Abstract
In our previous reports, we found polyclonal anti-double-stranded DNA antibodies (anti-dsDNA) purified from patients with active systemic lupus erythematosus (SLE) exerted inhibitory effect on [3H]thymidine incorporation of human mononuclear cells (MNC). However, the other immunological effects of anti-dsDNA on the functions of MNC have not yet been reported. In this study, two monoclonal antibodies, 12B3 and 9D7, with different anti-dsDNA activity were evaluated for their effects on the expression and release of different cytokines from human MNC. We confirmed absence of endotoxin in the two monoclonal antibody preparations and the used medium as detected by Limulus amoebocyte lysate test. The mRNA expression and release of different cytokines including interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) were measured. We found the two monoclonal anti-dsDNA not only dose-responsively suppressed the phytohaemagglutinin (PHA)-induced thymidine uptake of human MNC but stimulated the mRNA expression of IL-1β, IL-6 and IL-8 in normal human MNC detected by reverse transcription–polymerase chain reaction (RT–PCR). Enzyme-linked immunosorbent assay (ELISA) measurement of cytokines in MNC culture supernatants revealed that anti-dsDNA enhanced IL-1β, IL-8, TNF-α and IL-10 release from resting MNC. These effects of anti-dsDNA antibodies were not affected by polymyxin B, a potent binder and neutralizer of lipopolysaccharide (LPS). These in vitro studies suggest that anti-dsDNA possess a dual effect on normal human MNC: (a) to enhance the release of proinflammatory cytokines (IL-1β, IL-8 and TNF-α) from MNC to augment inflammatory reaction; and (b) to polarize the immune reaction towards the T helper 2 (Th2) (increased IL-10 production) pathway. This unique effect of anti-dsDNA may play a role in lupus pathogenesis by augmenting inflammatory reactions and autoantibody production which are commonly found in patients with active SLE.
Introduction
Anti-double-stranded DNA antibodies (anti-dsDNA) are the marker autoantibodies in systemic lupus erythematosus (SLE). Although the titre of anti-dsDNA in the serum of SLE can reflect the disease activity and especially the renal damage of the disorder, the role of the autoantibodies in lupus pathogenesis remains unclear. These antibodies can bind with DNA or DNA–histone conjugates to form circulating immune complexes and deposit in different tissues to elicit inflammation.1–3 However, many studies, including ours, demonstrated that anti-dsDNA antibodies cross-react with plasma membrane-associated antigens on different cell types and exert adverse effects on the cell functions.4–6 Furthermore, Yanase et al.7 reported that internalization of anti-DNA antibodies into the cells was mediated by binding to the brush border myosin expressed on the surface of cells. Recently, the effects of anti-dsDNA antibodies on the expression of cytokines in different cells have drawn much attention. Lai et al.8,9 found that polyclonal anti-dsDNA antibodies stimulated the release of interleukin (IL)-1 and IL-6 from endothelial cells and caused the vascular endothelial injury. Nevertheless, the effect of anti-dsDNA on cytokine gene expression in immunocompetent cells has not yet been reported in the literature.
Proinflammatory cytokines such as IL-1, IL-6, IL-8 and tumour necrosis factor-α (TNF-α) are produced primarily by activated macrophages and monocytes. These cytokines possess potent stimulatory effects on T and B lymphocytes, natural killer cells (NK), and neutrophils in increasing the biosynthesis of prostaglandins and acute-phase proteins by these cells.10 It is conceivable that type 1 helper T cells (Th1) produce IL-2 and interferon-γ (IFN-γ) whereas Th2 cells produce IL-4 and IL-10 to modulate cellular and humoral immune reactions, respectively.11 In our previous report, we found polyclonal anti-dsDNA purified from sera of patients with active SLE altered the proliferative capacity of mitogen-activated normal human mononuclear cells (MNC).12 Based on this observation, we deduce that anti-dsDNA can probably change the cytokine producing profile of MNC and subsequently affects the responses of these cells. To further confirm this possibility, the effects of two potent monoclonal anti-dsDNA antibodies, 9D7 and 12B3, obtained from autoimmune MRL-lpr/lpr mice were evaluated. We showed here that anti-dsDNA antibodies enhanced the expression and release of several proinflammatory cytokines (IL-1β, IL-6, IL-8 and TNF-α) and a Th2 cytokine (IL-10) from MNC. The significance of the findings relating to the pathogenesis of SLE is discussed
Materials and methods
Preparation of monoclonal anti-dsDNA antibody (mAb) from autoimmune MRL-lpr/lpr splenic cells
9A4, 9D7 and 12B3 were mouse monoclonal anti-dsDNA antibodies produced by fusion of NS-1 myeloma cells with spleen cells of autoimmune MRL-lpr/lpr mice following the method reported by Kohler and Milstein.13 The details of hybridization and screening procedures were described elsewhere in the literatures. The dsDNA binding activity of these autoantibodies were detected by using an anti-DNA antibody enzyme-linked immunosorbent assay (ELISA) and immunofluorescence stain of HEp-2 cells (described in the next paragraph). Hybridoma cells (9A4, 9D7, 12B3) and NS-1 cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (10% FBS-RPMI) and the supernatants collected from these cells were used for the following experiments. Purification of anti-dsDNA monoclonal antibody (mAb) from culture supernatants was carried out by protein A–agarose affinity chromatography (Sigma Chemical Company, St Louis, MO). The purity of immunoglobulin G (IgG) preparations was assessed by 13% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Only heavy and light chains of mouse IgG were found in the affinity-purified antibody. Determination of the IgG concentration and IgG subclass of the three monoclonal anti-dsDNA by ELISA, as described in the next paragraph, revealed IgG2b of 9A4 and 9D7, and IgG2a of 12B3. The IgG concentration in different anti-dsDNA was shown in Table 1. We used the same concentration of commercially available normal mouse IgG or IgG2b (Sigma) as a non-specific isotype or subclass-matched antibody control in the experiments. Endotoxin content in antibody preparations and culture media used in the experiments was measured by Limulus amoebocyte lysate assay (Sigma). The minimal detectable concentration of endotoxin was 0·05 EU/ml.
Table 1.
Immunologic properties of cultured supernatants from three hybridomas (9A4, 9D7 and 12B3) and NS-1 cells, and purified mouse IgG
| IgG concentration (ng/ml) | IgG subclass | HEp-2 staining pattern | dsDNA binding activity (OD410) | |
|---|---|---|---|---|
| 9A4 | 140 | IgG2b | Peripheral | 0·138 |
| 9D7 | 32 000 | IgG2b | Peripheral | 0·492 |
| 12B3 | 27 600 | IgG2a | Homogeneous | 1·85 |
| NS-1 | 0 | –* | – | 0·103 |
| Mouse IgG | 50 000 | ND† | – | 0·105 |
| RPMI-1640 | – | – | – | 0·102 |
Negative.
Not done.
ELISA for quantitation and subclass typing of monoclonal Abs
The commercial kits for mouse IgG and IgG subclass were purchased from Boehringer Mannheim Biochemicals (Mannheim, Germany) for quantitation and subclass typing of different culture supernatants. The minimal detectable concentration for mouse IgG was 6·25 ng/ml.
dsDNA binding assay
The dsDNA binding activity of the mAbs were measured by ELISA as described elsewhere.14 Briefly, polystyrene microtitre plates (Corning Co., New York, NY) were precoated with 150 µl of 0·5 mg/ml protamine chloride (Sigma) per well in a 2-hr incubation and washed subsequently with phosphate-buffered saline solution (PBS, pH 7·2). Commercially obtained calf thymus dsDNA (Sigma) was further purified by the method reported by Rauch et al.15 with some modifications. This purified dsDNA (5 µg/100 µl/well) was incubated in microwells overnight at room temperature. After washing for five times with PBS pH 7·2 containing 0·05% Tween-20 (washing buffer), 200 µl of 2% BSA in PBS were added and incubated for another 2 hr. The plates were washed five times with washing buffer and 100 µl of samples were added. After 1 hr incubation, the plates were washed five times with washing buffer. Then, 100 µl of diluted horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Lab. Inc., West Grove, PA) were added and incubated for another 30 min. The plates were washed again five times and 100 µl freshly prepared substrate solution (1·37 mg/ml of 2,2′-azinodi-13-ethylbenz-thiazoline sulphonic acid in 100 mm phosphate buffer, pH 4·2, containing 0·6 µl/ml 30% H2O2) were added to each well. After 15 min incubation, the signal was measured at 410 nm in a Dynatech EIA reader.
Immunofluorescence staining of HEp-2 cells with monoclonal Abs
The non-synchronized, prefixed HEp-2 cells in slide glass (SCIMEDX Corporation, Denville, NJ) were incubated with 50 µl of different monoclonal anti-dsDNA at 25° for 40 min and were washed three times with PBS, pH 7·2. Cells were then incubated with 1 : 200 diluted fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Jackson Immuno-Research) for 40 min. After three additional washes with PBS, the cells were examined under fluorescence microscopy and photographed.
Detection of [3H]thymidine incorporation of human mononuclear cells and rat glomerular mesangial cells (RMC)
Mononuclear cells were isolated from heparinized venous blood of normal subjects after centrifugation over a Ficoll–Hypaque cushion (specific gravity 1·077) at 300 g for 30 min. One hundred microlitres of MNC (1 × 106/ml) were placed in U-shaped flat-bottomed microwells in triplicate. Twenty microlitres of phytohaemagglutinin (PHA, 20 µg/ml) and 80 µl of individual hybridoma culture supernatant were added to the wells. The mixture was incubated at 37° in 5% CO2−95% air for 68 hr and then pulsed with 1 µCi methyl-[3H]thymidine/10 µl/well (specific activity 6·7 Ci/mmol, NEN Products, Boston, MA) for another 4 hr. The cells were harvested and the radioactivity was detected by a beta counter. RMC were isolated from 250 to 300 g Sprague–Dawley rat kidneys and were subcultured as described previously.16[3H]thymidine incorporation of RMC were carried out as the same as in MNC except that 100 µl of RMC (2 × 105/ml) and 100 µl of different concentration of anti-dsDNA were added into a microtitre well in triplicate. The mixture was incubated at 37° in 5% CO2−95% air for 54 hr and pulsed with 1 µCi methyl-[3H]thymidine/well for another 18 hr.
Binding and internalization of anti-dsDNA mAb to Jurkat T cells
Binding of anti-dsDNA to the surface of Jurkat T cells was analysed by the following method. Briefly, Jurkat T cells (3 × 106) were incubated with culture supernatant of anti-dsDNA hybridoma (9D7, 10 µg/ml) or mouse IgG2b (20 µg/ml) for 1 hr in an ice-bath followed by three washes with ice-cooled PBS. Cells were then reacted with 200× diluted FITC-labelled goat anti-mouse IgG (Jackson ImmunoResearch) for 40 min in an ice-bath. After three washes with ice-cooled PBS, cells were observed under fluorescence microscope. The percentage and mean fluorescence intensity of the positive cells were analysed with flow cytometry (Becton Dickinson, San Jose, CA).
Internalization of anti-dsDNA mAb was performed by the method described by Avrameas et al.17 Jurkat cells (3 × 105/ml) were incubated with culture supernatants of anti-dsDNA hybridomas (9D7, 10 µg/ml) or mouse IgG2b (20 µg/ml) for 18 hr at 37° in a 5% CO2−95% air. After three washes with PBS, cells were fixed in 2% paraformaldehyde in PBS (pH 7·4) for 25 min, washed with PBS and permeabilized with 0·1% Triton-X-100 in PBS for 5 min. The fixed cells were then stained with 200× diluted FITC-labelled goat anti-mouse IgG antibodies for 40 min. After three washes with PBS, the presence of intracellular mAb was observed under fluorescence microscope. The percentage and mean fluorescence intensity of the positive cells were analysed with flow cytometry.
Reverse transcription–polymerase chain reaction (RT–PCR) for detecting the expression of different cytokine mRNA in mononuclear cells
Three millilitres of MNC (2 × 106/ml), 0·6 ml of medium and 2·4 ml of anti-dsDNA were mixed and cultured at 37°, in 5% CO2−95% air. After 6, 18 and 30 hr incubation, the cells were harvested and the total RNA was extracted by using the Ultraspec RNA isolation system (BIOTECX Lab., Inc. Houston, TX). One microgram of RNA was reversely transcribed into cDNA in 30 µl reverse transcriptional buffer containing 50 mm Tris–HCl, 75 mm KCl, 3 mm MgCl2, 0·5 µg oligo-dT primer, 0·5 mm deoxynucleotide triphosphate (dNTP), 30 U RNasin, 10 mm dithiothreitol (DTT), and 400 U murine Moloney leukaemia virus (M-MLV) reverse transcriptase (Promega Corp., Madison, WI), pH 8·3, at 42° for 1 hr. After reverse transcription, 5 µl product was added to PCR buffer containing 10 mm Tris–HCl, 1·5 mm MgCl2, 50 mm KCl, 0·1% Triton-X-100, 100 ng forward primer, 100 ng reverse primer, 0·2 mm dNTP, 2 U DNA polymerase (Finnzymes Oy, Riihitontuntie, Finland) and 5% DMSO. The PCR was performed in a HYBAID OminGene DNA Thermal Cycler (Teddington, UK) with a program of denaturing at 95° for 45 s, annealing at 60° for 45 s and primer extension at 72° for 2 min. The amplification was carried out for 30 cycles. The reaction was stopped in a final extension at 72° for 10 min, followed by incubation at 25°. The forward and reverse primers for human IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, IFN-γ and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were purchased from Clontech Laboratories (Palo Alto, CA) and their nucleotide sequences are listed below:
IL-1β: forward primer 5′ATGGCAGAAGTACCTAAGCTCGC3′
reverse primer 5′ACACAAATTGCATGGTGAAGTCAGTT3′
IL-2: forward primer 5′CATTGCACTAAGTCTTGCACTTGTCA3′
reverse primer 5′CGTTGATATTGCTGATTAAGTCCCTG3′
IL-4: forward primer 5′CGGCAACTTTGACCACGGACACAAGTGCGATA3′
reverse primer 5′ACGTACTCTGGTTGGCTTCCTTCACAGGACAG3′
IL-6: forward primer 5′ATGAACTCCTTCTCCACAAGCGC3′
reverse primer 5′GAAGAGCCCTCAGGCTGGACTG3′
IL-8: forward primer 5′ATGACTTCCAAGCTGGCCGTGGCT3′
reverse primer 5′TCTCAGCCCTCTTCAAAAACTTCTC3′
IL-10: forward primer 5′AAGCTGAGAACCAAGACCCAGACATCAAGGCG3′
reverse primer 5′AGCTATCCCAGAGCCCCAGATCCGATTTTGG3′
TNF-α: forward primer 5′ATGAGCACTGAAAGCATGATCCGG3′
reverse primer 5′GCAATGATCCCAAAGTAGACCTGCCC3′
IFN-γ: forward primer 5′GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC3′
reverse primer 5′CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG3′
G3PDH: forward primer 5′ACCACAGTCCATGCCATCAC3′
reverse primer 5′TCCACCACCCTGTTGCTGTA3′
The size of PCR products were: 802 bp for IL-1β, 305 bp for IL-2, 344 bp for IL-4, 628 bp for IL-6, 289 bp for IL-8, 328 bp for IL-10, 695 bp for TNF-α, 427 bp for IFN-γ and 452 bp for G3PDH, respectively.
ELISA for measuring the concentraion of IL-1β, IL-2, IL-4, IL-8, IL-10, TNF-α and IFN-γ in culture supernatants
One hundred microlitres of MNC (2 × 106/ml), 20 µl of medium, and 80 µl of cultured supernatant of hybridoma, affinity-purified anti-dsDNA or NS-1 cells were mixed and cultured at 37°, in 5% CO2−95% air. After 1-, 2- or 3 -day incubation, the cells were centrifuged and the culture supernatants were collected. The concentration of different cytokines in cell-free culture supernatants were then measured by commercially available human cytokine ELISA kits. IL-1β, IL-8 and TNF-α ELISA kits were purchased from BioSource (Camarillo, CA). IL-2, IL-4, IL-10 and IFN-γ ELISA kits were purchased from Endogen (Woburn, MA). The minimal detectable concentration was 3·9 pg/ml for IL-1β, 38·4 pg/ml for IL-2, 10·24 pg/ml for IL-4, 31·2 pg/ml for IL-8, 15·36 pg/ml for IL-10, 15·6 pg/ml for TNF-α, and 25·6 pg/ml for IFN-γ, respectively.
Statistical analysis
Results represent mean±SD in this study. Statistical significance was assessed by non-parametric Wilcoxon rank sum test in the BMDP computer software system (BMDP Statistical Software Inc., Los Angeles, CA).
Results
Characterization of mouse monoclonal anti-dsDNA antibodies
The immunological properties of the three anti-dsDNA antibodies (9A4, 9D7 and 12B3) and NS-1 myeloma culture supernatant are listed in Table 1. mAbs 9D7 and 12B3 contained much higher concentration of IgG and possessed much more potent dsDNA binding activity than those of 9A4 and NS-1. Immunofluorescence staining of HEp-2 cells by mAbs 9D7 and 12B3 showed a peripheral and homogenous nuclear pattern, respectively. However, both dsDNA binding and HEp-2 staining activities of 9D7 and 12B3 disappeared after absorption with calf thymus dsDNA-cellulose column (Sigma) (data not shown). LPS contents of all culture supernatants, affinity-purified antibodies and culture media used in the experiments were below the detectable concentration (0·05 EU/ml) of the Limulus amoebocyte lysate assay.
Effect of monoclonal anti-dsDNA antibodies on [3H]thymidine incorporation of PHA-stimulated normal human mononuclear cells and cultured rat glomerular mesangial cells
It is conceivable that most of the autoepitopes are evolutionarily highly conserved and the autoantibodies are usually pan-reactive with the cognate antigens across species.18 Both rat mesangial cells and human mononuclear cells were used in the present study to determine the effect of monoclonal anti-dsDNA on the proliferation of these cells. The cells were incubated with mAb 9A4 (0·06 µg/ml), 9D7 (12·8 µg/ml), 12B3 (11·4 µg/ml), NS-1 culture supernatant (1 : 2·5× dilution), or mouse IgG (20 µg/ml) for 18 hr in the stimulation of PHA (2 µg/ml). The [3H]thymidine uptake of MNC was significantly suppressed by mAbs 9D7 and 12B3 (P < 0·05) whereas no effect was noted after incubation with mAb 9A4, NS-1 cultured supernatant or mouse IgG (Fig. 1a). In contrast, all of the supernatants seemed to enhance the growth of RMC (Fig. 1a). It is possible that NS-1 myeloma cells can produce an unknown nature growth factor for rat glomerular mesangial cells to promote the growth/proliferation of these cells. Accordingly, the culture supernatants of the three hybridomas which derived from the hybridization of autoimmune MRL-lpr/lpr spleen cells and NS-1 myeloma cells retains the capacity to produce the growth factor and enhances the thymidine uptake of RMC. The inhibitory effect of mAbs 9D7 and 12B3 on PHA-stimulated MNC proliferation was found to be dose-dependent (Fig. 1b).
Figure 1.
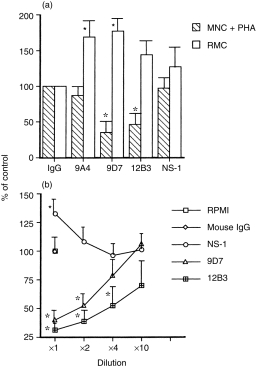
The effect of cultured supernatants from 9A4, 9D7, 12B3 and NS-1 cells and purified mouse IgG on [3H]thymidine incorporation of PHA (2 µg/ml)-stimulated normal human mononuclear cells (MNC + PHA) and cultured rat glomerular mesangial cells (RMC) after incubation for 3 days. (a) The concentration of different proteins: mouse IgG = 20 µg/ml, 9A4 = 0·06 µg/ml, 9D7 = 12·8 µg/ml, 12B3 = 11·04 µg/ml and NS-1 = 2·5× dilution. (b) Dose–response effect of different proteins on [3H]thymidine incorporation of PHA-stimulated MNC. The concentration of 1× dilution of different proteins was as the same as in (a). * denotes P < 0·05.
Binding and internalization of monoclonal anti-dsDNA antibody into Jurkat T cells
To determine whether anti-dsDNA mAb can bind and penetrate into cells, Jurkat T cells were incubated with culture supernatant of 9D7 mAb or mouse IgG2b at 4° for 1 hr in binding test and 37° for 18 hr in internalization test. After fixation and permeabilization, the cells were stained with fluorescein-conjugated anti-mouse IgG. Both surface binding and internalization of the anti-dsDNA into the cells were observed under fluorescence microscope and measured with flow cytometry. Compared to mouse IgG2b, anti-dsDNA mAb could bind more (15·57 versus 5·28%) to the cell surface and internalize more (31·87 versus 5·21%) into the cells as shown in Fig. 2. The same experiment was repeated three times with a similar tendency.
Figure 2.
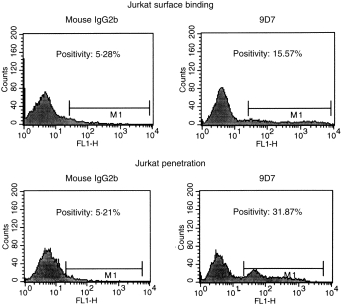
Binding and internalization of monoclonal anti-dsDNA antibody (9D7) and mouse IgG2b into Jurkat T cells were compared. Jurkat T cells (3 × 105/ml) were incubated with 9D7 (10 µg/ml) or mouse IgG2b (10 µg/ml) at 4° for 1 hr in binding test and at 37° for 18 hr in internalization test, respectively. After washing with PBS, percentage binding and internalization of anti-dsDNA antibodies were analysed by flow cytometry as described in Methods. The abscissa denotes fluorescence intensity and ordinate denotes cell number.
Effect of monoclonal anti-dsDNA antibodies on the transcription of cytokine genes in MNC
To determine whether anti-dsDNA monoclonal antibodies exert a modulatory effect on the transcription of different cytokines, the total cell RNA were extracted from 18 hr cultured MNC and the transcripts of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, IFN-γ, and ubiquitous G3PDH were detected by RT–PCR technology. Equal amounts of RNA detected by OD260 and ethidium bromide staining was used in the RT–PCR. Compared to NS-1 cultured supernatant (lane 3) and mouse IgG (lane 4), mAbs 9D7 (lane 1) and 12B3 (lane 2) enhanced the transcription of IL-1β, IL-6 and IL-8, but not the transcription of IL-10 and IFN-γ, in MNC after 18 hr incubation (Fig. 3a). There was no transcription of IL-2 and IL-4 genes at all in the cells after incubation with anti-dsDNA or NS-1 supernatant (data not shown). As demonstrated in Fig. 3(b), the kinetic study revealed that monoclonal anti-dsDNA antibodies 9D7 (lanes 1, 4 and 7) could stimulate the transcription of IL-1β and IL-8 in MNC in 6 hr, 18 hr and 30 hr incubation. In contrast, NS-1 supernatant (lanes 2, 5, 8) and mouse IgG (lanes 3, 6, 9) could only stimulate the transcription of the two cytokines in 6 hr and 18 hr, but not in 30 hr, in MNC. These results suggest that anti-dsDNA can enhance/prolong the expression of many proinflammatory cytokines (IL-1β, IL-6, IL-8 and TNF-α) in normal human MNC much more effective than mouse IgG or NS-1 cultured supernatant.
Figure 3.
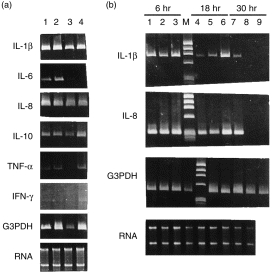
Expression of cytokine mRNA in normal human mononuclear cells (MNC) by RT–PCR after incubation with monoclonal anti-dsDNA antibody (9D7 and 12B3), NS-1 supernatant and purified mouse IgG. (a) Normal human MNC were incubated with different proteins for 18 hr. Lane 1: incubation with 9D7 (12·8 µg/ml), lane 2: incubation with 12B3 (11·04 µg/ml), lane 3: incubation with NS-1 supernatant (2·5× dilution) and lane 4: incubation with mouse IgG (20 µg/ml). (b) Kinetic expression of IL-1β and IL-8 mRNA in normal human MNC after incubation with different proteins for 6 hr (lanes 1–3), 18 hr (lanes 4–6) and 30 hr (lanes 7–9). Lanes 1, 4 and 7: incubation with 9D7 (12·8 µg/ml). Lanes 2, 5 and 8: incubation with NS-1 (2·5× dilution). Lanes 3, 6 and 9: incubation with mouse IgG (20 µg/ml). M: φx174/Hae III DNA size marker. Prolonged expression of IL-1β and IL-8 mRNA (lane 7) was found only after incubation with 9D7 for 30 hr.
Effect of monoclonal anti-dsDNA antibodies in the release of cytokines from MNC
Compared to mouse IgG and cultured supernatant of NS-1, mAbs 9D7 and 12B3 significantly enhanced the release of IL-1β, TNF-α and IL-8 from normal human mononuclear cells (Fig. 4, P < 0·05). However, these antibodies failed to induce the production and mRNA expression of IL-2, IL-4 and IFN-γ from MNC (data not shown).
Figure 4.
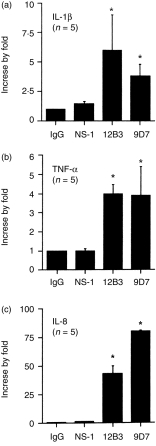
The effect of monoclonal anti-dsDNA antibody (9D7 and 12B3), NS-1 supernatant and purified mouse IgG on IL-1β, TNF-α and IL-8 cytokine release from normal human mononuclear cells (MNC) detected by ELISA after incubation for 3 days. The concentration of different proteins: 9D7 = 12·8 µg/ml, 12B3 = 11·04 µg/ml, mouse IgG = 20 µg/ml and NS-1 = 2·5× dilution. * denotes P < 0·05.
Although the increased expression of IL-10 mRNA after incubation with these two mAbs was not clearly found (Fig. 3a), the affinity-purified anti-dsDNA mAb could dose-dependently stimulate the release of IL-10 (Fig. 5a) but not IFN-γ from MNC (Fig. 5b).
Figure 5.
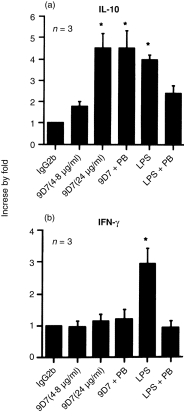
The effect of affinity-purified monoclonal anti-dsDNA antibody (9D7) and bacterial LPS (100 ng/ml) on the release of IL-10 and IFN-γ from normal human mononuclear cells (1 × 106/ml) of three individuals after stimulation for 2 days in the presence/absence of polymyxin B (PB, 10 µg/ml)). Anti-dsDNA monoclonal antibody was purified by protein A-agarose affinity chromatography. The concentration of mouse IgG2b was 40 µg/ml. LPS: lipopolysaccharide (E. coli, serotype 026:B6). * denotes P < 0·05.
It had been reported that LPS could induce the production of proinflammatory cytokines and IFN-γ from T lymphocytes.19 To further confirm the cytokine-stimulating effect of anti-dsDNA was not via the contaminating LPS in the antibody preparations (although undetectable amount of LPS in these preparations was found), effect of anti-dsDNA mAb and LPS in the presence/absence of polymyxin B (PB, 10 µg/ml, a potent LPS neutralizing agent) was compared. As shown in Fig. 5, the stimulating effect of purified mAb anti-dsDNA could not be neutralized by polymyxin B. In contrast, LPS could stimulate IL-10 and IFN-γ release from MNC but this effect could be neutralized by polymyxin B. These results suggest that the stimulating effects of anti-dsDNA mAb on cytokine production of human MNC were diverse and not due to LPS contamination in the preparations.
Discussion
Anti-dsDNA are found exclusively in patients with SLE and are clinically correlated with the diagnosis, disease activity and prognosis of SLE. Especially, the autoantibodies are thought to play a major role in the pathogenesis of lupus nephritis. However, the real mechanism(s) by which they participate in kidney damage is still not elucidated. Our previous studies suggest that the autoantibodies can bind to the cell membrane-expressed acidic ribosomal P proteins (P0, P1 and P2) and directly damage the glomerular mesangial cells via an apoptotic mechanism.5,6,20,21 Immunologically, polyreactivity is a common property of natural and disease-associated human autoantibodies.22 Accordingly, anti-dsDNA are expected to exert pleiopotential effects on different tissues other than mesangial20,21 and endothelial cells.8,9 We had found polyclonal anti-dsDNA purified from serum of patients with active SLE suppressed the [3H]thymidine incorporation of PHA-stimulated normal human MNC.12 But the molecular basis for this immunosuppression of anti-dsDNA is not clear. In the present study, two monoclonal anti-dsDNA autoantibodies were found to stimulate the mRNA expression/release ofIL-1β, IL-6, IL-8, IL-10 and TNF-α from normal human resting MNC (Fig. 4 and Fig. 5). Many reports had demonstrated that overexpression of the proinflammatory cytokines including IL-1, IL-6 and TNF-α occurred in both SLE patients and lupus-prone mice.23–32 Our findings are quite similar to that of them. These abnormal cytokine expressions were well correlated with the exaggerated inflammatory process in the disease.33 Finck et al.34 found that IL-6 accelerated the disease course of autoimmune NZB/NZW F1 mice. The administration of anti-IL-6 significantly prevented production of anti-dsDNA, reduced proteinuria, and prolonged life of the autoimmune prone mice.34 The present in vitro study suggests that anti-DNA autoantibodies not only stimulate the release of proinflammatory cytokines from monocytes/macrophages lineage but polarize the immune reaction towards the Th2 pathway. Subsequently, the released cytokines not only amplify the inflammatory reaction to mediate pleiotropic tissue damage but activate the humoral immunity which are the major abnormalities in patients with systemic lupus erythematosus. This observation has not been reported in the literature.
The cause for the abnormal modulation of these cytokines by anti-dsDNA in human or murine SLE is not clear at present. However, Okudaiya et al.35 found anti-dsDNA are capable of penetrating into the nuclei and affect the functions of mononuclear cells. Lai et al.8,36 demonstrated anti-dsDNA antibodies can stimulate the release of proinflammatory cytokines, IL-1 and IL-6, from endothelial cells via cytotoxic endothelial damage. Our data also revealed that anti-dsDNA can bind to the cells surface and penetrate into nuclei and subsequently induce proinflammatory cytokine release from functionally altered mononuclear cells. These findings further provide a possible explanation for the pathologic role of anti-dsDNA in causing pleiotropic adverse effects in tissues of patients with SLE.
The observation that anti-dsDNA deranged the balance of Th1/Th2 cytokine network in enhancing the production of IL-10 is quite interesting. By administration of human monoclonal anti-DNA antibody to BALB/c mice, Segal et al.33 demonstrated that the development of experimental SLE in these mice involved two stages: increased production of Th1-type cytokines followed by increased production of Th2-type cytokines. Nevertheless, high levels of the proinflammatory cytokines, TNF-α and IL-1, were produced throughout the disease course. This in vivo observation is quite consistent with our in vitro study. Moverover, Peng et al.37 and Nakajima et al.38 demonstrated that the expression IL-4 and IFN-γ play a prominent role in the pathogenesis of murine lupus. They showed that the administration of anti-IL-4 antibody was effective in preventing the onset of lupus nephritis. The IFN-γ−/− and IL-4−/− lupus-prone mice developed significantly reduced lymphadenopathy and end-stage organ disease. However, we could not detect IL-4 and IFN-γ gene expression in anti-dsDNA treated MNC. The role of IFN-γ and IL-4 in lupus pathogenesis can not be justified in our study. Richaud-Patin et al.29 had reported that high gene expression of IL-4 and IL-10 is present in SLE patients. We only found anti-DNA antibody increased IL-10, but not IL-4, production that denotes the autoantibodies deviated the immune response of normal MNC towards the Th2 pathway and subsequently enhanced the autoantibody production. Another interesting finding of the present study is that normal human MNC can spontaneously express IL-1β, IL-8, and IL-10 mRNA (Fig. 3). It is possible that the isolation procedure per se or in vitro 18 hr culture of MNC can elicit the transcription of these cytokine genes in these cells. The detection of the cytoplasmic and released cytokines immediately after isolation and after 18 hr incubation is now under investigation.
Undoubtedly, there are three unsolved problems remained in the present study. (a) Anti-dsDNA suppressed the [3H]thymidine incorporation of PHA-stimulated MNC but conversely enhanced that of rat mesangial cells. This contradiction in the growth/proliferation of the two different cells may reflect a difference in cell biology in response to anti-dsDNA. However, we hypothesize that an unknown nature of glomerular mesangial cell growth factor may probably be secreted from NS-1 cells and hybridomas (Fig. 1a). In addition, a similar tendency is also observed in the dilution test of NS-1 supernatant in promoting growth/proliferation of PHA-stimulated human MNC in 1× and 2× dilution but not in > 4× dilution (Fig. 1b). The purification and identification of this growth factor-like molecule is now under investigation. (b) Discrepancy exists between cell proliferation inhibition and IL-1β enhancing activity of anti-dsDNA on mononuclear cells. Our results indicate that anti-dsDNA can exert two different effects on mononuclear cells, a stimulating effect on monocytes/macrophages to increase IL-1β, IL-6, IL-8 and TNF-α expression but an inhibitory effect on T lymphocytes proliferation. Although the released IL-1β can stimulate T lymphocyte proliferation, the overall effect of anti-dsDNA on human MNC is suppressive. Lai et al.9,36 also reported a similar observation in that anti-DNA increased the gene expression of IL-8 and TGF-β in vascular endothelial cells on the one hand but injured the cells to release the von Willebrand factor on the other. (c) The molecular mechanism of anti-dsDNA to derange Th1/Th2 cytokine production by resting MNC is not investigated in the present study. However, this IL-10 stimulating effect of anti-dsDNA is quite important because increased IL-10 release may enhance autoantibody production in patients with SLE.39,40 The direct effect of anti-dsDNA on B-cell hyperreactivity was also suggested in our previous report.12 However, the molecular mechanism remains to be determined. In conclusion, monoclonal anti-DNA autoantibody stimulates the expression/release of IL-1β, IL-6,IL-8, IL-10 and TNF-α from normal human mononuclear cells to amplify the inflammatory reaction and polarize the immune response toward Th2 pathway which involve the lupus pathogenesis.
Acknowledgments
The authors are grateful to Miss Te-Wei Tang and Mr Yu-Shan Wang for technical assistance. This work was supported by grants from the National Science Council (NSC 86-2314-B-010-044, NSC 87-2314-B-010-122) and National Health Research Institute (DOH 86-HR-508, DOH 87-HR-508), ROC.
Abbreviations
- SLE
systemic lupus erythematosus
- MNC
normal human mononuclear cells
- Anti-dsDNA
mouse monoclonal antidouble stranded DNA antibody
- IL
interleukin
- TNF-α
tumour necrosis factor-α
- IFN-γ
interferon-γ
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126:607. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert P-H, Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968;127:507. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raz E, Ben-bassat H, Davidi T, Shlomai Z, Ba Eilat D. Cross-reactions of anti-DNA autoantibodies with cell-surface proteins. Eur J Immunol. 1993;23:383. doi: 10.1002/eji.1830230213. [DOI] [PubMed] [Google Scholar]
- 5.Sun K-H, Liu W-T, Tasi C-Y, Tang S-J, Han S-H, Yu C-L. Anti-dsDNA antibodies cross-react with ribosomal P proteins expressed on the surface of glomerular mesangialcells to exert a cytostatic effect. Immunology. 1995;85:262. [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K-H, Liu W-T, Tang S-J, et al. The expression of acidic ribosomal phosphoproteins on the surface membrane of different tissues in autoimmune and normal mice which are the target molecules for anti-double-stranded DNA antibodies. Immunology. 1996;87:362. [PMC free article] [PubMed] [Google Scholar]
- 7.Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest. 1997;100:25. doi: 10.1172/JCI119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai KN, Leung JC.K, Lai KB, Li PK.T, Lai CK.W. Anti-DNA autoantibodies stimulate the release of interleukin-1 and interleukin-6 from endothelial cells. J Pathol. 1996;178:451. [PubMed] [Google Scholar]
- 9.Lai KN, Leung JC.K, Lai KB, Lai FM, Wong KC. Increased release of von Willebrand factor antigen from endothelial cells by anti-DNA autoantibodies. Ann Rheum Dis. 1996;55:57. doi: 10.1136/ard.55.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan FM, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1996;8:872. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 12.Yu C-L, Chang KL, Chiu C-C, Chiang B-N, Han S-H, Wang S-R. Alteration of mitogenic responses of mononuclear cells by anti-dsDNA antibodies resembling immune disorders in patients with systemic lupus erythematosus. Scand J Rheumatol. 1989;18:265. doi: 10.3109/03009748909095029. [DOI] [PubMed] [Google Scholar]
- 13.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 14.Faaber P, Rijke GPM, van de Putte LBA, Capel PJA, Berden JHM. Cross-reactivity of human and murine anti-DNA antibodies with heparan sulfate: the major glycosaminoglycan in glomerular basement membranes. J Clin Invest. 1986;77:1824. doi: 10.1172/JCI112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch J, Massicotte H, Wild J, Tannenbaum H. Sensitive solid phase radioimmunoassay for the detection of anti-DNA autoantibodies. J Rheumatol. 1985;12:482. [PubMed] [Google Scholar]
- 16.Striker GE, Striker LJ. Glomerular cell culture. Lab Invest. 1985;53:122. [PubMed] [Google Scholar]
- 17.Avrameas A, Ternynck T, Nato F, Buttin G, Avrameas S. Polyreactive anti-DNA monoclonal antibodies and a derived peptide as vectors for the intracytoplasmic and intranuclear translocation of macromolecules. Proc Natl Acad Sci USA. 1998;95:5601. doi: 10.1073/pnas.95.10.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan EM. Autoimmunity and apoptosis. J Exp Med. 1994;179:1083. doi: 10.1084/jem.179.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591. [PubMed] [Google Scholar]
- 20.Tsai C-Y, Wu T-H, Sun K-H, Yu C-L. Effect of antibodies to double stranded DNA, purified from serum samples of patients with active systemic lupus erythematosus, on the glomerular mesangial cells. Ann Rheum Dis. 1992;51:162. doi: 10.1136/ard.51.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai C-Y, Wu T-H, Sun K-H, Liao T-S, Lin W-M, Yu C-L. Polyclonal IgG anti-dsDNA antibodies exert cytotoxic effect on cultured rat mesangial cells by binding to cell membrane and augmenting apoptosis. Scand J Rheumatol. 1993;22:162. doi: 10.3109/03009749309099265. [DOI] [PubMed] [Google Scholar]
- 22.Hurez V, Dietrich G, Kaveri SV, Kazatchkine MD. polyreactivity is a property of natural and disease-associated human autoantibodies. Scand J Immunol. 1993;38:190. doi: 10.1111/j.1365-3083.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 23.Prud’Homme GJ, Kono DH, Theofilopoulos AN. Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1 beta, interleukin-1 and interferon-gamma mRNA in the lymph nodes of lupus-prone mice. Mol Immunol. 1995;32:495. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- 24.Boswell JM, Yui MA, Endres S, Burt DW, Kelly VE. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988;141:118. [PubMed] [Google Scholar]
- 25.Lemay S, Mao C, Singh AK. Cytokine gene expression in the MRL/lpr model of lupus nephritis. Kid Int. 1996;50:85. doi: 10.1038/ki.1996.290. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C-Y, Wu T-H, Huang S-F, et al. Abnormal splenic and thymic IL-4 and TNF-α expression in MRL-lpr/lpr mice. Scand J Immunol. 1995;41:157. doi: 10.1111/j.1365-3083.1995.tb03548.x. [DOI] [PubMed] [Google Scholar]
- 27.Studnicka-benke A, Steiner G, Petera P, Smolen JS. Tumor necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- 28.Stuart RA, Littlewood AJ, Maddison PJ, Hall ND. Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol. 1995;13:17. [PubMed] [Google Scholar]
- 29.Richaud-patin Y, Alcocer-varela J, Ti Llorente L. High levels of TH2 cytokine gene expression in systemic lupus erythematosus. Rev Invest Clin. 1995;47:267. [PubMed] [Google Scholar]
- 30.Robak E, Sysa-jedrzejowska A, Stepien H, Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Netw. 1997;8:281. [PubMed] [Google Scholar]
- 31.Lacki JK, Leszcznski P, Kelemen JM, Muller W, MacKiewicz SH. Cytokine concentration in serum of lupus erythematosus patients: the effect on acute phase response. J Med. 1997;28:99. [PubMed] [Google Scholar]
- 32.Murray L, Martens C. The abnormal T lymphocytes in lpr mice transcribe interferon-γ and tumor necrosis factor-α genes spontaneously in vivo. Eur J Immunol. 1989;19:563. doi: 10.1002/eji.1830190325. [DOI] [PubMed] [Google Scholar]
- 33.Segal R, Bermas BL, Dayan M, Kalush F, Shearer GM.M, Mozes E. Kinetics of cytokine production in experimental systemic lupus erythematosus: involement of T helper cell 1/T helper cell 2-type cytokines in disease. J Immunol. 1997;158:3009. [PubMed] [Google Scholar]
- 34.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94:585. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okudaira K, Yoshizawa H, Williams RC., Jr Monoclonal murine anti-DNA antibody interacts with living mononuclear cells. Arthritis Rheum. 1987;30:669. doi: 10.1002/art.1780300610. [DOI] [PubMed] [Google Scholar]
- 36.Lai KN, Leung JC.K, Lai KB.L, Lai CK.W. Effect of anti-DNA autoantibodies on the gene expression of interleukin 8, transforming growth factor-beta, and nitric oxide synthase in cultured endothelial cells. Scand J Rheumatol. 1997;26:461. doi: 10.3109/03009749709065720. [DOI] [PubMed] [Google Scholar]
- 37.Peng SL, Moslehi J, Craft J. Role of interferon-γ and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima A, Hirose S, Okumura K. Role of IL-4 andIL-12 in the development of lupus in NZB/W F1 mice. J Immunol. 1997;158:1466. [PubMed] [Google Scholar]
- 39.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy Y, Brouet J-C. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]


