induction of TCR gene rearrangements in uncommitted stem cells by a subset of IL-7 producing MHC class II-expressing thymic stromal cells. Immunity. 1997;6:351. doi: 10.1016/s1074-7613(00)80337-4. [DOI] [PubMed] [Google Scholar]
- 34.Hameg A, Gouarin C, Gombert JM, et al. IL-7 up-regulates IL-4 production by splenic NK1.1+ and NK1.1– MHC class I-like/CD1-dependent CD4+ T cells. J Immunol. 1999;162:7067. [PubMed] [Google Scholar]
- 35.Moore NC, Anderson G, Smith CA, Owen JJT, Jenkinson EJ. Analysis of cytokine gene expression in subpopulations of freshly isolated thymocytes and thymic stromal cells using semiquantitative polymerase chain reaction. Eur J Immunol. 1993;23:922. doi: 10.1002/eji.1830230424. [DOI] [PubMed] [Google Scholar]
- 36.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel DD, Hale LP, Whichard LP, Radcliff G, MacKay CR, Haynes BF. Expression of CD44 molecules and CD44 ligands during human thymic fetal development: expression of CD44 isoforms is developmentally regulated. Int Immunol. 1995;7:277. doi: 10.1093/intimm/7.2.277. [DOI] [PubMed] [Google Scholar]
- 38.Inaba K, Turley S, Yamaide F, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hare KJ, Wilkinson RW, Jenkinson EJ, Anderson G. Identification of a developmentally regulated phase of postselection expansion driven by thymic epithelium. J Immunol. 1998;160:3666. [PubMed] [Google Scholar]
- 40.Altmann DM, Sansom D, Marsh SGE. What is the basis for HLA-DQ associations with autoimmune disease? Immunol Today. 1991;12:267. doi: 10.1016/0167-5699(91)90124-C. [DOI] [PubMed] [Google Scholar]


