Evidence is mounting to support the theory that ‘danger’ associated with tissue damage and stress is a level of control of antigen-specific immune responses. Heat shock proteins are emerging as key danger signals, but their influence on immune responses is not a simple one, particularly in affecting antigen processing and presentation. Such influences may extend to numerous immunological situations. Potential exists for heat shock proteins to be utilized in immune modulation, especially in cancer immunotherapy.
Introduction
Deficiencies of the self–non-self paradigm in explaining immune phenomena, such as the existence and breaking of immune tolerance, have lead the likes of Janeway1 and Matzinger2 to suggest additional levels of control of antigen-specific immune responses. In simple terms, the immune system is thought to be turned on by ‘danger’ associated with certain molecules of infectious organisms, or cell products released during tissue damage or stress. Antigen-presenting cells (APC) possess pattern-recognition receptors that can recognize such molecules. This recognition is thought to cause upregulation of costimulatory molecules on local APC, which is the second signal [in addition to antigenic peptide with major histocompatibility complex (MHC)] that is required to initiate an antigen-specific immune response. Candidate danger signals from infectious organisms include lipopolysaccharide, mannans, glycans and CpG DNA motifs, whilst those from mammalian cells include heat shock proteins (hsp; inducible and constitutive), mitochondria, mannose, RNA and DNA. With respect to hsp, recent evidence now suggests that their role in signalling danger may be wide ranging and, not surprisingly for immunology, more complicated than first thought.
Heat shock proteins
Initial immunological interest in hsp surrounded the concept of hsp being self antigens that are highly conserved throughout evolution and that cross-react with microbial hsp, giving rise to autoimmunity.3,4 This applied to diseases such as arthritis and diabetes. Microbial hsp were also thought to contribute adjuvant properties to vaccines, e.g. mycobacterial hsp70.5 More recently, hsp as antigens have been implicated in allograft rejection.6 The normal functions of hsp include binding and transporting of proteins in the endoplasmic reticulum and cytoplasm, whilst ensuring their correct folding.7 Thus, a role in normal antigen processing and presentation has been suggested.8 The antigen-chaperoning properties of hsp were highlighted in the tumour vaccination setting by the work of Srivastava (reviewed in 9). A number of hsp have now shown the ability to carry tumour antigens and generate protective immunity against live tumour challenge in murine models, including hsp70, hsp90 and gp96. In these experiments the hsp molecules were extracted and purified from tumours against which immunity was sought. Hsp from normal tissues or hsp from tumours, which were then stripped of their associated peptides by adenosine triphosphatase (ATPase) before vaccination, did not confer protection against tumour.10,11 Vaccination was very efficient, requiring only nanogram quantities of soluble hsp–peptide complex. Indeed, large amounts of hsp complex could induce immune tolerance.12 Hsp were also shown to transfer viral antigens,13 minor histcompatibility antigens and model antigens,14 thus generating immunity. In addition, hsp–peptide complexes capable of eliciting immunity in vivo, have been generated from hsp and peptide mixed together in vitro. 15 It was demonstrated that hsp complexes bind to professional APC via surface receptors, are taken up into the APC, and the peptides processed and presented with MHC class I.10,11 Thus hsp allow exogenous antigen access to the endogenous antigen-processing pathway, i.e. they promote antigen cross-priming. These findings were recently confirmed visually using electron microscopy.16
Death of a cell
The danger theory suggests that the mechanism by which a cell dies will dictate whether an immune response is initiated.2 Necrotic cell death and lysis often occurs during viral or bacterial infection, tissue damage and acute inflammation; situations where immune responses are beneficial and even critical to survival. Mechanisms of cell death, however, are often not clear cut and we have discussed these processes in more detail elsewhere.17 The upregulation of hsp in response to cell stress was initially described as a means of cytoprotection, where unfolded cell proteins are bound and protected from stress-induced damage (reviewed in 18). This response to stress is used by a wide range of organisms. It has been postulated that a response to released hsp (constitutive or induced) may be an evolutionary remnant from primitive ancestral immunity.9 It makes sense that hsp could be a first line signal to the innate immune system against danger. In our recent studies we showed that when certain tumour cells were killed by a non-apoptotic (necrotic) mechanism (mediated by thymidine kinase and ganciclovir) they expressed raised levels of inducible hsp70, and other hsp.19 Such killing in vivo gave rise to immune protection against subsequent tumour challenge. It is an accepted concept that normal cell turnover must not induce an (auto-) immune response, and apoptosis generally ensures this, as cell contents are not released (‘quiet’ death). Whole apoptotic cells are scavenged by macrophages, which present peptides in the absence of co-stimulatory molecules since no danger is perceived (Fig. 1). In addition, studies suggest that apoptotic cells can be actively inhibitory to immune responses, e.g. by eliciting interleukin-10 (IL-10) production by macrophages.20 However, other work has shown that antigen from apoptotic cells can be efficiently presented under appropriate (often in vitro) conditions.21 We found that certain tumour cells that died by thymidine kinase-induced apoptosis did not induce hsp and were not immunogenic in vivo. However, co-transfection with apoptosis-blocking bcl-2 drove these cells towards necrosis, accompanied by hsp expression and immunogenicity. We found that this hsp expression occurred in vivo and in vitro and so was not primarily influenced by other cell types. Interestingly, tumours expressing cytokines [IL-2, granulocyte–macrophage colony-stimulating factor (GM-CSF)] expressed hsp in vivo but not in vitro, 19 suggesting hsp mediation by inflammatory cells. When tumour cells were transfected to overexpress hsp 70 they were equally as immunogenic as during necrotic cell death. Thus, hsp expression could replace the necrotic mechanism in generating immunity.
Figure 1.
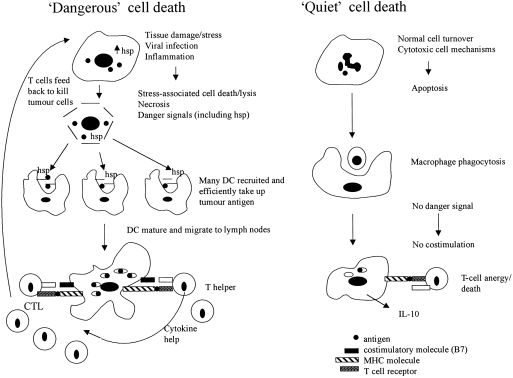
Summary of immune reactions to ‘dangerous’ and ‘quiet’ cell death. Cells dying by apoptotic, ‘quiet’ cell death are scavenged by macrophages. Peptides are presented in the absence of costimulation and T cells die or are anergized. Cells dying in ‘dangerous’ situations, induced by tissue damage/stress (physical/chemical), viral infection or inflammation (leading to necrotic cell death), cause danger signal upregulation and/or release, and cell lysis. DC are recruited in numbers and efficiently take up antigens, especially antigens chaperoned by hsp. DC mature and migrate to lymph nodes where they present peptides to T cells with co-stimulation. T cells primed/activated in numbers can feed back and kill tumour cells.
A number of studies describe the upregulation of hsp in response to adenoviral infection,22 and during inflammation associated with allograft rejection6,23 and rheumatoid arthritis.24 The mechanisms behind this upregulation remain to be deciphered. It is tempting to suggest that viral-, stress- or inflammation-mediated hsp expression may be involved in self antigen transfer to APC and cross-priming during autoimmunity or graft rejection. Although these conditions tend to be multifactoral, such a process may have a precipitating or potentiating role.
Refining the danger theory
A number of reactions must coincide to generate an antigen-specific immune response in a ‘dangerous’ situation. Evidence is now growing that hsp may have numerous effects on shaping antigen-specific immune responses. Recently, it has been demonstrated that hsp can directly induce macrophages25 and cytotoxic T cells26 to secrete inflammatory cytokines such as tumour necrosis factor-α (TNF-α). However, it appears that simply triggering dendritic cell (DC) maturation may in itself not be the ideal mechanism for inducing immunity. Indeed, it is logical that the DC needs to take up antigen before maturation and antigen presentation. In a follow up to our above studies19 we found that radio-labelled lysates from tumour cells over-expressing hsp70 were taken up by immature DC in vitro to a far greater degree than lysates of unmodified cells.27 This effect was lost when mature DC were used. Tagged hsp70 showed that the DC specifically took up the molecule. Incubation of DC with lysates from unmodified cells caused far greater MHC class I and II upregulation than hsp-cell lysate, suggesting reduced maturation when hsp was present. In vivo, hsp-expressing tumours were heavily infiltrated with DC. However, mixed cell tumours of hsp-expressing and unmodified tumour cells only elicited protection against the unmodified cell line that had been expressing hsp. Thus, hsp derived from stressed cells appear to combine the properties of being able to efficiently and specifically transfer antigen to APC,1 and recruiting and maintaining APC in an antigen-acquiring condition (Fig. 1).2 This may be preferable for presenting peptides that are less favourably presented or are in low quantity (causing epitope spreading). Peptides that are easily presented are more likely to have caused tolerance of T cells peripherally or during ontogeny, depending on the antigen. Inducing hsp expression may, in particular, be beneficial in overcoming immunosuppressive mechanisms in the tumour or tumour-vaccine microenvironment, in addition to breaking tolerance to tumour antigens. On the other hand, hsp expression in certain circumstances may induce unwanted autoimmune responses.
Concluding remarks
Greater flexibility in the way that immunologists view their field, together with mounting evidence, has resulted in the general acceptance of the danger hypothesis in influencing or controlling antigen-specific immune responses. Heat shock proteins are implicated as possible danger signals, but their influence may be more subtle and more wide-ranging than first thought. The potential clearly exists for hsp to be manipulated in immunological settings. This is particularly relevant in cancer immunotherapy, since hsp can make tumours appear dangerous to the immune system,28 and can transport tumour antigens for efficient T-cell priming.
References
- 1.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 4.Van Eden W, Van der Zee R, Paul AG, Prakken BJ, Wendling U, Anderton SM, Wauben MH. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998;19:303. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- 5.Barrios C, Lussow AR, Van Embden J, et al. Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guerin priming. Eur J Immunol. 1992;22:1365. doi: 10.1002/eji.1830220606. [DOI] [PubMed] [Google Scholar]
- 6.Birk OS, Gur SL, Elias D, et al. The 60-kDa heat shock protein modulates allograft rejection. Proc Natl Acad Sci USA. 1999;96:5159. doi: 10.1073/pnas.96.9.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 10.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;9:3077. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shockprotein-chaperoned peptides. Science. 1995;269:1585. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 12.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciupitu AM, Petersson M, O'donnell CL, et al. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blachere NE, Li Z, Chandawarkar RY, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold-schild D, Hanau D, Spehner D, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757. [PubMed] [Google Scholar]
- 17.Melcher A, Gough M, Todryk S, Vile R. Apoptosis or necrosis for tumor immunotherapy – what’s in a name? J Mol Med. 1999 doi: 10.1007/s001099900066. in press. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nature Biotechnol. 1998;16:833. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 19.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nature Med. 1998;4:581. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 20.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 21.Albert MLB, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 22.Melcher A, Murphy S, Vile R. Heat shock protein expression in target cells infected with low levels of replication-competent virus contributes to the immunogenicity of adenoviral vectors. Hum Gene Ther. 1999;10:1431. doi: 10.1089/10430349950017770. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo M, Alevy YG, Sundaresan S, et al. Increased expression of HDJ-2 (heat shock protein 40) and heat shock protein 70 in biopsy specimens of transplanted human lungs. J Heart Lung Transplant. 1998;17:241. [PubMed] [Google Scholar]
- 24.Schett G, Redlich K, Xu Q, et al. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest. 1998;102:302. doi: 10.1172/JCI2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212. [PubMed] [Google Scholar]
- 26.Breloer M, Fleischer B, von Bonin A. In vivo and in vitro activation of T cells after administration of Ag-negative heat shock proteins. J Immunol. 1999;162:3141. [PubMed] [Google Scholar]
- 27.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398. [PubMed] [Google Scholar]
- 28.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]


