Abstract
Nerve growth factor-β (NGF) is known as a growth factor for human basophils and murine mast cells and has recently been shown to also up-regulate mast cell characteristics in human leukaemic mast cells. We have examined here the effect of NGF on the differentiation of normal human mast cells from cord blood progenitors during culture with stem cell factor (SCF), NGF alone or in combination, or fibroblast supernatants. All these supplements induced mast cell immunoreactivity against tryptase, c-Kit and FcεRIα, but none of the cells reacted against the basophil specific antibody 2D7 before or during culture. Intracellular tryptase activity increased as well, with maximal levels on combined culture with SCF and NGF. On reverse transcription–polymerase chain reaction (RT–PCR), cells lacked tryptase and chymase and expressed low levels of FcεRI and c-Kit mRNA prior to culture, with marked up-regulation of FcεRI and c-Kit, and with de novo expression of mast-cell specific α- and β-tryptase by week 3, and of chymase by week 5. Only the TrkA and not the p75 NGF receptor was detected at m-RNA and protein level, and only the TrkA NGF receptor was up-regulated during NGF-driven culture. These findings show therefore that, like SCF, NGF is another growth factor that can induce and regulate human mast-cell development and differentiation.
Introduction
Nerve growth factor (NGF) was originally identified as a neurotrophic factor that plays a crucial role in the development and maintenance of sensory and sympathetic neurons.1 There is however, increasing evidence that NGF also exerts numerous biological effects on cells involved in the immune response or in inflammatory reactions, including wound healing.2,3 The factor is produced by a variety of cell types, such as fibroblasts, myelomonocytic cells, lymphocytes, mast cells and keratinocytes.4–8 Two distinct cellular receptors (NGFR) for NGF have been shown to mediate its biological activity, a 140 000 MW binding protein (p140) (NGFRH) and a 75 000 MW receptor (NGFRL).9 The NGFRH (or TrkA) belongs to the group of receptor tyrosine kinases and is encoded by the tyrosine receptor kinase A (TrkA) proto-oncogene.10 Cells found to express the p140 trk belong primarily to the central nervous system, but include also human monocytes and keratinocytes,4,11 whereas the NGFRL shows a wider distribution, including various cell types like neurons, fibroblasts, basal keratinocytes, lymphocytes and diverse tumours.12
In recent years, stem cell factor (SCF) has been shown to play a crucial role in the development, survival and function of human mast cells (reviewed in 13 and 14). The factor is produced by fibroblasts, keratinocytes and endothelial cells in the skin15,16 and was thus considered to explain the mast-cell marker up-regulation in immature human leukaemic mast cells (HMC-1 cells) in keratinocyte and fibroblast supernatants (FS).17 SCF by itself proved to be however, inactive on HMC-1 cell differentiation, probably due to the functional mutation of the SCF-receptor (c-Kit) in these cells,18,19 and NGF was subsequently considered as a possible alternative candidate to SCF as a mast-cell growth factor, particularly since it is also produced by fibroblasts and keratinocytes.4,5 In the meantime, NGF has been reported to indeed induce mast-cell marker up-regulation in HMC-1 cells.18
NGF has in fact been shown before to induce murine mast-cell development, survival and function, using both in vitro and in vivo models.20–31 Information on the effects of NGF on human mast cells is, however, sparse. In patients with scleroderma, an increase of cutaneous mast cells has been described, together with an increased NGF expression in the same tissue,32 and an enhancement of histamine production from rather undefined cells has been reported with cultures of umbilical cord blood cells.33 Otherwise, NGF has been viewed primarily as a human basophil growth factor.34 Although basophils share a number of properties with mast cells like the expression of the FcεRI and intracellular contents of histamine, they differ from mast cells in a number of properties, including their ontogeny, their growth factor requirements, their receptor expression – such as their at most low-level c-Kit expression, their low levels of α-tryptase, and their lack of mast cell specific β-tryptase.35–37
The present study was designed to clarify whether NGF, like SCF, induces normal human mast-cell development and whether NGF can operate independently of SCF because the NGF effects previously observed by us in HMC-1 cells might have been influenced by the constantly activated, mutated c-Kit in these cells.19 For this purpose, we studied in vitro mast-cell development from human umbilical cord blood-derived mast-cell precursors, a well established model for the study of human mast cell development,37–39 and we compared NGF effects to those of SCF and FS. In addition, we identified the type of NGF receptor expressed by the developing mast cells, and we distinguished these cells from human basophils with the basophil specific monoclonal antibody 2D7.40 For the same purpose, restriction analysis of the expression of tryptase was performed in order to differentiate between α-and β-tryptase.36,41
The data show for the first time that NGF is able to induce and up-regulate several mast cell characteristics during in vitro culture of human mast-cell precursors, with effects comparable to those of FS and SCF.
Materials and methods
Cells
Mononuclear cells from umbilical cord blood (cord blood derived mast cells; CBMC) of 12 healthy donors or from normal peripheral blood (PBMC) were prepared by differ-ential centrifugation on Ficoll–Hypaque (Biochrom, Berlin, Germany), followed by overnight adherence to polystyrol flasks and subsequent removal of non-adherent cells in order to eliminate contaminating cells like lymphocytes. Cells were seeded at 5 × 105 cells/flask and were routinely kept for up to 5 weeks in Iscove’s medium (Biochrom), containing 30% horse serum (HS) (Seromed, Berlin, Germany). These basic culture conditions, designated as control cultures, induce practically no effects concerning mast-cell differentiation by themselves, but they have been shown in the past to be superior to fetal calf serum (FCS) in optimally supporting mast cell cultures from their precursors in combination with mast-cell growth factors contained in fibroblast supernatants.42–44
NGF-β (Boehringer Mannheim, Mannheim, Germany) was added to control medium at 0·1–50 ng/ml in preliminary studies and, after optimal up-regulation of mast cell tryptase was observed at 10–50 ng/ml, concentrations used routinely were 10 ng/ml. FS, generated from 105 murine fibroblasts/ml (l-cell line) cultured to confluency, was used as positive control at the previously established optimal concentrations of 30%.42–44 SCF (Peprotech, Rocky Hill, NJ) was added to cultures at 50 ng/ml, based on previous studies of mast cell cultures from human peripheral blood precursors.15 Cells were seeded into the different culture media at 1 × 105 cells/ml and fed every other day. Viability of cells was determined by trypan blue exclusion.
Monoclonal antibodies
The monoclonal antibody against mast cell tryptase (AA1)45 was kindly provided by A. Walls, Southampton, UK, that against the α-chain of the FcεRI (29C6) by J. Hakimi, Nutley, NJ,46 that against CD117 (c-Kit) (YB5.B8) by L. Ashman, Adelaide, Australia,47 and the basophil specific antibody (2D7) by L.B. Schwartz, Richmond, VA.40 The antibody against NGFRL was purchased from Boehringer Mannheim, that against NGFRH from Santa Cruz Biotechnology (Santa Cruz, CA), and that against desmin (D33) from Dianova, Hamburg, Germany.
Immunohistochemical staining
Immunohistochemistry was performed on cytocentrifuge preparations of cells before and on days 10 or 21 of in vitro ulture, as previously described, using the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique.48 Antibody reactivity was evaluated by two independent observers, counting at least 100 cells, recording distinctly stained cells only.49
Tryptase activity
As a complementation to tryptase immunoreactivity, the enzyme activity of mast-ell tryptase was measured by cleavage of the peptide Z-Gly-Pro-Arg-pNA (4 mm) in the presence of heparin (5 mg/ml) and α1-antitrypsin (2 mg/ml) (all from Sigma, St. Louis, MO as described before.50 Cells were lysed by three cycles of freezing and thawing for determination of intracellular tryptase contents. Activity was calculated as mU/106 cells and expressed as percentage change from CBMC cultured in control medium.
Reverse transcription–olymerase chain reaction (RT–CR)
Cells were lysed and total RNA prepared using the RNeasy-total-RNA-kit (selective binding at silica gel-based membrane) (Quiagen, Hilden, Germany). Potential contaminations with genomic DNA were removed by treatment with DNAase. cDNA was synthesized by reverse transcription of 5 µg total RNA, using a cDNA synthesis kit (InVitrogen, San Diego, CA). The following sets of oligonucleotide primers were used to amplify cDNA (expected fragment lengths are given in parentheses):
tryptase: 5′GGA GCT GGA GGA GCC CGT GA and 5′ACC TGG GTA AGG AAG CAG TGG TG (531 bp),51 FcεRIα 5′CTG TTC TTC GCT TCC AGA TGG CGT and 5′TAC AGT AAT GTT GAG GGG CTG AG (536 bp),52 GAPDH: 5′GAT GAC ATC AAG AAG GTG GTG and 5′GCT GTA GCC AAA TTC GTT GTC (197 bp),53 c-Kit: 5′CGT TGA CTA TCA GTT CAG CGA G and 5′CTA GGA ATG TGT AAG TGC CTC C (369 bp),54 chymase: 5′AAG GAG AAA GCC AGC CTG ACC and 5′TCC GAC CGT CCA TAG GAT ACG (321 bp),55 5′TGA GTG CTG CAA AGC CTG CAA and 5′TCT CAT CCT GGT AGT AGC CGT (230 bp) for the p75 NGF receptor, and 5′GGC TCC TCG GGA CTG CGA TG and 5′CAG GAG AGA GAC TCC AGA GCG (260 bp) for the TrkA NGF-receptor.56 Amplification was performed using taq polymerase (Gibco, Berlin, Germany) over 24–35 cycles with an automated thermal cycler (Perkin Elmer, Weiterstadt, Germany). Each cycle consisted of the following steps: denaturation at 94°, annealing at 58°, and extension at 72° for 1 min each. The different PCR products were analysed by agarose gel electrophoresis and enzymatic digestion, using standard techniques.57 All used primer pairs produce PCR products with lengths specific only for cDNA. Restriction digest analysis of all RT–PCR products yielded breakdown bands of the predicted molecular size. The numbers of cycles were varied to obtain conditions with a linear correlation between signal intensities of RT–PCR product and c-DNA concentration.
For semiquantitative RT–PCR analysis, cDNA from freshly isolated CBMC or from cells cultured under various conditions was adjusted to equal quantities by serial dilutions (also in the range of linear correlation betweenc-DNA concentration and density of RT–PCR-signal), controlled by PCR amplification using primers of the housekeeping gene for reduced glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as previously described.16 All experiments for semiquantitative RT–PCR were repeated at least three times.
Restriction analysis of PCR amplification products with the tryptase primers was performed using two different enzymes, FokI and PstI (Gibco, Eggenstein, Germany). FokI cuts α-tryptase at two sites, yielding fragments of 100, 131 and 300 bp, and β-tryptase at one site, yielding only two fragments of 100 and 431 bp. With PstI, α-tryptase is not affected at all, whereas β-tryptase is cleaved into two bands of 193 and 338 bp (data from the EMBL-bank).
Statistics
Statistical significance was calculated using the two-tailed Student’s t-test or the Wilcoxon test.
Results
Expression of mast-cell markers
Freshly isolated mononuclear cells of the adherent fraction from cord blood failed to stain with an antibody against tryptase, and fewer than 1% of the cells were positive using antibodies against c-Kit or FcεRI. During culture with SCF, NGF and FS, immunoreactivity for mast-cell markers was increased significantly, compared to control cultures, except for c-Kit expression in the presence of FS (Fig. 1a–c).
Figure 1.

Immunocytochemical reactivity of mast-cell markers in CBMC cultured for 5 weeks under standard conditions (control) or in the presence of FS (30%), SCF (50 ng/ml), NGF (10 ng/ml) and the last two factors combined, at the same concentrations used with the individual factors. Data are expressed as means ± SD of the percentage of positive cells in cultures from n = 8 different donors) (*P < 0·01). (a) FcεRIα (antibody 29C6); (b) c-Kit (antibody YB5.B8); (c) tryptase (antibody AA1).
There was not only an increase in the percentage of positive cells, but individual cells exhibited also more intense staining, as illustrated for tryptase in Fig. 2. In the control culture, there are only few cell granules staining positively for tryptase (Fig. 2a), whereas addition of FS resulted in a clear signal in most cells (Fig. 2b) and that of SCF or NGF caused most cells to be densely filled with tryptase-positive granules (Fig. 2c–d). Furthermore, tryptase activity/cell was significantly increased as well (Table 1). The data are expressed as percentage change from control cultures because of the variation between different donors prior to culture (range from 5 to 30 mU/106 cells).
Figure 2.
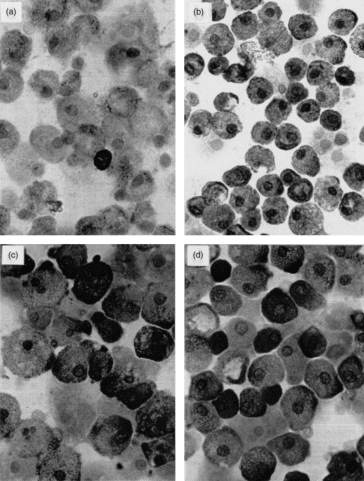
Immunocytochemical staining (APAAP technique) for tryptase (antibody AA1) in CBMC cultured for 5 weeks under control conditions (a) or with 30% FS (b), 50 ng/ml SCF (c) or 10 ng/ml NGF (d) added. (magnification 1000×)
Table 1.
Tryptase activity and total cell numbers after three weeks in CBMC cultured under standard conditions (control) or in the presence of different conditioning and recombinant growth factors
| Culture conditions | Tryptase activity (% of control) | Cell number (× 106 cells) |
|---|---|---|
| Control | 100 ± 21 | 1·5 ± 0·25 |
| range 5–30 mU/106 cells | ||
| FS (30%) | 152 ± 17* | 0·8 ± 0·20† |
| SCF (50 ng/ml) | 216 ± 38* | 0·5 ± 0·10† |
| NGF (10 ng/ml) | 196 ± 26* | 1·1 ± 0·20† |
| SCF (50 ng/ml) + NGF (10 ng/ml) | 315 ± 65*‡ | 1·0 ± 0·15† |
Significant increases
decreases compared to control, with P < 0·01.
Significant increase compared to culture with NGF or SCF alone, with P < 0·05, using the Wilcoxon test).
Total cell numbers at day 0: 1 × 106 cells. No tryptase activity at day 0.
Means ± SD of n = 8 independent experiments.
Although viability of cells always remained above 80% during culture, cell numbers were significantly lower in the presence of FS, SCF and NGF, compared to controls (Table 1).
In order to explore whether SCF in the presence of NGF might have additive or inhibitory effects, the two factors were also studied in combination. As is evident from Fig. 1, cultures with both factors failed to further increase the number of positive cells, compared to the individual factors alone, although a marked increase (significantly higher compared to cultures with NGF or SCF alone: P < 0·05) of tryptase enzyme activity was observed (Table 1), suggesting partially additive effects of the factors when combined.
Evaluation of specific mRNAs for mast-cell markers
Possible changes in the expression of mast-cell markers at the mRNA level were studied next by semiquantitative RT–PCR analysis for FcεRIα, tryptase and c-Kit, using GAPDH as control and the respective specific sets of oligonucleotide primers. As shown in Fig. 3 and Table 2, after amplification of the cDNA from freshly isolated CBMC, only weak signals were detected with FcεRIα and c-kit primers prior to culture, and none at all with the tryptase primers (Fig. 3, lane 3). After three weeks of culture with NGF (lane 5), SCF (lane 8) or FS (lane 9), an increased expression of mRNA of all investigated markers was observed, compared to control cultures (lane 4). The latter showed only low amounts of FcεRI and c-Kit, but no tryptase specific signals.
Figure 3.
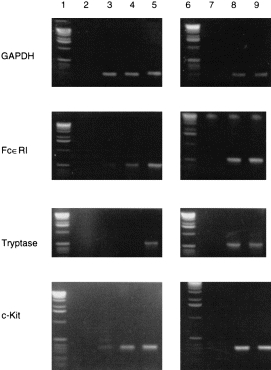
Expression of mRNA for GAPDH, FcεRIα, tryptase andc-Kit, demonstrated by RT–PCR with cDNA from CBMC before and 3 weeks after culture with NGF: lane 1, 6: DNA-ladder; lane 2, 7: negative control for PCR (without c-DNA); lane 3: CBMC day 0; lane 4: CBMC day 21 under standard control culture conditions; lane 5: CBMC at day 21 cultured with NGF (10 ng/ml); lane 8: CBMC at day 21 cultured with SCF (50 ng/ml); lane 9: CBMC at day 21 cultured with FS (30%) (representative results with cells from one of five different donors).
Table 2.
Densitometric analysis of RT–PCR signal intensities using cDNA for FcεRI, c-Kit and mast cell-specific tryptase, isolated from CBMC cultured under standard conditions (control), in the presence of recombinant growth factors, or with FS added, which contains these factors
| Culture conditions | FcεRI | Tryptase | c-Kit |
|---|---|---|---|
| Day 0 | 50 ± 30 | 0 ± 0 | 60 ± 40 |
| Day 21, control | 120 ± 40 | 0 ± 0 | 160 ± 60 |
| Day 21, NGF (10 ng/ml) | 290 ± 80 ** | 80 ± 40 ** | 210 ± 110 |
| Day 21, SCF (50 ng/ml) | 330 ± 100* | 150 ± 35 ** | 270 ± 90 * |
| Day 21, FS (30%) | 280 ± 95 * | 90 ± 50** | 240 ± 80 * |
Means ± SD of n = 5 independent experiments).
Significant compared to the control (day 21) with
P < 0·05
P < 0·01.
Since tryptase was the only de novo expressed mast cell marker during culture with NGF, the kinetics of its mRNA expression were studied in more detail (Fig. 4a,Table 3). As can be seen, a signal was detected only after 3 weeks of culture, with its further up-regulation by week 5. Mast cell chymase specific mRNA was expressed only after five weeks of culture (Fig. 4b,Table 3).
Figure 4.
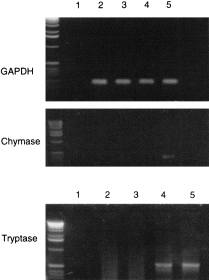
RT–PCR of cDNA from CBMC cultured with NGF (10 ng/ml) for different time periods using primer specific for GAPDH and mast cell tryptase or chymase: lane 1, negative control for PCR (without c-DNA); lane 2, CBMC day 0; lane 3, CBMC, day 14; lane 4, CBMC, day 21; lane 5, CBMC, day 35 (representative results with cells from one out of three different donors).
Table 3.
Densitometric analysis of RT–PCR signal intensities, using cDNA isolated from CBMC cultured with NGF (10 ng/ml) for different time periods with primers specific for mast cell tryptase or chymase
| Culture period (weeks) | Tryptase | Chymase |
|---|---|---|
| 0 | 0 | 0 |
| 2 | 0 | 0 |
| 4 | 150 ± 50 | 0 |
| 5 | 180 ± 40 | 40 ± 20 |
Means ± SD of n = 3 independent experiments.
Differentiation of mast cells from basophils
Since NGF was hitherto merely regarded as a basophil growth factor, it was necessary to confirm the mast cell identity of the FcεRIα-and tryptase-positive cells. No reactivity with the basophil-specific monoclonal antibody 2D7 was detected in cells prior to, during and at the end of culture, whereas basophils in normal blood were strongly positive (not shown). In addition, in order to determine the type of tryptase expressed during culture of CBMC with SCF or NGF, restriction analysis of the PCR amplification products obtained with the tryptase primers was performed, using the enzymes FokI and PstI (Fig. 5). Agarose electrophoresis showed fragment sizes compatible with a mixture of cDNA for α- and β-tryptase from cells cultured with either SCF or NGF (FokI: 430, 300, 130, 100 bp; PstI: 531, 340, 200 bp).
Figure 5.
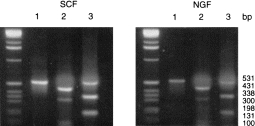
Restriction analysis of PCR products obtained with tryptase oligonucleotide primers and cDNA from CBMC cultured for 3 weeks with SCF (50 ng/ml) or NGF (10 ng/ml). (bp, base pairs): lane 1, uncleaved fragment; lane 2, digestion with FokI; lane 3, digestion with PstI.
Expression of growth factor receptors
With the clear demonstration of NGF effects on the development of mast-cell characteristics in cultured CBMC, it was of interest to determine the type of receptor expressed on the cells that might thus be potentially involved in mast-cell differentiation. As shown in Fig. 6, a clear expression of NGFRH (gp140trk) was detectable at the mRNA level, but no detectable band was observed with the primers for NGFRL. Peripheral blood mononuclear cells (PBMC) were used as positive control and expressed mRNA specific for both receptors.
Figure 6.
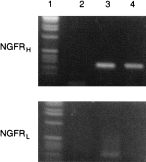
Expression of mRNA for NGFRH and NGFRL demonstrated by PCR with cDNA from CBMC cultured with NGF (10 ng/ml) for 21 days and freshly isolated PBMC as positive control (representative results with cells from one out of three different donors): lane 1, DNA-ladder; lane 2, negative control for PCR (without c-DNA); lane 3, PBMC; lane 4, CBMC, cultured with NGF (10 ng/ml).
These results were confirmed at the protein level by immunocytochemical staining with an antibody against NGFRH(Fig. 7a) in control cultures (80% positive cells), with a slight increase in the presence of NGF (95% positive cells) (Fig. 7b). No reactivity with the antibody against the NGFRL was noted under these same conditions (not shown).
Figure 7.

Immunocytochemical staining (APAAP technique) for NGFRH in CBMC at day 21, cultured (a) without or (b) with NGF (10 ng/ml (magn. 1000×).
Discussion
The present findings provide several lines of evidence for the ability of NGF to induce in vitro mast cell differentiation from human mast-cell precursors, confirming in vitro and in vivo findings in the rodent system20,24–26,30 and our own recent observations in the immature human mast-cell line (HMC-1).18 They also fit with recent data by Tam et al. who described higher numbers of chymase-positive cells after the addition of NGF to the culture of CBMC for 3 weeks58 and with findings of this group and Nilsson et al.59 demonstrating signal transduction in HMC-1 cells via the high-affinity receptor for NGF TrkA.
Since the mast cell markers studied here are also expressed on other cells,13,14 none of them provides convincing proof by itself that the cells grown in culture are indeed mast cells. Unrelated cells like FcεRI-positive Langerhans cells or c-Kit-positive melanocytes represent no problem because none of these cells express the respective other cell’s receptor, nor do they contain mast cell tryptase or chymase. The closely similar percentages of cells expressing both tryptase and c-Kit (Fig. 1) suggest furthermore, that these two molecules are expressed by the same cells. FcεRIα is on the other hand positive in lower percentages of cells, in agreement with recent findings from our group in SCF-driven mast cell cultures human from peripheral blood mononuclear cells. FcεRIα up-regulation was furthermore later in time than that of tryptase, although histamine release occurred on receptor-specific stimulation (manuscript in revision). Because the majority of FcεRI positive cells still expressed however, monocytic markers, namely CD14 in 90%, CD64 in 82%, and CD68 in 52%, the exact association of FcεRI with cultured mast cells vs. monocytes is not clear from the present data.
The clarification of this point is even more important with regard to basophils, also in view of previous reports that NGF induces only human basophil and no mast-cell growth on in vitro culture.34 The high level of c-Kit expression in the present cultures speaks, however, against the presence of basophils among the cells, as does the expression of both α- and β-tryptase in cells cultured with NGF or SCF, in agreement with previous data with SCF-induced CBMC-derived mast cells.41 Both tryptases are also expressed in normal human lung and cutaneous mast cells, but basophils contain only α-tryptase.36 Even more convincing proof that no basophils were present among the CBMC prior to and during culture with NGF is provided with the lack of reactivity of cells with the basophil-specific monoclonal antibody 2D7.40
The reason for the failure of other groups to observe NGF-driven in vitro development of human mast cells in the absence of SCF remains unclear. In an older study, Matsuda et al.60 reported on NGF stimulation of mixed basophil/mast cell and eosinophil colonies in T-cell dependent cultures of human peripheral blood, using a methylcellulose assay. Because we studied only adherent cells of CBMC, we possibly eliminated basophil precursors among the seeded cells. In a later study,33 addition of NGF to interleukin-3 (IL-3)-driven human CBMC cultures was shown to enhance histamine contents of the cells, but the exact cell type producing histamine was not identified. In a more recent study of human bone marrow cultures, murine NGF, at 100 ng/ml, failed to induce tryptase in separate cultures from two different human donors, whereas SCF was effective.61 The combination of both factors even reduced SCF-induced tryptase up-regulation by more than 50%.61 Several factors may account for the negative results with NGF in this study, compared to our data, such as differences in the source of mast cell precursors, the culture conditions, and the source and concentration of NGF. The very different culture conditions employed by us, using, e.g. HS instead of FCS,61 may be a possible explanation for our current findings. Since both murine and human NGF have been shown to be biologically active in basophils62 and since interspecies DNA sequences are highly homologous,1 the use of murine NGF in the studies of Valent et al.61 are on the other hand unlikely to account for the negative findings of these authors.
After 5 weeks of culture, SCF, NGF and FS had comparable effects on mast-cell marker expression in CBMC cultures, yielding maximally 50% positive cells (Fig. 1). The combination of SCF and NGF failed to strikingly improve the number of positive cells, although tryptase activity was markedly increased in the cultures, suggesting up-regulation of tryptase synthesis in the individual cells. Interestingly, the detectable tryptase mRNA expression took however, more than 2 weeks of culture and that of chymase 5 weeks (Fig. 4). This may indicate a slow recruitment of cells into differentiation, independent of their stage in the cell cycle. Alternatively, a complex interplay of several factors may be required for mast-cell differentiation, including the up-regulation of receptors like c-Kit and NGFRH (Figs 1 and 6), in order to enhance the efficacy of the individual growth factors. From murine studies, NGF is known to induce mast-cell mediator release which in turn enhances mast-cell development.63 An autocrine loop, involving NGF or SCF secretion by mast cells7,64 might be another means to gradually increase the efficacy of mast-cell differentiation during culture.
The effects observed here are most likely mediated via the NGFRH since only this receptor was identified on CBMC in control and NGF-driven cultures (Fig. 6). In HMC-1 cells, we have also demonstrated primarily the NGFRH both at the level of gene transcription and protein expression, with only minor amounts of mRNA specific for NGFRL and lack of detectable protein.18 The NGFRH has been shown to be present and functional also on rat peritoneal mast cells and on human basophils, with no or only minor expression, respectively, of the NGFRL.29,63 Effects mediated via the NGFRH in these cells include priming for and potentiation of mediator release as well as prevention of apoptosis.29,61,65 The latter effects as well as increased survival of mast cells has not only been described for NGF,27,29 but also for SCF.66 The reasons for the lower cell numbers in cultures with NGF, SCF or FS, compared to control cultures (Table 1), are unclear but may relate to differential effects of the factors on mast cells versus other cell types.
To sum up, the present data add several new pieces to the puzzle of the neural–immunological connection, showing that NGF, originally defined as a neurotrophic factor, can affect not only murine mast cells and human basophils, but also human mast cells. NGF must thus be viewed as a potent growth factor for human mast cells, in addition to SCF. The effect of these two growth factors on mast-cell precursors from sources other than cord blood, their differential or joint action during various stages of human mast cell maturation, and their possible role as paracrine or autocrine stimuli will have to be explored in further studies.
Acknowledgments
The authors wish to thank Mrs. Regina Nordheim and Mrs. Elke Bauer for excellent technical assistance. Supported by grants from the German Research Foundation (He2686/8-3) and the Ministry for Research (01GC8702/TP7).
Abbreviations
- CBMC
cord blood derived mast cell
- FcεRI
high affinity IgE receptor
- FCS
fetal calf serum
- FS
fibroblast supernatants
- HS
horse serum
- NGFRL
p75 NGF-receptor
- NGFRH
p140 NGF-receptor (TrkA)
- PBMC
peripheral blood mononuclear cells
- NGF
nerve growth factor-β
- SCF
stem cell factor
- RT–PCR
reverse transcription–polymerase chain reaction
References
- 1.Ulrich A, Gray A, Berman C. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983;303:821. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L, Bracci-laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunological diseases. Allergy. 1997;52:883. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda H, Koyama H, Sato H, et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pincelli C, Sevignani C, Manfredini R, et al. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol. 1994;103:13. doi: 10.1111/1523-1747.ep12388914. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright M, Mikheev AM, Heinrich G. Expression of neutrotrophic genes in human fibroblasts: differential regulation of the brain-derived neutrotrophic factor gene. Int J Dev Neurosci. 1994;12:685. doi: 10.1016/0736-5748(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 6.Patrick CW, Jr, Kukreti S, McIntire LV. Quantitative effects of peripheral monocytes and nerve growth factor on CNS neural morphometric outgrowth parameters in vitro. Exp Neurol. 1996;138:277. doi: 10.1006/exnr.1996.0066. [DOI] [PubMed] [Google Scholar]
- 7.Leon A, Buriani A, Dal-toso R, et al. Mast cell synthesize, store and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santambrogio L, Benedetti M, Chao MV, et al. Nerve growth factor production by lymphocytes. J Immunol. 1993;153:4488. [PubMed] [Google Scholar]
- 9.Raffioni S, Bradshaw RA. The receptors for nerve growth factor and other neurotrophins. Annu Rev Biochem. 1993;62:823. doi: 10.1146/annurev.bi.62.070193.004135. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Jing S, Venkata N, O’Rouke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 11.Ehrhard PB, Ganter U, Stalder A, Bauer J, Otten U. Expression of functional trk protooncogene in human monocytes. Proc Natl Acad Sci. 1993;90:5423. doi: 10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bothwell M. Neurotrophin function in skin. J Invest Dermatol, Symp Proc. 1997;2:27. doi: 10.1038/jidsymp.1997.7. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 14.Artuc M, Hermes B, Steckelings MU, Grützkau A, Henz BM. Mast cells and their mediators in wound healing – active participants or innocent bystanders? Exp Dermatol. 1999;8:1. doi: 10.1111/j.1600-0625.1999.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 15.Grabbe J, Welker P, Dippel E, Czarnetzki BM. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch Dermatol Res. 1994;287:78. doi: 10.1007/BF00370723. [DOI] [PubMed] [Google Scholar]
- 16.Grabbe J, Welker P, Rosenbach T, et al. Release of stem cell factor (SCF) from human keratinocytes (HaCaT) is increased in differentiating versus proliferating cells. J Invest Dermatol. 1996;107:219. doi: 10.1111/1523-1747.ep12329664. [DOI] [PubMed] [Google Scholar]
- 17.Grabbe J, Welker P, Grützkau A, et al. Induction of human leukemic mast cell differentiation by fibroblast supernatants, but not by stem cell factor. Scand J Immunol. 1998;47:324. doi: 10.1046/j.1365-3083.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 18.Welker P, Grabbe J, Grützkau A, Henz BM. Effects of NGF and other fibroblast-derived growth factors on immature human mast cells (HMC-1) Immunology. 1998;94:310. [PMC free article] [PubMed] [Google Scholar]
- 19.Furitsu T, Tsujima T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;99:1736. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloe L, Levi-montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 21.Bruni A, Bignon E, Boarato E, Leon A, Toffano G. Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS Lett. 1982;138:190. doi: 10.1016/0014-5793(82)80438-9. [DOI] [PubMed] [Google Scholar]
- 22.Tal M, Liberman R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neurosci Lett. 1997;221:129. doi: 10.1016/s0304-3940(96)13318-8. [DOI] [PubMed] [Google Scholar]
- 23.Pearce FL, Thompson HL. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J Physiol (Lond) 1986;372:379. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda H, Kannan Y, Ushio H, et al. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174:7. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jippo T, Ushio H, Hirota S, et al. Poor response of cultured mast cells derived from mi/Mi mutant mice to nerve growth factor. Blood. 1994;84:2977. [PubMed] [Google Scholar]
- 26.Kannan Y, Matsuda H, Ushio H, Kawamoto K, Shimada Y. Murine granulocyte–macrophage and mast cell colony formation promoted by nerve growth factor. Int Arch Allergy Immunol. 1993;101:362. doi: 10.1159/000236584. [DOI] [PubMed] [Google Scholar]
- 27.Horigome K, Bullock ED, Johansson EM., Jr Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate early gene induction. J Biol Chem. 1994;269:2695. [PubMed] [Google Scholar]
- 28.Bullock ED, Johnson EM., Jr Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells: potential role in survival promotion. J Biol Chem. 1996;271:27500. doi: 10.1074/jbc.271.44.27500. [DOI] [PubMed] [Google Scholar]
- 29.Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trk proto-oncogene receptor. Blood. 1995;86:4638. [PubMed] [Google Scholar]
- 30.Getchell ML, Kulkarni-narly A, Takami S, Albers KM, Getchell TV. Age-dependent phenotypic switching of mast cells in NGF transgenic mice. Neuroreport. 1995;6:1261. doi: 10.1097/00001756-199506090-00008. [DOI] [PubMed] [Google Scholar]
- 31.Aloe L, Probert L, Kollias G, Micera A, Tirassa P. Effect of NGF antibodies on mast cell distribution, histamine and substance P levels in the knee joint of TNF arthritic transgenic mice. Rheumatol Int. 1995;14:249. doi: 10.1007/BF00262091. [DOI] [PubMed] [Google Scholar]
- 32.Tuveri MA, Passiu G, Mathieu A, Aloe L. Nerve growth factor and mast cell distribution in the skin of patients with systemic sclerosis. Clin Exp Rheumatol. 1993;11:319. [PubMed] [Google Scholar]
- 33.Richard A, McColl SR, Pelletier G. Interleukin 4 and nerve growth factor can act as co-factor for interleukin-3-induced histamine production in human umbilical cord blood cell in serum-free culture. Br J Haematol. 1992;81:6. doi: 10.1111/j.1365-2141.1992.tb08162.x. [DOI] [PubMed] [Google Scholar]
- 34.Denburg JA. Differentiation of human basophils and mast cells. In: Marone G, editor. Human Basophils and Mast Cells: Biological Aspects. Basel: Karger; 1995. p. 49. [Google Scholar]
- 35.Schwartz LB, Kepley C. Development of markers for human basophils and mast cells. J Allergy Clin Immunol. 1994;94:1231. doi: 10.1016/0091-6749(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 36.Xia HZ, Kepley CL, Sakai K, Chelliah L, Irani A, Schwartz LB. Quantitation of tryptase, chymase, Fc epsilon RI alpha and Fc epsilon RI gamma mRNAs in human mast cells and basophils by competitive reverse transcription–polymerase chain reaction. J Immunol. 1995;154:5472. [PubMed] [Google Scholar]
- 37.Mitsui H, Furitsu T, Dvorak AM, et al. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-Kit ligand. Proc Natl Acad Sci USA. 1993;90:735. doi: 10.1073/pnas.90.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furitsu T, Saito H, Dvorak AM, et al. Development of human mast cells in vitro. Proc Natl Acad Sci USA. 1989;86:100039. doi: 10.1073/pnas.86.24.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito H, Ebisawa M, Tachimoto H, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343. [PubMed] [Google Scholar]
- 40.Kepley CL, Craig SS, Schwartz LB. Identification and partial characterization of a unique marker for human basophils. J Immunol. 1994;154:6548. [PubMed] [Google Scholar]
- 41.Nilsson G, Blom T, Harvima I, Kusche-gullberg M, Nilsson K. Stem cell factor-dependent human cord blood derived mast cells express α- and β-tryptase, heparin and chondroitin sulphate. Immunology. 1996;88:308. doi: 10.1111/j.1365-2567.1996.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czarnetzki BM, Krüger G, Sterry W. In vitro generation of mast cell-like cells from human peripheral mononuclear phagocytes. Int Arch Allergy Appl Immunol. 1983;71:161. doi: 10.1159/000233381. [DOI] [PubMed] [Google Scholar]
- 43.Czarnetzki BM, Figdor CG, Kolde G, Vroom T, Aalberse R, de Vries JE. Development of human connective tissue mast cells from purified blood monocytes. Immunology. 1984;51:549. [PMC free article] [PubMed] [Google Scholar]
- 44.Grabbe J, Welker P, Möller A, Dippel E, Ashmann LF, Czarnetzki BM. Comparative cytokine release from human monocytes, monocyte-derived immature mast cells and a human mast cell line (HMC1) J Invest Dermatol. 1993;103:504. doi: 10.1111/1523-1747.ep12395649. [DOI] [PubMed] [Google Scholar]
- 45.Walls AF, Jones DB, Williams JH, Church MK, Holgate ST. Immunohistochemical identification of mast cells in formaldehyde-fixed tissue using monoclonal antibodies specific for tryptase. J Pathol. 1990;162:119. doi: 10.1002/path.1711620204. [DOI] [PubMed] [Google Scholar]
- 46.Riske F, Hakimi J, Mallamaci M, et al. High affinity human IgE receptor (FcεRI). Analysis of functional domains of the α-subunit with monoclonal antibodies. J Biol Chem. 1991;266:11245. [PubMed] [Google Scholar]
- 47.Lerner NB, Nocka KH, Cole SR, et al. Monoclonal antibody YB5.B8 identifies the human c-kit protein product. Blood. 1991;77:1876. [PubMed] [Google Scholar]
- 48.Schadendorf D, Tiedemann K-H, Haas N, Czarnetzki BM. Detection of human papilloma viruses in paraffin-embedded condylomata accuminata – comparison of immunhistochemistry, in situ hybridization, and polymerase chain reaction. J Invest Dermatol. 1991;97:549. doi: 10.1111/1523-1747.ep12481884. [DOI] [PubMed] [Google Scholar]
- 49.Hamann K, Grabbe J, Welker P, Haas N, Algermissen B, Czarnetzki BM. Phenotypic evaluation of cultured human mast and basophilic cells and of normal human skin mast cells. Arch Dermatol Res. 1994;286:380. doi: 10.1007/BF00371797. [DOI] [PubMed] [Google Scholar]
- 50.Harvima IT, Schechter NM, Harvima RJ, Fraeki JE. Human skin tryptase: purification, partial characterization and comparison with human lung tryptase. Biochim Biophys Acta. 1998;957:71. doi: 10.1016/0167-4838(88)90158-6. [DOI] [PubMed] [Google Scholar]
- 51.Miller JS, Westin EH, Schwartz LB. Cloning and characterisation of complementary DNA for human tryptase. J Clin Invest. 1989;84:1188. doi: 10.1172/JCI114284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson MW, Mehl VS, Richards ML, Fu-tong L. m-RNA variants encoding multiple forms of the high-affinity IgE receptor alpha subunit in transformed and nontransformed mast cells. Int Arch Allergy Appl Immunol. 1991;96:289. doi: 10.1159/000235511. [DOI] [PubMed] [Google Scholar]
- 53.Tokunaga K, Nakamura Y, Sakata K, et al. Enhanced expression of glyceraldehyde-3-phosphate. Cancer Res. 1987;47:5616. [PubMed] [Google Scholar]
- 54.Ratajczak MZ, Luger SM, De Riel K, Abraham J, Calabretta B, Gewirtz AM. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci USA. 1992;89:1710. doi: 10.1073/pnas.89.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caughey GH, Zerweck EH, Vanderslice P. Structure, chromosomal assignment and deduced amino acid sequence of a human gene for mast cell chymase. J Biol Chem. 1991;266:12956. [PubMed] [Google Scholar]
- 56.Herrmann JL, Menter DG, Hamada J, Marchetti D, Nakajima N, Nicolson GL. Mediation of NGF-stimulated extracellular matrix invasion by the human melanoma low-affinity p75 neurotrophin receptor: melanoma p75 functions independently of trkA. Mol Biol Cell. 1993;4:1205. doi: 10.1091/mbc.4.11.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrock J, Fritsch EF, Maniatis T. Analysis of RNA. In: Ford N, Nolan C, Ferguson M, editors. Molecular Cloning. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. p. 743. [Google Scholar]
- 58.Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807. [PubMed] [Google Scholar]
- 59.Nilsson G, Forsberg K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda H, Caughlin MD, Bienenstock J, Denburg JA. Nerve growth factor promotes human hemopoietic colony growth and differentiation. Proc Natl Acad Sci USA. 1988;85:6508. doi: 10.1073/pnas.85.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valent P, Spanböchl E, Sperr WR, et al. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood. 1992;80:2237. [PubMed] [Google Scholar]
- 62.Burgi B, Otten UH, Ochsenberger B, et al. Basophil priming by neurotrophic factors. Activation through the trk receptor. J Immunol. 1996;157:5582. [PubMed] [Google Scholar]
- 63.Marshall JS, Stead RH, McSharry C, Nielsen L, Bienenstock J. The role of mast cell degranulation products in mast cell hyperplasia. Mechanism of action of nerve growth factor. J Immunol. 1990;144:1886. [PubMed] [Google Scholar]
- 64.Welker P, Grabbe J, Henz BM. Human mast cells produce and differentially express soluble and membrane-bound stem cell factor. Scand J Immunol. 1999;49:495. doi: 10.1046/j.1365-3083.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- 65.Bischoff SC, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662. [PubMed] [Google Scholar]
- 66.Mekori YA, Oh CK, Metcalfe DD. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3. Prevention of apoptosis by c-kit ligand. J Immunol. 1993;151:3775. [PubMed] [Google Scholar]


