Abstract
Reorganization of the extracellular matrix is important in many biological and pathophysiological processes, including tissue remodelling, wound healing, or cancer metastasis. The ability of cultured fibroblasts to reorganize and contract three-dimensional type I collagen gels is regarded as an in vitro model for this process. In tissue fibrosis, complex interactions among fibroblasts, inflammatory cells and the extracellular matrix are taking place. Mast cells have often been discussed to play a role in several fibrotic conditions including scleroderma, scar formation, or wound healing. In this study, we examined the effects of mast cells on contraction of collagen lattices. The results demonstrate that co-culture of dermal fibroblasts with a human mast cell line (HMC-1) significantly enhanced contraction of the three-dimensional collagen lattices, whereas mast cells alone failed to contract the gel. Addition of culture supernatants of mast cells did not enhance the speed of gel contraction, indicating the importance of cell–cell contact. Morphological analysis showed that mast cells were incorporated into the lattices. Histological examination also demonstrated that within the lattices, mast cells were localized in close contact to, or attached to, fibroblasts. As fibroblasts and mast cells are known to attach via stem cell factor (SCF)/c-kit interaction when co-cultured in monolayers, we also examined the effect of antibodies against SCF and c-kit in this system. Addition of both antibodies inhibited gel contraction up to 70%. In contrast, antibodies against interleukin-4 (IL-4) and IL-4 receptor did not affect gel contraction. These results indicate that mast cells enhance fibroblast-mediated contraction of collagen lattices via direct cell–cell contact, mediated in part by SCF/c-kit interactions.
Introduction
Interaction of fibroblasts with the extracellular matrix (ECM) controls their behaviour and several functions, such as morphology, growth, motility, differentiation and gene expression.1,2 Three-dimensional ECM culture systems have been developed to simulate natural interactions between cells and ECM more closely than traditional in vitro monolayer culture systems.3,4 Fibroblasts incubated in gels consisting mainly of type I collagen contract the initially loose network to a dense, tissue-like structure. This process is accompanied by a fundamental reprogramming of fibroblast morphology and metabolism, resulting in down-regulation of type I collagen, induction of collagenase, or the collagen receptor α2/β1 integrin. The contraction of collagen lattices is enhanced by various physiological mediators, such as transforming growth factor-β,5 platelet-derived growth factor,6,7 endothelin,8 or insulin.9
Experimental evidence for involvement of mast cells in wound healing has been derived from observations of increased numbers of mast cells and increased histamine release in rats undergoing incisional wounding.10 Wound healing is a complex process involving interactions between various cell types. Mast cell–fibroblast interactions may take place predominantly during the phase of granulation tissue and matrix formation. In this stage, fibroblasts migrate and proliferate in the wound space and produce the ECM consisting of collagen, fibronectin and proteoglycans. Increased numbers of mast cells have also been observed in fibrotic tissues, such as scleroderma or hypertrophic scars.11–13 In scleroderma skin, up-regulation of collagen, as well as reduction of collagenase, have been shown, which is supportive of the pathological accumulation of collagen in scleroderma.14–16 Mast cells have been found to interact with different cell types, including fibroblasts, vascular endothelial cells, epithelial cells, or immunocytes. There are reports demonstrating that mast cells tightly attach to fibroblasts when co-cultured in monolayer systems,17,18 however, there are contradicting reports on the receptors involved.19,20 In monolayer co-culture, fibroblasts support mast cell viability and induce enhanced spontaneous release of histamine.21 In this study, to determine the potential interactions between fibroblasts and mast cells, we investigated the effect of mast cells on fibroblasts when co-cultured in three-dimensional collagenous environment, using the human mast cell line HMC-1, which was established from a patient with mast cell leukaemia22 and was shown to exhibit a phenotype resembling that of human mast cells.23 We provide evidence first, that fibroblast-mediated contraction of three-dimensional lattices is enhanced by co-cultures with mast cells and second, that fibroblast–mast cell contact is mediated via stem cell factor (SCF)/c-kit interaction.
Materials and methods
Cell culture
Fibroblasts were established by outgrowth from skin biopsies of healthy donors as previously described.24 Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 50 µg/ml sodium ascorbate, 100 U/ml penicillin, 100 µg/ml streptomycin, and grown in the moist atmosphere of a CO2 incubator (5% CO2, 95% air) at 37°. The human mast cell line, HMC-1 (generous gift from Dr Butterfield, Mayo Clinic, NY) was maintained in Iscove’s medium (Gibco, Life Technologies Inc, Grand Island, NY) supplemented with 10% FCS, α-thioglycerol (Sigma, St Louis, MO), glutamine (300 µg/ml) penicillin (400 U/ml) and streptomycin (50 µg/ml) in the same CO2 incubator. Mast cells were fed every 3–4 days.
Preparation of collagenous matrices
Crude extracts of type I collagen from calf skin were further purified by dialysis and lyophilization as previously described,25 and dissolved in sterile 0·1% acetic acid at a concentration of 3 mg/ml. Gels 100 mm in diameter were cast in bacteriological Petri dishes by combining 9 ml of Iscove’s medium, 4·5 ml collagen solution, neutralized with 0·75 ml of 0·1 m NaOH, 0·75 ml FCS, containing equivalent to 5 × 106 skin fibroblasts, or to 5 × 106 fibroblasts and an equal number of mast cells (1:1) or to 5 × 106 fibroblasts and 25 × 106 mast cells (1:5). Gel contraction was measured by determining the diameter of the gel in triplicate, in more than five independent experiments.
Blocking experiments
Antibodies against SCF (R & D Systems, Minneapolis, MN), c-kit (Calbiochem, Hamburg, Germany), interleukin-4 (IL-4; Genzyme, Cambridge, MA), IL-4 receptor (Genzyme) were dissolved in phosphate-buffered saline (PBS)/1% bovine serum albumin. Antibodies against SCF (0·1–10 µg/ml) and c-kit (0·05–1 µg/ml) were added individually or in combination immediately before gel casting. In separate experiments, antibodies against IL-4 (0·1–10 µg/ml) and IL-4 receptor (0·1–10 µg/ml) were similarly added.
Histological and histochemical analysis
Twenty-four hours after gel casting, contracted gels were fixed in 5% formalin solution, and embedded in paraffin. Sections were routinely stained with haematoxylin and eosin (H & E), and for the identification of mast cells, with toluidine blue at pH 7·0.
Statistics
Results were expressed as mean ± SD. Significance testing was assessed by Student’s t-test. P-values < 0·05 were considered to be significant.
Results
Effect of mast cells on gel contraction by fibroblasts
Collagen gel alone was not capable of contracting. Co-culture of HMC-1 mast cells with primary dermal fibroblasts significantly enhanced gel contraction in a dose-dependent manner (Fig. 1). When seeded at a ratio of 1:1, the contraction was significantly faster than contraction by fibroblasts alone. When seeded at a 1:5 ratio, gel contraction was enhanced even further. Mast cells alone did not contract the gels at all. In contrast, supernatants from cultured HMC-1 cells (25 × 106) did not alter the contraction of collagen lattices by fibroblasts (data not shown).
Figure 1.
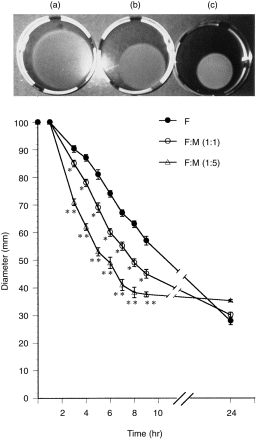
Kinetics of collagen lattice contraction by human skin fibroblasts, co-cultured with or without human mast cells (HMC-1). Lattices contained 5 × 106 fibroblasts (F) and mast cells at 1 : 1 (F/M 1 : 1) or in fivefold excess (F/M 1 : 5). Cultures were photographed at 6 hr after culture initiation: (a) fibroblasts alone; (b) fibroblasts co-cultured with mast cells at a 1 : 1 ratio; and (c) at a 1 : 5 ratio. Contraction of type I collagen matrices by fibroblasts was assessed by measuring gel diameters (graph). *P < 0·01, **P < 0·005, compared with fibroblasts alone. Representative data of the five independent experiments are shown.
This suggests that fibroblast–mast cell attachment is necessary for the enhancement of gel contraction in this system.
Morphological analysis of co-cultures in collagen lattices
When fibroblasts are seeded in a three-dimensional matrix consisting mainly of type I collagen, the cells adhere to the collagen fibres and contract the initially loose network to a dense tissue-like structure.4,25 Morphological features of the different cultures in three-dimensional lattices are shown in Fig. 2. Mast cells were found tightly attached to fibroblasts inside the lattices (Fig. 2a–c), and almost no mast cells were detectable outside the gels after the gel started to contract. Toluidine blue stain confirmed that mast cells were attached to, or in close contact with, fibroblasts (Fig. 2f).
Figure 2.
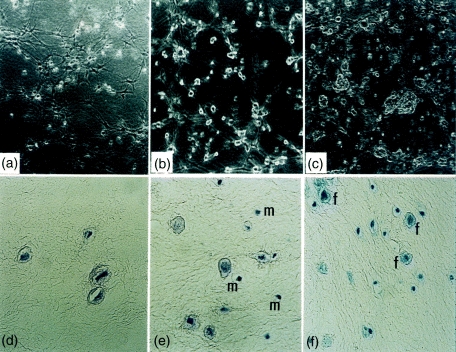
Morphology of fibroblasts and mast cells, co-cultured in three-dimensional lattices by phase contrast microscopy (a–c): (a) fibroblasts alone, (b) fibroblasts and mast cells at a 1 : 1 ratio, (c) fibroblasts and mast cells at a 1 : 5 ratio. Mast cells are indicated by arrows. Histopathological analysis of three-dimensional lattices 24 hr after the gel casting (d–f): H & E stain of fibroblasts alone (d), and fibroblasts and mast cell (m) co-cultures (1 : 1 ratio) (e), (f) toluidine blue stain of fibroblasts (f) and mast cell co-cultures (1 : 5 ratio). Magnifications: a–c, ×40; d, ×400; e,f, ×200.
Inhibitory effect of antibodies against SCF/c-kit
It is known that fibroblasts and mast cells form tightly heterotypical adhesions when co-cultured in monolayer systems.17,18 This effect has been suggested to be mediated by SCF/c-kit interaction.19 In order to investigate whether a similar mechanism is required for the enhancement of gel contraction, we examined the effects of anti-SCF/c-kit antibodies on the interaction of fibroblasts with mast cells in this system. We first confirmed spontaneous SCF production in the culture supernatants of normal skin fibroblasts (n = 5) (72·4 ± 20·2 pg/ml) by enzyme-linked immunosorbent assay (ELISA; Yamamoto et al. manuscript in preparation). Both antibodies against SCF (0·1–10 µg/ml) and c-kit (0·05–1 µg/ml) inhibited the enhancement of gel contraction in a concentration-dependent manner (Fig. 3). Although the combined application of both antibodies showed up to 70% inhibition, they could not completely inhibit the gel contraction. Since IL-4 is expressed by mast cells and the IL-4 receptor is expressed on fibroblasts, we next examined the effect of antibodies against IL-4 and its receptor.26,27 However, these antibodies did not alter collagen gel contraction (data not shown). As a further control, we examined the effect of an isotype-matched control immunoglobulin G (IgG) and an irrelevant antibody against human leucocyte antigen (HLA) -ABC (Chemicon International Inc., Temecula, CA) and no change of the gel contraction was noted (data not shown). Langholz et al.28 also used the antibody against HLA-ABC and showed that it does not affect contraction by fibroblasts alone.
Figure 3.
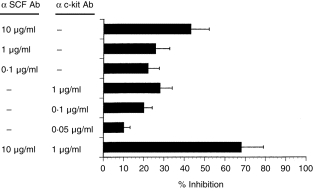
Inhibitory effect of antibodies against SCF and c-kit on collagen lattice gel contraction. Fibroblasts and mast cells were seeded at a 1 : 5 ratio. Antibodies (Ab) against SCF (0·1–10 µg/ml) and c-kit (0·05–1 µg/ml) were added singly or in combination prior to gel casting. As gel contraction was maximally enhanced at 4–6 hr after gel casting, gel diameters were determined at 6 hr and compared with control (fibroblasts only). Inhibition of gel contraction was assessed by the comparison of the alteration of gel diameter between those with and without antibodies.
Discussion
Recent studies provided evidence that mast cells play a crucial role for tissue remodelling and fibrosis.29 In this study, we confirmed that addition of mast cells to fibroblasts cultured in three-dimensional collagen lattices significantly enhanced gel contraction. In contrast, mast cells alone did not contract the gels at all. Cell–cell attachment is necessary for this effect, because cultured supernatants of mast cells did not show any promoting effect for gel contraction. Microscopy showed that mast cells attach to fibroblasts prior to starting the contraction in the three-dimensional lattices. During contraction, nearly all mast cells became incorporated in the collagen network. Histological examination with toluidine blue stain demonstrated that mast cells attached to or were in close contact with fibroblasts.
In monolayer co-cultures, it is well-known that mast cells attach to fibroblasts.17,18 Adachi et al.19 showed that this effect is mediated in part by SCF/c-kit interaction, and nearly 70% inhibition was noted when antibodies against SCF and c-kit were added. On the other hand, Trautmann et al.20 showed that the attachment of the human mast cell line, HMC-1, to fibroblasts was not mediated by SCF/c-kit interaction. We observed that in monolayers, when added, mast cells began to attach to fibroblasts within 20 min (data not shown). Our experiments of inhibition of attachment after pre-incubation with these antibodies in monolayers also showed a similar result to that of Adachi et al.19 (data not shown). In three-dimensional lattices, up to 70% inhibition of the enhancement of gel contraction by addition of mast cells was noted by a combination of antibodies against SCF and c-kit, indicating that this mechanism is mediated, in part, by SCF/c-kit interaction. However, even the combined addition of both antibodies could not completely block the enhancement of gel contraction. One explanation might be that even higher concentrations of antibodies are needed; or another receptor/counter-receptor is involved in fibroblast–mast cell attachment. To explore the latter, we examined the effect of antibodies against IL-4 and IL-4 receptor. This approach was based on two observations; First, IL-4 is expressed by CD4+ T lymphocytes or mast cells, and the IL-4 receptor is expressed on fibroblasts.26,27 Second, Trautmann et al.27 recently demonstrated that the release of IL-4 by mast cells induced by cell–cell contact between mast cells and fibroblasts is important for the regulation of fibroblast proliferation. However, our results indicate that these antibodies did not alter the kinetics of gel contraction, suggesting that an IL-4–IL-4 receptor interaction is not involved in gel contraction.
In conclusion, we demonstrated that mast cells enhance gel contraction by fibroblasts within type I collagen lattices via strong cell–cell attachment, and that this enhancement is partially mediated by SCF/c-kit interaction. This study indicates that mast cells may play an important role in wound contraction in vivo.
Acknowledgments
We wish to thank Gabriele Huppe for excellent technical assistance. This study was supported in part by a grant from Köln Fortune Program, Faculty of Medicine, University of Cologne (T.Y. and K.H).
References
- 1.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell E, Ivarsson B, Merill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckes B, Krieg T, Nusgens BV, Lapiere CM. In vitro reconstituted skin as a tool for biology, pharmacology and therapy: a review. Wound Rep Reg. 1995;3:248. doi: 10.1046/j.1524-475X.1995.30304.x. [DOI] [PubMed] [Google Scholar]
- 5.Montesano R, Orci L. Transforming growth factor beta stimulates collagen matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85:4894. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gullber D, Tingstrom A, Thuresson AC, et al. β1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- 7.Clark RA.F, Folkvord JM, Hart CE, Murray MJ, McPherson JM. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989;84:1036. doi: 10.1172/JCI114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidry C, Hook M. Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J Cell Biol. 1991;115:873. doi: 10.1083/jcb.115.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson SN, Ruben Z, Fuller GC. Cell-mediated contraction of collagen lattices in serum-free medium: effect of serum and nonserum factors. In Vitro Cell Dev Biol. 1990;26:61. doi: 10.1007/BF02624156. [DOI] [PubMed] [Google Scholar]
- 10.Gottwald T, Coerper S, Schaffer M, Koveker G, Stead RH. The mast cell–nerve axis in wound healing: a hypothesis. Wound Rep Reg. 1998;6:8. doi: 10.1046/j.1524-475x.1998.60104.x. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins RA, Claman HN, Clark RAF, Steigerwald JC. Increased dermal mast cell populations in progressive systemic sclerosis: a link in chronic fibrosis? Ann Intern Med. 1985;102:182. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- 12.Nishioka K, Kobayashi Y, Katayama I, Takijiri C. Mast cell numbers in diffuse scleroderma. Arch Dermatol. 1987;123:205. [PubMed] [Google Scholar]
- 13.Kischer CW, Bunce H, Shetlar MR. Mast cell analysis in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol. 1978;70:355. doi: 10.1111/1523-1747.ep12543553. [DOI] [PubMed] [Google Scholar]
- 14.Botstein GR, Sherer GK, Leroy EC. Fibroblast selection in scleroderma. An alternative model for fibrosis. Arthritis Rhem. 1982;25:189. doi: 10.1002/art.1780250212. [DOI] [PubMed] [Google Scholar]
- 15.Krieg T, Perlish JS, Mauch C, Fleischmajer R. Collagen synthesis by scleroderma fibroblasts. Ann NY Acad Sci. 1985;460:375. doi: 10.1111/j.1749-6632.1985.tb51184.x. [DOI] [PubMed] [Google Scholar]
- 16.Mauch C, Eckes B, Hunzelmann N, Oono T, Kozlowska E, Krieg T. Control of fibrosis in systemic scleroderma. J Invest Dermatol. 1993;100:92. doi: 10.1111/1523-1747.ep12356293. [DOI] [PubMed] [Google Scholar]
- 17.Levi-schaffer F, Austen KF, Gravallese PM, Stevens RL. Co-culture of interleukin-3 dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci USA. 1986;83:5. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levi-schaffer F, Austen KF, Caulfield JP, Hein A, Bloes WF, Stevens RL. Fibroblasts maintain the phenotype of rat heparin containing mast cells in vitro. J Immunol. 1985;135:3454. [PubMed] [Google Scholar]
- 19.Adachi E, Ebi Y, Nishikawa S, et al. Necessity of extracellular domain of W (c-kit) receptors for attachment of murine cultured mast cells to fibroblasts. Blood. 1992;79:650. [PubMed] [Google Scholar]
- 20.Trautmann A, Feuerstein B, Ernst N, Brocker E-B, Klein CN.E. Heterotypic cell–cell adhesion of human mast cells to fibroblasts. Arch Dermatol Res. 1997;289:194. doi: 10.1007/s004030050180. [DOI] [PubMed] [Google Scholar]
- 21.Levi-schaffer F. Mast cell/fibroblast interactions in health and disease. Chem Immunol. 1995;61:161. [PubMed] [Google Scholar]
- 22.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leukemia Res. 1996;12:345. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson G, Blom T, Kusche-gullberg M, et al. Phenotypic characterization of the human mast cell line HMC-1. Scand J Immunol. 1994;39:489. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 24.Krieg T, Aumailley M, Dessau W, Wiestner M, Muller PK. Synthesis of collagen by human fibroblasts and their SV40 transformants. Exp Cell Res. 1980;125:23. doi: 10.1016/0014-4827(80)90184-6. [DOI] [PubMed] [Google Scholar]
- 25.Mauch C, Hatamochi A, Scharffetter K, Krieg T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp Cell Res. 1988;178:493. doi: 10.1016/0014-4827(88)90417-x. [DOI] [PubMed] [Google Scholar]
- 26.Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors: role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606. [PubMed] [Google Scholar]
- 27.Trautmann A, Krohne G, Brocker EB, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998;160:5053. [PubMed] [Google Scholar]
- 28.Langholz O, Rockel D, Mauch C, et al. Collgaen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol. 1995;131:1903. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothe MJ, Nowak M, Kerdel FA. The mast cell in health and disease. J Am Acad Dermatol. 1990;23:615. doi: 10.1016/0190-9622(90)70264-i. [DOI] [PubMed] [Google Scholar]


