Abstract
The production of cytokines by lymphoid cells, isolated from non-infected and Haemonchus contortus-infected lambs, was investigated. Particular attention was paid to differences in T helper 1- (Th1) and Th2-type immune profiles between genetically resistant and random-bred animal groups. Non-infected resistant and random-bred lambs produced equivalent levels of interferon-γ (IFN-γ) and interleukin-5 (IL-5), from isolated abomasal lymph node cells (ALN), mesenteric lymph node cells (MLN) and spleen cells (SC), in response to in vitro stimulation with T-cell mitogen (concanavalin A) or larval parasite antigen. ALN and MLN cells derived from infected resistant and random-bred lambs produced relatively lower levels of IFN-γ, following in vitro stimulation with parasite antigen, when compared with their uninfected counterparts. In contrast, infected lambs of both groups showed enhanced mitogen- and antigen-stimulated production of IL-5, in comparison with uninfected controls, at days 5 and 28 postinfection (p.i.). Mitogen- and antigen-stimulated IL-5 responses were higher among resistant lambs compared with random-bred lambs, with the highest overall production of IL-5 by parasite antigen-stimulated ALN and MLN cells. Among day 28 p.i. lambs, levels of cell culture-derived parasite-specific immunoglobulin G1 (IgG1) and IgE antibodies were higher in resistant lambs than in random-bred lambs, following in vitro stimulation of SC or ALN cells with parasite antigen. Finally, after 28 days p.i., histological examination of abomasal tissue revealed higher densities of mast cells and eosinophils in the mucosa of resistant lambs than in random-bred lambs. Taken together, these data support the notion of a strong Th2-type immune response to Haemonchus infection in genetically resistant sheep, and support the claim for a Th1/Th2 dichotomy in ruminants.
Introduction
Elucidation of mechanisms that underlie genetic variations in resistance to nematode parasites is critical for the design of effective parasite vaccines, and for the development of methods for the identification of genetically resistant breeding livestock. In sheep, genetic resistance to the nematode parasites Trichostrongylus colubriformis and Haemonchus contortus is immunologically mediated.1,2 Resistance is correlated with gastrointestinal (GI) tract mucosal mastocytosis, tissue or blood eosinophilia,2–6 and parasite-specific immunoglobulin A (IgA) and IgG1 antibody responses.7,8 These responses suggest the development of a T helper 2 (Th2) -type immune response, yet definitive evidence of a Th1/Th2 dichotomy in ruminants is lacking, and furthermore the underlying patterns of cytokine production (which may characterize a polarized Th response) have yet to be defined in ovine helminthic infections.
That CD4+ T helper (Th) cells are important in the genetic control of sheep immunity to H. contortus has been shown by Gill.9 Significantly higher lymphocyte blastogenic responses and delayed-type hypersensitivity responses to parasite antigen have been reported in sheep with genetic resistance to haemonchosis. While CD4+ Th cells play a central role in the expression of protective immune responses,6 the mechanisms through which they regulate infection have not been defined. Studies in laboratory animals suggest that CD4+ Th cells may contribute to resistance by releasing a variety of cytokines which amplify and regulate the recruitment, proliferation and differentiation of effector cells, such as mast cells, eosinophils and antibody-secreting cells.10
The present study examined the production of key indicator Th1-type (interferon-γ; IFN-γ) and Th2-type (interleukin-5; IL-5) cytokines by H. contortus-infected genetically resistant and random-bred lambs, following in vitro stimulation of spleen cells and regional (GI tract) lymph node cells with parasite antigen or T-cell mitogen. Furthermore, associated Th2-type accessory responses were investigated, including parasite antigen-driven production of IgE and IgG1 in in vitro cell culture, and mast cell/eosinophil infiltration into the abomasal mucosa of infected lambs in vivo.
Materials and methods
Animals and parasites
Ten-month-old, fine wool Merino lambs with genetic resistance11 or with average susceptibility to H. contortus (random-bred), born and raised at the University of New England Kirby Research Station, were used for this study. Two weeks before the start of the experiment, lambs were drenched with ivermectin (200 µg/kg body weight) and housed indoors under conditions which precluded accidental infection with worms. Lambs were infected with 20 000 H. contortus infective larvae per os (Kirby strain) on day 0, as described previously.2 Uninfected lambs served as controls.
Preparation of lymphoid cells
Groups of resistant or random-bred lambs were killed on day 0 (uninfected, two animals/group), day 5 and day 28 after infection (four animals/group) and their spleen, abomasal lymph nodes (ALN) and mesenteric lymph nodes (MLN) were removed under sterile conditions. Single cell suspensions from spleen, ALN, or MLN were prepared in Hanks’ balanced salt solution containing 5% fetal calf serum (FCS), 100 i.u./ml penicillin and 100 µg/ml streptomycin (Gibco, Grand Island, NY). Spleen cell (SC) suspensions were depleted of erythrocytes by tris-ammonium chloride lysis. Mononuclear cells were isolated by Ficoll–paque (Pharmacia LKB, Uppsala, Sweden) centrifugation, washed three times in RPMI-1640 medium supplemented with FCS (10%), NaHCO3 (7·5%), l-glutamine (2 mm), HEPES (10 mm), 2-mercaptoethanol (5 × 10−5 m), penicillin (100 i.u./ml) and 100 µg/ml streptomycin (Gibco), and resuspended in fresh medium to a concentration of 4 × 106 cells/ml. Cell viability, as determined by trypan blue dye exclusion, was greater than 90% in all cases.
Cell culture
To induce cytokine production, SC, ALN, or MLN cells (2 ml/well) were cultured in 24-well flat-bottomed culture plates (Costar, Cambridge, MA) with either 100 µl of T-cell mitogen, [concanavalin A (ConA) 10 µg/ml, Calbiochem-Behring Corp., La Jolla, CA], or 100 µg/ml H. contortus larval antigen (prepared as described previously2). Cells cultured with an unrelated antigen (ovalbumin; Sigma, St Louis, MO) served as unstimulated controls. Plates were incubated for 24 hr at 37° in a humidified atmosphere containing 5% CO2. The supernatants from replicate cultures (three/sample) were pooled, filter-sterilized and stored at – 20° until tested.
Cytokine and IgG1/IgE measurements
IFN-γ activity (units/ml) in the culture supernatants was measured using double antibody sandwich enzyme-linked immunosorbent assays (ELISA), as described by Rothel et al.12 A sandwich ELISA was also used to quantify IL-5 (units/ml) in culture supernatants, using commercially available anti-murine IL-5 monoclonal and polyclonal antibodies (Pharmingen, San Diego, CA). Parasite-specific IgG1 and IgE antibodies present in culture supernatants were measured using a standard ELISA protocol;6 mouse anti-sheep IgG1 and IgE monoclonal antibodies (kindly provided by Dr Ken Beh, CSIRO) were used in the assays.
Histology
Paraformaldehyde or Carnoy’s fluid-fixed abomasal tissues were stained with toluidine blue or alcian blue dye, and subsequently counter-stained with safranin O or Lendrum’s carbol chromotrope, as described previously.2 Mean numbers of eosinophils or mast cells in the abomasal mucosa were estimated by counting a minimum of 10 random fields per animal sample under low-power microscopy, and were subsequently expressed as densities of cells per mm2 tissue surface area.
Statistical analysis
Student’s t-test was used to assess the significance of differences between resistant and random-bred lambs. A probability level of < 0·05 was taken to indicate a statistically significant difference in the means between pairs of data.
Results
Parasitology
To re-affirm the resistance or susceptibility of the lambs used in this study, and also to confirm the infectivity of the parasite larvae, postinfection (p.i.) adult worm counts were performed on the abomasal contents of six resistant or random-bred lambs, as described previously.2 As expected, resistant lambs had significantly lower mean worm burdens than random-bred lambs (2016 ± 771 versus 5333 ± 894, respectively).
Cytokine production
Mean levels of IFN-γ and IL-5 produced by SC, ALN, or MLN cells, in response to stimulation with Con A or parasite antigen, are shown in Figs 1 and 2. Cell cultures derived from non-infected resistant or random-bred lambs produced similar amounts of IFN-γ. Production of IFN-γ in response to Con A mitogen varied little between uninfected and postinfected animal groups. In contrast, production of IFN-γ by ALN or MLN cells, in response to parasite antigen, was markedly lower among day 5 and day 28 p.i. lambs in comparison to the corresponding uninfected lambs of both resistant or random-bred groups.
Figure 1.
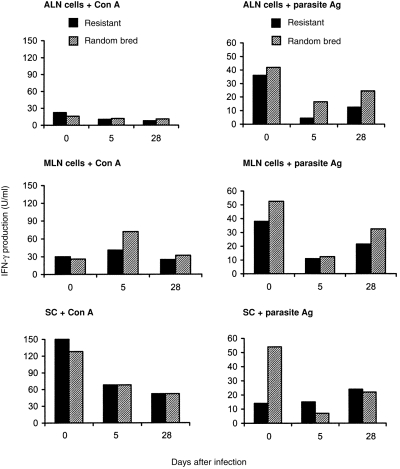
Interferon-γ production by SC, ALN and MLN cells and from uninfected (day 0) and H. contortus-infected genetically resistant and random-bred lambs, after in vitro stimulation with Con A or larval H. contortus antigen.
Figure 2.
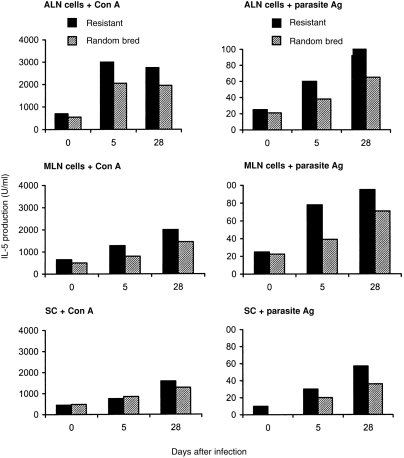
Interleukin-5 production by SC, ALN and MLN cells from uninfected (day 0) and H. contortus-infected genetically resistant and random-bred lambs, after in vitro stimulation with Con A or larval H. contortus antigen.
Cell cultures derived from non-infected resistant or random-bred lambs produced similar amounts of IL-5, following in vitro stimulation with mitogen or parasite antigen. Among day 5 and day 28 p.i. lambs, levels of mitogen- and antigen-stimulated production of IL-5 were higher than in the corresponding uninfected lambs of both resistant and random-bred groups. The highest amounts of IL-5 overall were produced by ALN and MLN cells, following in vitro cell stimulation with parasite antigen. ALN and MLN cells from resistant lambs produced significantly more IL-5 than cells from random-bred lambs, at 5 and 28 days p.i.
Stimulation with ovalbumin, an unrelated antigen, did not induce production of IFN-γ or IL-5 by cells from infected or uninfected lambs at any time-points (data not shown).
IgG1 and IgE production
In vitro parasite-specific IgG1 and IgE production was measured among day 28 p.i. lambs, following stimulation of cells in culture with parasite antigen. SC and ALN cells from resistant lambs produced significantly more IgG1 and IgE than cells from random-bred lambs (Fig. 3).
Figure 3.
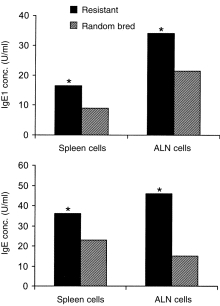
IgG1 and IgE antibody production by SC and ALN cells from H. contortus-infected genetically resistant and random-bred lambs (28 days p.i.), following in vitro stimulation of cell cultures with larval H. contortus antigen. *P < 0·05 (resistant vs. random bred).
Mast cell and eosinophil responses in the mucosa
Abomasal tissues from both resistant and random-bred lambs were examined for mean densities of mucosal mast cells and eosinophils (cells/mm2 tissue surface area) 28 days after infection. The mucosa of resistant lambs contained significantly higher densities of cells than those of random-bred lambs (Fig. 4).
Figure 4.
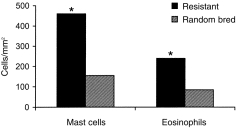
Tissue densities of mast cells and eosinophils in the abomasal mucosa of H. contortus-infected genetically resistant and random-bred lambs, at 28 days p.i. *P < 0·05 (resistant vs. random bred).
Discussion
The control of helminth infections through selective breeding is a major aim of veterinary health improvement.13 In the case of H. contortus infection in sheep, the genetic control of resistance is known to have a specific (lymphoid) component, involving CD4+ Th cells.8 While resistance has been shown to correlate with enhanced peripheral blood lymphocyte reactivity to parasite antigens,1,5,7 until now there have been no studies which have assessed these responses qualitatively. Such information is important not only for determining which genes are important in immune protection, but also for the development of effective prophylactic vaccines, that may offer enhanced resistance to already ‘resistant’ strains, and even to ‘non-resistant’ animals.
In the present study, our initial observation was that lymphoid cells of both genetically resistant and random-bred non-infected lambs produced small amounts of cytokines in response to H. contortus antigen in vitro, which was most likely due to sensitization of these animals to helminth antigens, while on pasture, prior to ivermectin treatment. In contrast, we demonstrated that infection with H. contortus initiated strong production of a Th2-type cytokine (IL-5) by isolated lymphoid cells. Both genetically resistant and random-bred lambs exhibited this pattern of cytokine production p.i., although it was noteworthy that overall levels of IL-5 production were highest in the regional (GI tract) lymph nodes of resistant animals. This pattern is similar to that observed in murine models of the genetic control of acquired immunity to helminth infection.14 For example, in mice genetically resistant to Heligmosomoides polygyrus, immune-mediated protection is associated with the preferential expansion of IL-4- andIL-5-secreting CD4+ Th-cell populations, with associated Th2-type effector mechanisms that include parasite-specific GI tract IgA antibodies and mucosal mastocytosis, and blood eosinophilia. The evidence from the present report, where infected resistant lambs also produced elevated levels of cell-derived parasite-specific IgE and IgG1 antibodies and had elevated abomasal mucosal mast cell infiltrations, would support the notion that an underlying CD4+ Th2-type immune response to H. contortus exists in sheep. Definitive evidence that ruminants can display polarized Th1/Th2 responses is scarce, however, there is strong support for this notion from our study. Similarly, it has recently been shown that sheep genetically resistant to Trichostrongylus colubriformis mount a Th2-type cytokine response following natural exposure to T. colubriformis (i.e. via grazing on infected pasture), with elevated expression of IL-4 mRNA in GI tract lymphatic tissue and peripheral blood.15
In murine models, immune reactions to on-going infections can become strongly dichotomized into Th1- or Th2-type responses, the latter corresponding with Th2-associated effector mechanisms that have already been outlined (IgA, IgG1, IgE antibody; mast cell and eosinophil infiltration; IL-4 and IL-5 production). These responses have been shown to be cross-regulatory in the murine system, and it is generally acknowledged that GI tract helminth infections initiate the selective expansion of Th2-type CD4+ Th cells with a concomitant down-regulation of Th1-type responses.16 Regulation is thought to take place via the production of mutually exclusive cytokines, such that IL-4 and IL-5 can actively suppress the expansion of Th1-type IFN-γ-secreting T-cell responses.17 Advanced anti-cytokine reagents necessary for Th phenotyping at the lymphocyte population level are currently unavailable in veterinary research. Nevertheless, the evidence from our study suggests that a Th regulatory mechanism may exist in ruminants also, in that enhanced secretion of IL-5 was associated with a decrease in secretion of IFN-γ p.i. with H. contortus, and this was particularly noticeable in regional lymph node cells stimulated specifically with parasite antigen. Furthermore, overall levels of parasite antigen-stimulated production of IL-5 by regional (ALN, MLN) cells were significantly higher among resistant than random-bred lambs. Taken together with the supporting histological and serological data, which indicated that p.i. Th2-associated effector responses were significantly higher among resistant sheep than random-bred sheep, these results strongly argue that the protective immune response in sheep genetically resistant to H. contortus involves the selective expansion of Th2-type subset cells with strong IL-5-secreting activity, with minimal activation (and possibly active down-regulation) of Th1-type IFN-γ-secreting activity.
The precise effector mechanism by which sheep are able to control H. contortus infection remains to be defined. However, results from the present study have demonstrated a reduced parasite burden in the abomasum of resistant sheep, and higher densities of infiltrating mast cells and eosinophils into the mucosa of these animals. Furthermore, among the various lymphoid tissues examined, parasite antigen-driven production of IL-5 was highest in the ALN cells isolated from resistant lambs. Together, these data could suggest that mucosal recruitment of granular leucocytes to the site of infection is T-cell-mediated, and that a strongly polarized Th2-type response (involving high local production of IL-5, which is known to be stimulatory for mast cells and eosinophils) would account for the observed histopathological response to infection. Reduction in helminth burden is known to be facilitated by degranulating leucocytes in murine models of helminthiasis,14 and in many cases worm expulsion is aided by the presence of parasite-specific antibodies. The role of antibodies interacting with granular leucocytes in the localized immune-mediated control of haemonchosis in sheep remains uncertain. However, a recent report has indicated that eosinophils isolated from mammary washes of H. contortus-infected sheep are efficient at killing infective third-stage parasite larvae in vitro,18 and that killing can be enhanced by the presence of parasite-specific antibody, complement and exogenous IL-5. In the present study, the fact that antigen-stimulated levels of specific IgE and IgG1 antibodies were higher for ALN cells from genetically resistant sheep, in comparison to random-bred sheep, would suggest an active role for antibody produced locally. This remains to be determined by controlled in vivo studies in sheep.
Acknowledgments
We thank Dr P. R. Wood for providing hybridomas producing anti-IFN-γ monoclonal antibodies and Ms S. K. Burgess for skillful technical assistance. This work was supported by the Australian Wool Research and Promotion Organisation.
Abbreviations
- ALN
abomasal lymph node
- Con A
concanavalin A
- GI
gastrointestinal
- MLN
mesenteric lymph node
- p.i
post-infection
- SC
spleen cell
- Th
T-helper (CD4+) cell
References
- 1.Windon RG, Dineen JK, Wagland BM. Genetic control of immunological responsiveness against the intestinal nematode Trichostrongylus colubriformis in lambs. In: Mcguirk BJ, editor. Merino Improvement Programs in Australia. Melbourne: Australian Wool Corporation; 1987. p. 371. [Google Scholar]
- 2.Gill HS. Genetic control of acquired resistance to haemonchosis in Merino lambs. Parasite Immunol. 1991;13:617. doi: 10.1111/j.1365-3024.1991.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 3.Dawkins HJS, Windon RG, Eagleson GK. Eosinophil responses in sheep selected for high and low responsiveness to Trichostrongylus colubriformis. Int J Parasitol. 1989;19:199. doi: 10.1016/0020-7519(89)90008-8. [DOI] [PubMed] [Google Scholar]
- 4.Dineen JK, Windon RG. The effect of sire selection on the response of lambs to vaccination with irradiated Trichostrongylus colubriformis larvae. Int J Parasitol. 1980;10:189. doi: 10.1016/0020-7519(80)90048-x. [DOI] [PubMed] [Google Scholar]
- 5.Gill HS, Gray GD, Watson DL. Breeding for Disease Resistance. Melbourne: Australian Wool Corporation; 1991. Mechanisms underlying genetic resistance to Haemonchus contortus in sheep; p. 67. [Google Scholar]
- 6.Gill HS, Gray GD, Watson DL, Husband AJ. Isotype-specific antibody responses to Haemonchus contortus in genetically resistant sheep. Parasite Immunol. 1993a;15:61. doi: 10.1111/j.1365-3024.1993.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 7.Gill HS, Watson DL, Brandon MR. Monoclonal antibody to CD4+ T cells abrogates genetic resistance to Haemonchus contortus in sheep. Immunology. 1993b;78:43. [PMC free article] [PubMed] [Google Scholar]
- 8.Gill HS, Husband AJ, Watson DL, Gray GD. Antibody-containing cells in the abomasal mucosa of sheep with genetic resistance to Haemonchus contortus. Res Vet Sci. 1994;56:41. doi: 10.1016/0034-5288(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 9.Gill HS. Cell-mediated immunity in Merino lambs with genetic resistance to Haemonchus contortus. Int J Parasitol. 1994;24:749. doi: 10.1016/0020-7519(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 10.Miyajima A, Miyatake S, Schreurs J, et al. Co-ordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988;2:2462. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- 11.Albers GAA, Gray GD, Piper LR, Le Jambre LF, Barger IA. The genetics of resistance and resilience to Haemonchus contortus in young Merino sheep. Int J Parasitol. 1987;17:1355. doi: 10.1016/0020-7519(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 12.Rothel JS, Jones SL, Corner LA, Cox JC, Wood PR. A sandwich enzyme immunoassay for bovine interferon-gamma and its use in the detection of tuberculosis in cattle. Austr Vet J. 1990;67:134. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 13.Gray GD, Gill HS. Host genes, parasites and parasitic infection. Int J Parasitol. 1993;23:485. doi: 10.1016/0020-7519(93)90037-y. [DOI] [PubMed] [Google Scholar]
- 14.Wakelin D. Immunity to Parasites: How Parasitic Infections are Controlled. 2. Cambridge: Cambridge University Press; 1996. p. 204. [Google Scholar]
- 15.Pernthaner A, Cabaj W, Stankiewicz M, Davies J, Maas D. Cytokine mRNA expression and IFN-gamma production of immunised nematode-resistant and susceptible lambs against natural poly-generic challenge. Acta Parasitol. 1997;42:180. [Google Scholar]
- 16.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and function of helper T cells. Adv Immuol. 1989;46:111. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 17.Grencis RK. Th2-mediated host protective immunity to intestinal nematode infections. Phil Transact Royal Soc London, Series B: Biol Sci. 1997;352:1377. doi: 10.1098/rstb.1997.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainbird MA, Macmillan D, Meeusen EN. Eosinophil-mediated killing of Haemonchus contortus larvae: effect of eosinophil activation and role of antibody, complemment and interleukin-5. Parasite Immunol. 1998;20:93. doi: 10.1046/j.1365-3024.1998.00132.x. [DOI] [PubMed] [Google Scholar]


