Abstract
Salmonella bacteria are a major cause of food-borne infectious diarrhoea and there is great interest in understanding the pathogenesis of Salmonella infection and in vaccine development. Potential vaccines include the aromatic mutants of S. typhimurium. Such non-lethal Aro mutants have also been useful for studying Salmonella infections in mouse models. Studies of systemic infection, using these Aro mutants, in both normal and cytokine gene knockout mice, indicate that interferon-γ (IFN-γ) plays a key role in the resolution of Salmonella infection. The present studies have investigated the outcome of oral infection in mice with attenuated Salmonella because this infection route mimics natural infection in humans. In IFN-γ gene knockout (IFN-γ−/−) mice, intestinal immunity was impaired and oral challenge resulted in disseminated septicaemia 2 weeks later. No dissemination of infection was seen in wild-type mice. In wild-type mice, both CD4 and CD8 cell numbers increased in the gut following Salmonella challenge, together with increased expression of major histocompatibility complex (MHC) II and vascular cell adhesion molecule-1 (VCAM-1). No such changes were seen in IFNγ−/− mice. Following oral challenge, antilipopolysaccharide (LPS) and antiphosphoryl choline antibodies increased by more than 100-fold in both serum and faecal pellet extracts of IFNγ−/− mice compared with wild-type mice. Our data show that IFN-γ production is essential for resolution of enteric Salmonella infection and that antibody has little effect on this process.
Introduction
Salmonella spp. are a major cause of enteric infections in humans and are associated with significant morbidity and mortality, especially in developing countries. It is therefore of particular interest to investigate the mechanisms involved in host immunity against Salmonellosis, and to develop effective vaccines to prevent the outbreak of such disease. Salmonellae are facultative intracellular bacteria, and the natural route of infection for most pathogenic Salmonellae is the oral route. Following ingestion, Salmonellae replicate directly in the mucosa-associated lymphoid tissues (e.g. Peyer’s patches [PP]) and thereafter disseminate via the mesenteric lymph nodes to systemic sites (e.g. spleen and liver). This characteristic dissemination pattern, i.e. growth in mucosal and systemic sites, allows Salmonellae to induce broad-based immune responses, including cell-mediated, humoral and secretory immunoglobulin A (IgA) antibody responses. Salmonella typhimurium infection in mice is a useful model for studying the pathogenesis and immune responses associated with human Salmonella infections. Development of non-lethal Aro mutants of S. typhimurium1 as potential live attenuated vaccines has further enhanced the utility of the mouse model.
Interferon-γ (IFN-γ), produced by activated T cells and natural killer (NK) cells, has been shown to play an important role in host defence against intracellular pathogens such as S. typhimurium.2 Following Salmonella challenge, production of IFN-γ by PP lymphocytes has been reported in experiments conducted both in vitro and in vivo. Early production of IFN-γ mRNA has been demonstrated in gut-associated lymphoid tissues and spleens of mice challenged orally with S. typhimurium. 3 In vitro studies have shown that epithelial cells and fibroblasts are resistant to S. typhimurium invasion in the presence of IFN-γ,1 and that IFN-γ activates mouse peritoneal macrophages, resulting in enhanced S. typhimurium killing.4 In vivo experiments have shown that intraperitoneal (i.p.) administration of IFN-γ can protect mice against a lethal S. typhimurium infection,5 but in contrast, administration of anti-IFN-γ antibody is reported to enhance greatly the susceptibility of mice to intravenous (i.v.) bacterial challenge.6 More recently, Hess et al. showed that mice rendered IFN-γ deficient by targeted gene deletion are susceptible to i.v. challenge with S. typhimurium aroA deletion mutants.7 These mice also demonstrated elevated serum-specific antibody levels compared with normal mice: the patterns of serum antibody were shifted from immunoglobulin G2a (IgG2a) to IgG1, and the production of T helper 2 (Th2) cytokines (interleukin [IL]-4 and IL-5) was increased compared with normal mice.8 Therefore it is believed that activation and/or recruitment of lymphocytes which produce IFN-γ is an important factor in determining the outcome of Salmonella clearance following i.v. challenge.
Whereas, in the studies referred to above, challenge was performed by systemic injection, a more definitive assessment of the role of IFN-γ would be provided by oral challenge (the natural route of infection) of IFN-γ gene knockout (IFN-γ−/−) mice. Therefore we have investigated the course of S. typhimurium infection and associated immune responses in IFN-γ−/− and wild-type mice after oral challenge. We report here that host intestinal immunity is impaired in IFN-γ−/− mice following oral S. typhimurium challenge, which resulted in widespread septicaemia, despite elevated antibody responses in both mucosal and systemic compartments.
Materials and methods
IFN-γ gene knockout mice
Mice rendered deficient for IFN-γ (IFN-γ−/−) by targeted disruption of the gene for IFN-γ,9 originally from Genentech Inc. (Genetech Inc., South San Francisco, CA), were kindly provided by Professor N. Hunt (University of Sydney Department of Pathology, Sydney, Australia). C57Bl/6 wild-type mice were obtained from The University of Sydney Blackburn Animal House, and all mice were kept under specific pathogen-free (SPF) conditions. All procedures were approved by the Animal Care and Ethics Committee, University of Sydney.
Inoculation of mice with S. typhimurium
An attenuated aroA/aroD deletion mutant of S. typhimurium was kindly provided by Dr Richard Strugnell (Department of Microbiology, University of Melbourne, Australia).10 The bacterial storage and inoculation protocols were as described previously.11 The experimental mice (10 gene knockout and 10 controls) were anaesthetized with avertin and 200 µl of 0·75% sodium bicarbonate was administered intragastrically 15 min prior to challenge with high (5 × 108) or low (2·5 × 107) doses of viable organisms.
Histopathology
Samples of liver (including gall bladder), spleen, mesenteric lymph node, thymus, several levels of small intestine (including some PP), caecum, ascending colon, kidney, heart and lung were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 4 µm and stained with haematoxylin and eosin. Selected sections were also stained for bacteria with Brown & Brenn’s modification of the Gram stain or the Warthin–Starry silver method. Histopathological findings were recorded in detail for each group and a visual scoring system was used in which tissues were scored from 0 to 5 (normal to most severe, respectively) to assess the onset and extent of change in those organs most affected. In the liver, scores of 1, 2 or 3 indicated scattered focal lesions with maximum diameters of 80 µm or less, 80–240 µm and greater than 240 µm, respectively; a score of 4 indicated more numerous lesions (at least one per high power field; lesions in these animals were often greater than 240 µm in diameter); and a score of 5 was intended to indicate numerous coalescing lesions with extensive disruption of hepatic architecture although it was found that no mice exhibited changes of this severity. In mesenteric lymph node, PP and spleen, a score of 1 indicated reactive hyperplasia or scant, small focal infiltrates of leucocytes; 2 indicated conspicuous lesions in the sinuses or peripheral to the main lymphoid elements but not affecting overall tissue architecture; 3 indicated prominent lesions starting to impinge on the normal lymphoid elements of the tissue; 4 indicated extensive coalescing lesions with few areas of normal tissue; and 5 indicated complete effacement of normal architecture.
Serum collection
At each time-point, mice were anaesthetized with avertin and blood was collected from the orbital sinus. The blood was held at room temperature for 1 hr, then serum was collected and stored at − 70° until assayed.
Intestinal contents and faecal pellet collection
Intestinal wash and faecal pellet collections were performed as described previously.11 Briefly, the small intestinal contents and fresh faecal pellets were collected and resuspended in phosphate-buffered saline (PBS) containing 0·1 mg/ml of soybean trypsin inhibitor. Four-hundred microlitres of supernatant was transferred into a tube containing 100 µl of glycerol to which was added 10 µl of phenylmethylsulphonylfluoride (Sigma, St. Louis, MO) solution (20 mg in absolute ethanol). Samples were vortexed briefly and stored at − 70° until assayed.
Bacterial counts in spleen, liver, faecal pellets and intestinal contents
Fresh tissues from spleen and liver of both IFN-γ−/− and wild-type control mice were collected and weighed aseptically. The tissues were minced mechanically in sterilized PBS and finally passed through 27-gauge needles. Faecal pellets were weighed and diluted in sterilized PBS. The preparations from spleen and liver tissue, faecal dilutions and intestinal contents were serially diluted and plated onto Luria–Bertani (LB) plates containing ampicillin (50 µg/ml) and streptomycin (100 µg/ml), and cultured at 37° overnight. Bacterial counts were evaluated on the plates the following day.
Enzyme-linked immunosorbent assays (ELISAs)
ELISA plates (Polysorb; Nunc, Sydney, Australia) were coated with phosphoryl choline–bovine serum albumin (PC–BSA) (synthesis of PC–BSA is described in reference 11) or Salmonella lipopolysaccharide (LPS) (cat no. L6511; Sigma, Sydney, Australia) at a dilution of 1:1000 in coating buffer (Na2CO3/NaHCO3, pH 9·4), stored overnight at 4° then incubated with blocking buffer (0·05% Tween-20, 0·25% gelatin in PBS) for 30 min at room temperature and then washed twice with distilled water. The samples were diluted in blocking buffer, 50 µl was added to the plates and then incubated for 1 hr at room temperature. Plates were then washed (0·05% Tween-20 in PBS) and biotin-conjugated goat anti-mouse IgA, anti-mouse IgG, anti-mouse IgM, or anti-mouse total immunoglobulin (all supplied by Zymed, South San Francisco, CA) were diluted 1:500 in blocking buffer and 50 µl was added to corresponding wells for 1 hr. After washing, avidin-horseradish peroxidase conjugate (Bio-Rad, Sydney, Australia) was added at a 1:500 dilution in blocking buffer. After a further wash, 50 µl of z,z-azinobis(benthyl-benzthiazoline)sulphonate (ABTS) peroxidase substrate (Kirkegaard & Perry Labs Inc., Parkville, Australia) was added to the plates, incubated for 30 min at room temperature, then 50 µl of peroxidase stop solution (Kirkegaard & Perry Labs Inc.) was added to each well. The plates were read at 405 nm using a Bio-Rad 3560 ELISA plate reader. Differences in antibody levels were tested for significance by using a two-tailed, unpaired Student’s t-test.
Immunohistochemistry
The intestinal tissues were snap-frozen and stored in liquid nitrogen. Sections (5 µm) were cut using a cryostat microtome and fixed with 95% ethanol and air dried. The sections were rehydrated and labelled with cell culture supernatant of the American Type Culture Collection (ATCC; Rockville, MD) cell line TIB 120 (rat anti-mouse major histocompatibility complex [MHC] II), rat anti-mouse vascular adhesion molecule-1 (VCAM-1, Pharmingen, Sydney, Australia), rat anti-mouse CD4, rat anti-mouse CD8 (Immunodiagnostics, Sydney, Australia) antibodies, followed with biotin-conjugated sheep anti-rat antibody, and avidin-conjugated horseradish peroxidase (Dako, Glostrup, Denmark). The slides were finally developed for 3–6 min using the 3,3′-diaminobenzidene (DAB) kit (Dako), as instructed by the manufacturer. The sections were lightly counterstained with haematoxylin. The localization and counting of CD4+ and CD8+ cells in the intestine was performed by manual examination of sections under light microscopy. The data were examined for significance by using a two-tailed, unpaired Student’s t-test.
Image analysis
The immunohistochemically stained sections were analysed using the Leica Q500 MC (Leica, Sydney, Australia) image processing and analysis system for the light microscope, as described previously.12 Positive targets of MHC II and VCAM-1 in the gut were identified and designated on the displayed image. This definition of positivity was used for analysis of all subsequent samples. Twenty randomly selected fields per gut section were analysed. The image analysis results are presented as the average of MHC II- and VCAM-1-positive areas per section from both IFN-γ−/− and IFN-γ+/+ mice. The data obtained from the image analysis were examined for significance by using a two-tailed, unpaired Student’s t-test.
Results
Bacterial counts
S. typhimurium were not detected at any time-point in any samples collected from wild-type mice challenged with either high or low doses. In IFN-γ−/− mice, S. typhimurium were first detected in the intestinal washes from both high- and low-dose challenged mice from week 1 postchallenge (Fig. 1), at which time bacterial numbers were 20-fold higher in the high-dose challenged animals. Bacteria were detected in the spleen, liver, faecal pellet and intestinal washes from the gene knockout mice on week 2 postchallenge, when bacterial counts were almost fourfold higher in the liver following high-dose challenge compared with low-dose, eightfold higher in spleen, 17-fold higher in the pellets and 40-fold higher in the intestinal wash. At week 4 postinfection, large numbers of S. typhimurium were detected in all these samples. At both 3 and 4 weeks postinfection, the numbers of Salmonella recovered from all tissues were ≈ 20-fold higher in higher-dose challenged animals.
Figure 1.
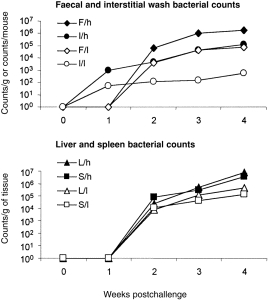
Bacterial counts from (a) faecal pellets (♦, ◊) and intestinal washes (•, ○), and from (b) liver (▴, ▵) and spleen (▪, □) from interferon-γ gene knockout (IFN-γ−/−) mice after oral challenge with viable Salmonella typhimurium at high (5 × 108) and low (2·5 × 107) doses. The filled symbols represent mice receiving high inoculum, open symbols represent mice receiving low inoculum. The data represent the means of observations from 10 mice per group; vertical bars depict the standard error of the mean. (a) F/h, faecal bacteria recovered from high inoculum; I/h, intestinal bacteria recovered from high inoculum; F/l, faecal bacteria recovered from low inoculum; I/l, intestinal bacteria recovered from low inoculum. (b) L/h, liver bacteria recovered from high inoculum; S/h, spleen bacteria recovered from high inoculum; L/l, liver bacteria recovered from low inoculum; S/l, spleen bacteria recovered from low inoculum.
Histopathology
No histological differences were found between wild-type and IFN-γ−/− mice in the absence of challenge. In the infected mice, consistent histological changes were found in sections of liver, spleen, mesenteric lymph node and PP, but only occasional foci of suppurative inflammation and ulceration were seen in the small intestines in a minority of sections from the IFN-γ−/− mice and from one wild-type mouse. Intra- and extracellular Gram-negative bacilli were seen in sections of tissues with severe lesions stained using the Brown & Brenn and Warthin–Starry methods (data not shown). A summary of the semiquantitative lesion scores for consistently affected organs is shown in Table 1, and a more detailed account of the lesions is presented below.
Table 1.
Lesion scores in wild-type and interferon-γ gene knockout (IFN-γ−/−) mice during experimental infection with avirulent Salmonella typhimurium
| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| Liver | ||||
| IFN-γ+/+ | 1* | 2* | 2* | 1* |
| IFN-γ−/− | 0/1 | 2* | 4 | 4 |
| Spleen | ||||
| IFN-γ+/+ | 1* | 0 | 0 | 0 |
| IFN-γ−/− | 0 | 2* | 4* | 5 |
| Mesenteric lymph node | ||||
| IFN-γ+/+ | 0 | 2 | 2 | + |
| IFN-γ−/− | 0 | 4 | 5 | 5 |
| Peyer’s patches | ||||
| IFN-γ+/+ | 0 | 2 | 1 | 1 |
| IFN-γ−/− | 2 | 4 | 4 | 4 |
All scores refer to mice inoculated with 5 × 108 organisms. Scores in mice inoculated with 2·5 × 107 organisms were similar except where marked
in which cases the score was slightly lower.
Liver
At each time-point after inoculation, livers of the wild-type mice contained scattered, small foci of leucocyte infiltration, which were slightly more numerous in mice given the larger inoculum. The number and size of foci reached a peak at week 2 (Fig. 2a, 2b) but had decreased slightly by week 4 (Fig. 2e, 2f). The composition of the leucocyte infiltrate varied little throughout the course of the experiment, consisting of macrophages, lymphocytes and smaller numbers of neutrophils. In the IFN-γ−/− mice, no consistent changes were found in the livers at week 1, although occasional prominent microabscesses were present in the livers of one mouse at each inoculation rate. By week 2, however, moderate to large foci of leucocyte infiltration were scattered throughout the parenchyma and perivascularly in livers of all IFN-γ−/− mice (Fig. 2c, 2d). These increased in number at subsequent time-points, and their composition changed from mixed leucocytes at week 2 to a predominantly macrophage (granulomatous) infiltrate in most mice in the later stages. Some foci also contained areas of necrosis, and thromboses were often present in larger vessels with perivascular lesions (Fig. 2g, 2h). While at week 2 fewer lesions were present in mice receiving the lower dose of organisms, the number of lesions in the two dosage groups was similar thereafter. Occasional small foci of extramedullary haemopoiesis were present in some of the most severely affected mice.
Figure 2.
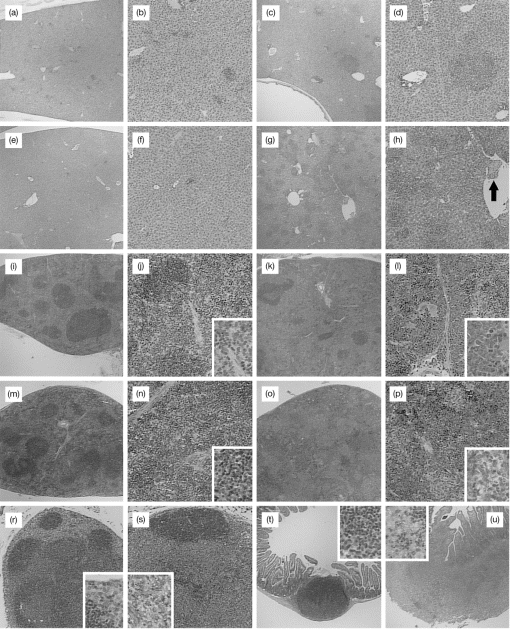
Histopathological findings in interferon-γ gene knockout (IFN-γ−/−) and wild-type mice inoculated with Salmonella typhimurium. At week 2 postinoculation, liver lesions in wild-type mice (a, × 200; b, × 400) were smaller and less prominent than those in IFN-γ−/− mice (c, × 200; d, × 400). By 4 weeks, liver lesions in the wild-type mice had largely resolved (e, × 200; f, × 400) but continued to progress in IFN-γ−/− mice (g, × 200) and were associated with thromboses in nearby blood vessels (arrow) (h, × 400). In the spleen, there were increased numbers of lymphocytes in the red pulp of wild-type mice at week 1 (i, × 200; j, × 400, inset × 800) but no significant changes thereafter. The spleens of IFN-γ−/− mice were normal until week 3 when pronounced extramedullary haemopoiesis was present (o, × 200; p, × 400, inset × 800). At 4 weeks, the normal spleen of wild-type mice (m, × 200; n, × 400, inset × 800) contrasted markedly with that of IFN-γ−/− mice in which there were numerous granulomas and intense haemopoiesis (o, × 200; p, × 400, inset × 800). The mesenteric lymph nodes of wild-type mice exhibited a sinusoidal accumulation of macrophages at week 2 (r, × 200, inset × 800) while severe pyogranulomatous lymphadenitis was present in IFN-γ−/− mice from this time-point onwards (s, × 200, inset × 800). A mild, mixed leucocyte infiltrate was present in Peyer’s patches of wild-type mice at week 2 (t, × 200, inset × 800) in contrast to the severe pyogranulomatous inflammation in IFN-γ−/− mice (u, × 200, inset × 800). All sections were stained with haematoxylin and eosin.
Spleen
There were no significant findings in the spleens from wild-type mice except at week 1 when the red pulp of spleens from mice given the larger dose of organisms contained large numbers of lymphocytes (Fig. 2i, 2j, 2m, 2n). The spleens from IFN-γ−/− mice were normal at week 2 but after this time splenomegaly was evident and was associated with increasing amounts of haemopoietic tissue and scattered granulomas (Fig. 2k, 2l). By week 4, most of the parenchyma consisted of broad, dense bands of haemopoietic tissue and coalescing granulomas with very little lymphoid tissue still evident (Fig. 2o, 2p). These changes were more pronounced in mice given the larger inoculum at weeks 2 and 3, but by week 4 spleens from the two dose groups were virtually indistinguishable.
Mesenteric lymph node
During the course of infection in wild-type mice, coalescing sheets of macrophages accumulated in the subcapsular sinus and deeper within the node to a varying degree (Fig. 2r). This change was only ever moderate in extent and had diminished slightly by week 4. In the IFN-γ−/− mice, however, marked lymphadenomegaly was present by week 2 and an intense, diffuse infiltrate of macrophages and neutrophils had effaced most of the node architecture (Fig. 2.). At later time-points, microabscessation had developed and the inflammatory process extended into surrounding fat. No clear distinction was observed between dose levels in either mouse genotype.
Peyer’s patches
In the wild-type mice, minor changes were present from week 2, consisting of light infiltrates of macrophages, neutrophils and plasma cells in the superficial lamina propria of the patch and in immediately adjacent villi (Fig. 2t). In the IFN-γ−/− mice, however, there was focal ulceration and suppurative exudate in the superficial lamina propria of the PP at week 1, and from week 2 onwards the PP were greatly enlarged and obliterated by an inflammatory process similar to that in the lymph nodes (Fig. 2u). A dense infiltrate of macrophages and neutrophils was also present in the lamina propria of villi adjacent to the patch, although villi on the opposite (mesenteric) side of the gut were normal.
Anti-LPS antibodies
There were low, but detectable, levels of IgA anti-LPS antibody in the faecal pellets of both IFN-γ−/− and wild-type mice prior to challenge (Fig. 3). IgA anti-LPS antibody in the faecal pellets of both high- and low-dose challenged IFN-γ−/− mice continued to increase until week 4 following challenge. In the high-dose challenged group, IgA anti-LPS antibody levels in the faecal pellet on week 4 were elevated more than 100-fold compared with prechallenge levels, and were almost 50-fold higher than prechallenge levels in the low-dose IFN-γ−/− group. There were no significant differences in faecal IgA levels between high- and low-dose challenged groups of wild-type mice, but faecal IgA anti-LPS antibody was almost fourfold lower in wild-type than in high-dose challenged IFN-γ−/− mice at week 4.
Figure 3.
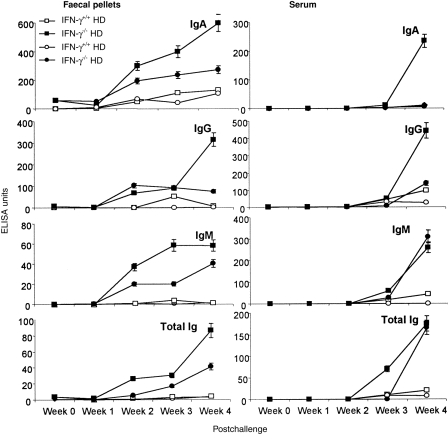
Total anti-lipopolysaccharide (LPS) antibody and immunoglobulin (Ig)A-, IgG- and IgM-specific anti-LPS antibody in faecal pellets and serum collected at weekly intervals following oral challenge with viable Salmonella typhimurium at high (5 × 108) and low (2·5 × 107) doses in wild-type (IFN-γ+/+) and interferon-γ gene knockout (IFN-γ−/−) mice. Data represent the means of observations from 10 mice per group expressed as enzyme-linked immunosorbent assay (ELISA) units, and vertical bars depict the standard error of the mean. HD, high dose; LD, low dose.
Faecal IgG anti-LPS antibody levels also increased with time after infection in both high- and low-dose challenged groups, reaching high levels by week 4. In IFN-γ−/− mice, high-dose challenged animals showed higher levels of IgG anti-LPS antibody than low-dose challenged mice at all time-points, with levels more than 50-fold higher than those in the corresponding wild-type mice. Only low levels of IgG anti-LPS were detected in wild-type mice, and with high-dose challenge, antibody increased at week 3 but fell by week 4 postinfection, which perhaps was a result of elimination of S. typhimurium in the gut by this time-point. IgM and total anti-LPS antibody responses in faecal pellets followed a similar pattern.
In serum, IgA anti-LPS antibody levels postchallenge only increased by week 4 in IFN-γ−/− mice challenged with the high dose of S. typhimurium, but IgG, IgM and total immunoglobulin antibodies increased in IFN-γ−/− mice with both high- and low-dose challenge. Only low levels of antibody were detected by week 4 in wild-type mice.
Anti-PC antibodies
The level of anti-PC antibody of all isotypes also increased in faecal pellets following S. typhimurium challenge, with the highest levels always found in the high-dose challenged IFN-γ−/− group (Fig. 4: IgA, 23-fold increase; IgG, 14-fold increase; IgM, 130-fold increase; total immunoglobulin, 65-fold increase).
Figure 4.
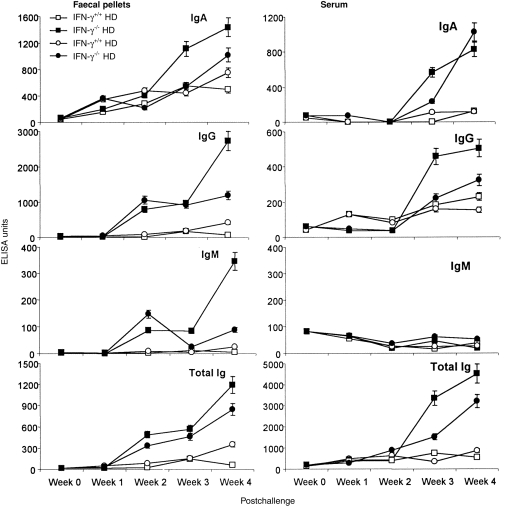
Total anti-phosphoryl choline (PC) antibody and immunoglobulin (Ig)A-, IgG- and IgM-specific anti-PC antibody in faecal pellets and serum collected at weekly intervals following oral challenge with viable Salmonella typhimurium at high (5 × 108) and low (2·5 × 107) doses in wild-type (IFN-γ+/+) and interferon-γ gene knockout (IFN-γ−/−) mice. Data represent the means of observations from 10 mice per group expressed as enzyme-linked immunosorbent assay (ELISA) units, and vertical bars depict the standard error of the mean. HD, high dose; LD, low dose.
IgA, IgG and total immunoglobulin anti-PC in serum all increased with time after challenge in IFN-γ−/− mice but no increase in serum IgM antibodies occurred in any group. In wild-type mice only low levels of anti-PC antibody were detected in serum, except for IgG which showed a significant increase after challenge in both high- and low-dose challenged IFN-γ−/− mice.
MHC class II, VCAM-1 expression, CD4+ and CD8+ cells in the gut
Low levels of constitutive MHC class II and VCAM-1 antigen expression were detected in the intestine prior to challenge, with no significant difference observed between IFN-γ−/− and wild-type mice (Fig. 5). Only a small increase in MHC II antigen expression was detected in the intestine of IFN-γ−/− mice at week 3 postinfection but, in contrast, high levels of MHC II antigen expression had been induced in the intestine of wild-type mice by weeks 2, 3 and 4 postchallenge, which were significantly higher than in the knockout mice at corresponding time-points (P < 0·01).
Figure 5.
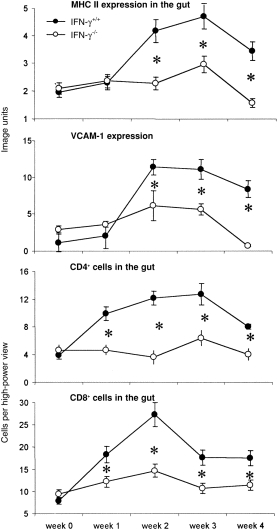
Major histocompatibility complex (MHC) II (a) and vascular cell adhesion molecule-1 (VCAM-1) (b) expression in the gut from wild-type (IFN-γ+/+) and interferon-γ gene knockout (IFN-γ−/−) mice from week 0 to week 4 postchallenge. The y-axis is image analysis units. CD4+ (c) and CD8+ (d) cells in the gut from IFN-γ+/+ and IFN-γ−/− mice from week 0 to week 4 postchallenge. The y-axis is expressed as cells per high power field (× 40) CD8+ cells in the gut from IFN-γ+/+ and IFN-γ−/− mice from week 0 to week 4 postchallenge. Data represent the means of observations from 10 mice per group, and vertical bars depict the standard error of the mean
Similarly there was no significant induction of VCAM-1 expression in the intestine of IFN-γ−/− mice in response to infection at any time-point. On the other hand, VCAM-1 expression in wild-type mice increased gradually with time after challenge and was eightfold higher than the background level by weeks 2–4.
Low numbers of CD4+ cells were observed in the gut of both IFN-γ−/− and wild-type mice prior to challenge, with no significant difference between groups (Fig. 5). Most CD4+ cells were located in the lamina propria, whereas the majority of CD8+ cells were within the intraepithelial compartment. No significant changes in CD4+ or CD8+ cell numbers were detected in the gut of IFN-γ−/− mice from week 0 to week 4, but in wild-type mice the number of both subsets of cells increased by ≈ threefold between weeks 1–3, but had decreased to near-baseline levels by week 4 postinfection.
Discussion
The results presented above demonstrate that, following oral challenge, IFN-γ−/− mice are more susceptible to infection with an aroA−/aroD− mutant of S. typhimurium than are wild-type mice. The susceptibility is both dose- and time-dependent and is characterized by generalized bacteraemia with lesions in multiple organs. Histopathological examination showed development of lesions in the liver, spleen, mesenteric lymph nodes and PP of wild-type mice, although these were modest in extent and were less severe at later time-points, suggesting that resolution was underway. Furthermore, no organisms could be cultured from the gut or elsewhere and no other ill-effects were noted in challenged wild-type mice.
This was in marked contrast to the findings in the same organs in the IFN-γ−/− mice, where severe pyogranulomatous lesions developed over 2–3 weeks and often progressed to efface much of the normal tissue architecture. Liver lesions and spleen and mesenteric lymph node hyperplasia were first detected as early as 1 week postinfection, increased markedly with time and were correlated with increasing numbers of recoverable S. typhimurium from organ homogenates. All experiments had to be terminated at 4 weeks postinfection owing to mortality in the high-dose challenge group of knockout mice.
Attenuated aro mutants of S. typhimurium cannot manufacture aromatic amino acids and cannot obtain these from eukaryotic hosts and thus are supposedly unable to replicate in the host. However, small amounts of p-amino-benzoic acid and 2,3-dihydroxybenzoate are present in mouse chow1 and this may be sufficient to permit low levels of bacterial replication. In wild-type mice this low level of replication is obviously not sufficient to overcome the immune response and bacteria are cleared as a result of phagocytosis and intracellular destruction by IFN-γ-activated macrophages following activation of T helper 1 (Th1) CD4+ cells.7 The small focal lesions noted in livers and spleens of wild-type mice at 2 weeks postinfection are probably caused by the entry of bacterial LPS into the hepatic circulation. These lesions resolve as bacteria are cleared and endotoxin levels decrease at week 4. In IFN-γ−/− mice, however, this bacterial replication cannot be contained within the gut, presumably because of impaired macrophage function. This is consistent with the findings in a model of Bacillus Calmette–Guérin (BCG) infection in IFN-γ−/− mice9 where macrophages showed impaired ability to produce nitric oxide and superoxide anion and as a result were unable to destroy intracellular BCG. In S. typhimurium-infected IFN-γ−/− mice, bacteria were observed within macrophages in some visceral lesions. While these cells may have been recruited locally, it is also possible that they were of gut origin, which would indicate that macrophages are a means of extraintestinal dissemination.
Studies by others have shown that MHC class II knockout and CD4 knockout mice are also susceptible to infections with aro mutants of S. typhimurium.7 The data reported here show no increase in MHC II expression or CD4+ cell numbers in the gut of IFN-γ−/− mice, following S. typhimurium challenge, which is consistent with findings in IFN-γ−/− mice of reduced class II MHC expression on macrophages following infection with BCG.9 These data all further support a critical role for CD4 Th1 T-cell mediated activation of macrophages as the mechanism of bacterial clearance in both infection models.
Hess et al.7 demonstrated that CD8 knockout mice were no more susceptible to systemic S. typhimurium infection than wild-type mice following bacterial challenge via the i.p. route. Therefore, our results demonstrating increased numbers of both CD8+ intraepithelial lymphocytes and CD8+ lamina propria lymphocytes following oral challenge suggest that CD8+ cells may play a role in mucosal clearance of S. typhimurium but have little, if any, role in clearance of systemic bacteria.
The expression of VCAM-1 on the endothelium is closely correlated with leucocyte recruitment into effector sites via their ligand, very late activation antigen-4 (VLA-4),13 and IFN-γ is able to increase VCAM-1 expression.14 Our data confirm that high expression of VCAM-1 on the gut tissue is closely associated with increased CD4+ and CD8+ cell numbers in the wild-type mice in response to challenge, and is correlated with improved resistance to Salmonella infection.
IFN-γ−/− mice showed increasing levels of anti-LPS and anti-PC antibodies in faecal pellets and serum with time after infection and compared to wild-type mice (Fig. 3, Fig. 4) and IgG and IgA antibodies, both of which are T-dependent isotypes, increased in all samples, particularly at weeks 3 and 4. This suggests that, despite the decreased levels of class II MHC expression found in IFN-γ−/− mice, antigen-presenting cell function is still intact but that the T-cell response is predominantly a Th2 response. This is supported by the findings of others15 who showed that following oral infection with attenuated Salmonella, IFN-γ−/− mice have increased numbers of IL-4-, IL-5- and IL-6-secreting cells in PP. Continued persistence of antigen (bacteria) in infected macrophages results in continued activation of Salmonella-specific Th2 cells and chronic activation of non-protective humoral immunity. In wild-type mice, however, activation of Th1 IFN-γ-producing cells results in clearance of bacteria by macrophages. IFN-γ also suppresses antibody production,16 consistent with our findings of lower antibody levels in wild-type mice.
Recent studies reviewed by Erickson & Waldschmidt17 have shown that antibody secretion (IgM and IgG1) and proliferation of B1 cells is enhanced by coculture with Th2 clones. This process was CD40–CD40 ligand (CD40L) independent and required T-cell production of both IL-4 and IL-5. Thus, immunoglobulin secretion by B1 cells can occur in the absence of classical T-cell help but can be further enhanced by Th2 cells through the secretion of these two cytokines. Hence, B1 cell responses can be thought of as having both T-independent and T-dependent components. Marinaro et al. have shown that the number of IL-4- and IL-5-secreting cells are increased in Salmonella-infected IFN-γ−/− mice.15 Therefore it is possible that this form of CD40–CD40L independent T-cell help for B1 cells may be increased in knockout mice compared with wild-type controls.
Another possible reason for the increased antibody responses to PC and LPS observed in IFN-γ−/− mice could be that these mice have increased numbers of B1 B cells. Our previous studies11 have shown that B cells of this lineage are responsible for the majority of anti-PC and anti-LPS antibody production following S. typhimurium challenge, and in IL-5-deficient mice both B1 cell numbers and antibodies for PC and LPS are reduced. In IL-10-depleted mice, Ishida et al.18 showed that B1 cells fail to develop as a result of increased production of IFN-γ. Thus IFN-γ is a negative regulator of B1 cell development and in its absence B1 cell numbers may be increased.
Acknowledgments
This work was supported by grants from the Australian National Health and Medical Research Council, Ramaciotti foundation and the Australian Research Council. The authors acknowledge the skilled technical assistance of Mr M. Allanson.
Abbreviations
- IFN-γ−/−
interferon-γ gene knockout mice
- LPS
lipopolysaccharide
- PC
phosphoryl choline
- PP
Peyer’s patch
- VCAM-1
vascular cellular adhesion molecule-1
References
- 1.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 2.Benbernou N, Nauciel C. Influence of mouse genotype and bacterial virulence in the generation of interferon-gamma-producing cells during the early phase of Salmonella typhimurium infection. Immunology. 1994;83:245. [PMC free article] [PubMed] [Google Scholar]
- 3.Ramarathinam L, Shaban RA, Niesel DW, Klimpel GR. Interferon gamma (IFN- gamma) production by gut-associated lymphoid tissue and spleen following oral Salmonella typhimurium challenge. Microb Pathog. 1991;11:347. doi: 10.1016/0882-4010(91)90020-b. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Nakane A, Minagawa T. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect Immun. 1989;57:2345. doi: 10.1128/iai.57.8.2345-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiderlen AF, Kaufmann SH, Lohmann-matthes ML. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984;14:964. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- 6.Nauciel C, Espinasse-maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321. [PubMed] [Google Scholar]
- 8.Benmerah A, Patey N, Cerf-bensussan N. Adhesion molecules on mucosal T lymphocytes. In: Kagnoff MF, Kiyono H, editors. Essentials of Mucosal Immunology. San Diego: Academic Press; 1996. p. 263. [Google Scholar]
- 9.Dalton DK, Pitts-meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Turner SJ, Carbone FR, Strugnell RA. Salmonella typhimurium delta aroA delta aroD mutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect Immun. 1993;61:5374. doi: 10.1128/iai.61.12.5374-5380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao S, Beagley KW, Murry A, et al. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology. 1998;94:181. doi: 10.1046/j.1365-2567.1998.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence D, Bao S, Canfield PJ, Allanson M, Husband AJ. Elevation of immunoglobulin deposition in the synovial membrane of dogs with cranial cruciate ligament rupture. Vet Immunol Immunopathol. 1998;65:89. doi: 10.1016/s0165-2427(98)00173-1. [DOI] [PubMed] [Google Scholar]
- 13.Chin JE, Hatfield CA, Winterrowd GE, et al. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 14.Rosenman SJ, Shrikant P, Dubb L, Benveniste EN, Ransohoff RM. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995;154:1888. [PubMed] [Google Scholar]
- 15.Marinaro M, Kiyono H, Vancott JL, et al. Vaccines for slective induction of Th1- and Th2-cell response and their roles in mucosal immunity. In: Kagnoff MF, Kiyono H, editors. Essential of Mucosal Immunology. San Diego: Academic Press; 1996. p. 461. [Google Scholar]
- 16.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation. IV. Murine CTL clones produce IL-3 and GM-CSF, the activity of which is masked by the inhibitory action of secreted IFN-gamma. J Immunol. 1990;144:548. [PubMed] [Google Scholar]
- 17.Erickson LD, Waldschmidt TJ. T helper cell activation of B-1 cells. Mucosal Immunol Update. 1998;6:10. [Google Scholar]
- 18.Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992;175:1213. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]


