Abstract
Dendritic cells (DC) are specialized antigen-presenting cells. DC can acquire and process antigens in the periphery before maturing and migrating to secondary lymphoid tissues where they present the antigens and deliver co-stimulatory signals to T cells. We describe an immunostimulatory oligonucleotide containing a CpG motif that stimulated murine DC to up-regulate co-stimulatory molecules, induce T-cell proliferative responses and secrete interleukin-12 in vitro. Administration of this oligonucleotide, but not of a control oligonucleotide lacking this motif, to mice led to the disappearance of DC from the marginal zone and T-cell areas of spleen, but not from heart or kidney. The same CpG did not cause maturation of monocyte-derived human DC in vitro, but lipopolysaccharide-treated monocyte-derived DC showed enhanced functional activity and up-regulated co-stimulatory molecules.
Introduction
It has been shown that bacterial DNA (prokaryotic DNA) that has a relative abundance of CpG motifs and CpG oligonucleotides can activate immune responses.1In vitro, CpG activates natural killer (NK) cells,2 macrophages,3 B cells1 and dendritic cells (DC)4 to up-regulate certain surface molecules, such as CD69, major histocompatibility complex (MHC) class I, MHC class II, as well as co-stimulatory molecules such as CD80 and CD86. In addition, CpG stimulates antigen-presenting cells (APC) to secrete interleukin-1 (IL-1), IL-6, tumour necrosis factor-α (TNF-α), IL-12 and interferon-γ (IFN-γ).4–9 For this process to occur, leucocytes take up DNA via adsorptive endocytosis into an acidified intracellular compartment, which results in generation of reactive oxygen species. This process leads to NF-κB activation and subsequent proto-oncogene and cytokine expression.10 CpG activation of APC is mediated, at least in part, by the stress kinase pathway, which requires endosomal translocation and maturation.11
In vivo, administration of CpG has been shown to induce TNF-α production by macrophages in mice8 and IFN-γ production by NK cells in humans in vitro.12 Immunization of mice with CpG and antigen drives the immune response towards a T helper type 1 (Th1) type of response, as measured by increased production of immunoglobulin G2a (IgG2a) and IFN-γ.13,14 In addition, it has been shown that CpG oligonucleotides are effective adjuvants in induction of protective immunity to tumours15 and viral infections.16 On this solid background of in vitro observation, and in vivo murine experiments, it has been suggested that oligonucleotides may play an important role in stimulating vaccine-induced immunity, and acting as immunostimulating adjuvants in other situations.
The present study shows that CpG induces murine DC maturation, as measured by up-regulation of surface as well as co-stimulatory molecules, and induces IL-12 production in vitro. In vivo, the administration of CpG leads to disappearance of DC from spleen in a manner analogous to that caused by lipopolysaccharide (LPS). The same CpG which is capable of activating human B cells does not induce maturation of human monocyte-derived DC.
Materials and methods
Mice
The mice used were 5–7-week-old female BALB/c and C57BL/10 mice, kept under specific pathogen-free conditions. BALB/c mice were injected intravenously with 25 μg/dose LPS (Sigma, St Louis, MO), 5 nmol/dose control oligonucleotide containing CpG motif (CpG) or 5 nmol/dose oligonucleotide containing GpC (control GpC). One group of mice received PBS and was used as control group.
Oligonucleotides
The oligodeoxynucleotides (ODN) sequences 1668 (TCCATGACGTTCCTGATGCT) (CpG) and 1745 (TCCATGAGCTTCCTGATGCT) (GpC-ODNs) were purchased from MWG Biotech (Munich, Germany).
Antibodies
Anti-mouse antibodies included the following: rat IgG2b:B21.2 (TIB 229) anti-IAb,d; rat IgG2a: biotinylated anti-CD40, GL-1 anti-B7-2 (anti-CD86), NLDC 145 anti-DEC 205: hamster IgG; N418 (HB 224) anti-CD11c: rat IgG1; phycoerythrin (PE) -conjugated anti-mouse IL-12 (p40/p70), PE-conjugated rat IgG1 isotype control (Pharmingen, San Diego, CA). Secondary antibodies were PE-conjugated monkey anti-rat IgG (Jackson, Bar Harbour, ME) or fluorescein isothiocyanate (FITC) -conjugated goat anti-rat IgG (Sigma). Mouse anti-human antibodies included the following: HB15a (anti-CD83) (Serotec, Oxford, UK), IgG2b isotype control (Dako, A/S, Glostrup, Denmark), FITC-conjugated rabbit anti-mouse F(ab)2 (Dako).
Bone marrow-derived DC culture and in vitro stimulation
The tibia and femur from BALB/c mice were removed and both ends of the bones were cut and the marrow flushed out using RPMI-1640 (Gibco BRL, Paisley, UK) with a syringe and 25-gauge needle. The bone marrow cells, 5 × 105 cells/ml, were cultured in RPMI-1640 containing 10% fetal calf serum (FCS; Labtech, Intl. Uckfield, UK) and 1 ng/ml recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF) and recombinant IL-4 (rIL-4; Peprotech, Rocky Hill, NJ) for 6 days. The culture was fed with rGM-CSF and rIL-4 (0·5 ng/ml each) on days 2 and 4 of the culture. The bone marrow-derived DC (1 × 106) were harvested and plated in 24-well plates and stimulated for 3 days for analysis of maturation and 24 hr for IL-12 production with 25 ng/ml LPS (Sigma), CpG, or control GpC. The cells cultured in plain medium were used as non-activated DC. Golgi stop (Pharmingen) was added to the cell culture 3 hr before staining for intracellular cytokine.
Generation of human cultured DC
Sixty millilitres of blood was taken from healthy volunteers and peripheral blood mononuclear cells were isolated using Lymphoprep (Nycomed, Oslo, Norway) and following the manufacturer’s instructions. Human monocyte-derived DC were generated as described by Bender et al.17 up to day 6 to obtain immature DC. The cells were fed on days 1, 3 and 5 with fresh medium and cytokines. The cells were fed with fresh medium and cytokines on day 6 and cultured for 2 days with CpG (1, 10, or 100 nmol/ml), control GpC (1, 10, or 100 nmol/ml), LPS (50 ng/ml) or medium only. Culture medium was RPMI-1640 (Life Technologies, Paisley, UK) supplemented with 1% autologous plasma, 50 ng/ml GM-CSF (Novartis, Camberley, UK) and 50 ng/ml IL-4 (Peprotech, Boston, MA).
Allogeneic mixed leucocyte reaction (MLR)
Nylon wool-passed T cells from C57BL/10 mouse spleen and graded numbers of stimulator DC irradiated at (5000 rads 137Cs) were co-cultured in RPMI containing 10% FCS in U-bottom 96-well microplates. After 3 days of culture, cells were pulsed with 25 µl of 25 µCi/ml [3H]thymidine (Amersham Life Science, Buckinghamshire, UK; TRA310) for an additional 18 hr before harvesting and scintillation counting. Human T cells were prepared and used following the same protocol as for mouse T cells.
Cell surface and intracellular antigens
DC were placed in 96-well plates and stained by incubation with primary rat monoclonal antibodies (mAbs) for 45 min at 4°. They were then washed, followed by incubation with FITC- or PE-conjugated anti-rat antibodies for another 45 min. Intracellular IL-12 was detected with PE-conjugated anti-mouse IL-12 (p40/p70) using CytoStain™ Kits according to the manufacturer’s instructions (Pharmingen).
Immunocytochemical staining
Spleens were removed and embedded in tissue-Tek OCT compound and were frozen in liquid nitrogen. Frozen sections were cut at 6-μm thickness, air-dried, fixed in acetone for 1 min, air-dried and rehydrated in phosphate-buffered saline (PBS) containing 5% FCS and 10% sodium azide. After blocking endogenous peroxidase in 0·3% H2O2, sections were incubated with N418 supernatant (anti-CD11c) or an isotype-matched control, followed by horseradish peroxidase (HRP)-conjugated goat anti-Armenian hamster (Jackson ImmunoResearch Laboratories). HRP localization was revealed using 3-amino-9-ethylcarbozole (AEC substrate kit, Vector Laboratories, Burlingame, CA).
Results
CpG induces murine DC maturation in vitro
Non-adherent bone marrow-derived DC were plated in medium containing GM-CSF and IL-4 and treated with CpG, control GpC, or LPS for 3 days. Non-adherent cells were washed, and tested in a MLR using T cells from C57BL/10 mice. Treatment of DC with CpG or LPS but not control GpC induced significantly higher T-cell proliferation than control DC (Fig. 1). The increase in the potency of allostimulating DC treated with CpG was dose dependent, with the highest level of stimulation observed at 100 nmol/ml and followed by that at 10 nmol/ml CpG. Lower concentration of CpG (1 or 0·1 nmol/ml) did not increase the allostimulatory effects of murine DC (data not shown). LPS levels in oligonucleotides were less than 1 ng/ml as measured by limulus assay, indicating that the effects of oligonucleotides on DC were not due to LPS contamination. DC cultured in the presence of GM-CSF and IL-4 were more efficient in inducing allogeneic T-cell proliferation than those cultured in the presence of GM-CSF alone and stimulation was further increased after culture in CpG (data not shown).
Figure 1.
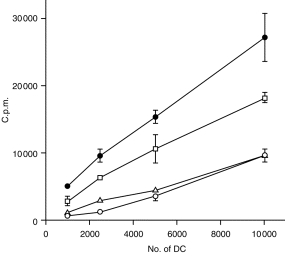
CpG oligonucleotide induces functional maturation of DC. Allogeneic spleen T cells were incubated with graded numbers of DC stimulated with CpG (10 nmol/ml) (□), LPS (25 ng/ml) (•), or control GpC (10 nmol/ml) (▵), or given no stimulation (○). [3H]TdR was added (2 µCi/well) for the final 18 hr of the culture period. Data represent the mean and SD from one of three experiments.
The level of expression of co-stimulatory molecules on DC stimulated with CpG, control GpC, or LPS was determined by flow cytometry. DC activated with the CpG or LPS (25 ng/ml) expressed higher levels of MHC class II, CD86, DEC 205 and CD40 than DC cultured in the presence of the control GpC or immature DC (Fig. 2). The CpG increased expression at 100 and 10 nmol/ml concentrations while the control GpC, even at a high concentration (100 nmol/ml), did not up-regulate the expression levels of co-stimulatory molecules on DC. The results in the present study suggest that CpG cause murine DC maturation, as assessed by up-regulation of immunostimulatory surface molecules and functional properties.
Figure 2.
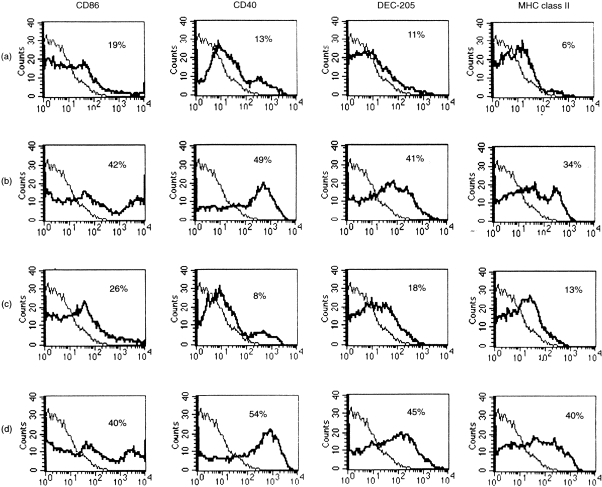
Up-regulation of MHC class II, CD40, CD86 and DEC-205 expression on murine DC treated with CpG. DC incubated for 3 days: (a) in medium only as controls, (b) LPS (25 ng/ml), (c) control GpC (10 nmol/ml), or (d) CpG (10 nmol/ml). DC were analysed for expression of the indicated surface molecules using flow cytometry. Solid line indicates the isotype control and thick solid line indicates the antibody of interest. Representive data from one of three (n = 3) experiments are shown.
CpG induces IL-12 production by murine DC in vitro
Bone marrow-derived DC were cultured for 24 hr with CpG (from 0·1 to 100 nmol/ml), control GpC (from 0·1 to 100 nmol/ml), LPS (25 ng/ml) or in medium (control). CpG (10 or 100 nmol/ml) induced IL-12 production that was detected by intracellular staining specific for IL-12 (p40/p70). DC cultured with control GpC (from 0·1 to 100 nmol) and inactivated DC did not produce IL-12 (Fig. 3).
Figure 3.
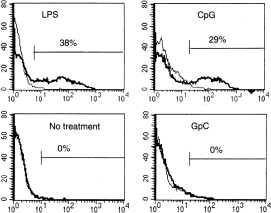
Induction of IL-12 production by CpG-containing ODN. DC were treated with CpG ODN, GpC ODN, or LPS for 24 hr and intracellular IL-12 p40 was quantified using flow cytometry as described in the Materials and Methods. The thinner solid line indicates the isotype control and the thicker solid line indicates the antibody of interest.
CpG does not induce human monocyte-derived DC maturation in vitro
Monocyte-derived human DC (day 6) were cultured with CpG (from 1 to 100 nmol/ml), control GpC (from 1 to 100 nmol/ml), LPS (50 ng/ml), monocyte conditioned medium (MCM), or medium only (control). CpG did not cause maturation of human monocyte-derived DC (from eight different donors) as assessed by up-regulation of co-stimulatory molecules (Fig. 4a) and allo-MLR assay (Fig. 4b). LPS and MCM both induced DC maturation. Control GpC did not induce DC maturation (data not shown).
Figure 4.
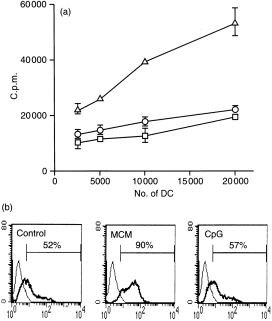
CpG does not induce human DC maturation as detected by up-regulation of CD83 molecules (a) and funtional assay by MLR (b). (a) Human DC were treated for 2 days with CpG (100 nmol/ml) (○), MCM (▵), or medium only as controls (□). (b) DC were analysed for expression of CD83 molecules using flow cytometry. The thinner solid line indicates the isotype control and the thicker solid line indicates the antibody of interest. Representative data from one of eight (n = 8) experiments (eight individuals) are shown. T cells were incubated with graded numbers of DC stimulated with CpG (100 nmol/ml) or LPS. DC incubated in medium only were used as control. [3H]TdR was added (2 µCi/well) for the final 18 hr of the culture period.
CpG injection results in the loss of CD11c-positive cells from mouse spleen after 2 days
It has been shown that intravenous administration of 5 nmol CpG induces TNF-α production by macrophages similar to the effects of LPS. Since administration of LPS resulted in the loss of CD11c-positive cells in spleen.18 We further studied the effects of CpG on MHC class II and CD11c-positive cells in vivo. The staining of murine spleen cryosections (Fig. 5) shows a reduction in CD11c-positive cells 48 hr after intravenous administration of LPS (25 µg/dose) or CpG (5 nmol/dose). No reduction in the number of CD11c-positive cells was observed in the spleen of mice which received control GpC (5 nmol/dose) or PBS. The number of MHC class II-positive cells in the heart and kidney and CD11c-positive cells in spleen were substantially reduced in mice 48 hr after subcutaneous or intramuscular injection of a 100 µg/dose LPS (data not shown). This effect was not observed after subcutaneous or intramuscular injection of low (10 nmol) or high (100 nmol) doses of CpG or control GpC (data not shown).
Figure 5.
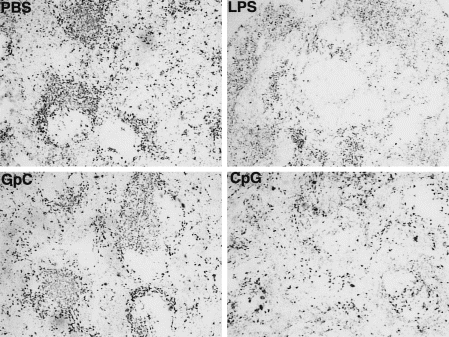
Depletion of CD11c+ cells from spleen after systemic administration of CpG-containing ODN. Immunoperoxidase labelling of BALB/c spleen cryosections for CD11c: 48 hr after intravenous injection of PBS, LPS, CpG ODN 1668, or control GpC ODN 1745.
Discussion
To determine the biological effects of oligonucleotides on murine bone marrow-derived DC and human monocyte-derived DC and to compare their effects with that of LPS, we used a proven immunostimulatory sequence (CpG) and an inactive oligonucleotide (control GpC) in an in vitro system. Comparisons were also made in vivo on murine DC.
The results presented in this study indicate that CpG induces maturation and activation of murine DC. This process, in turn, is required for migration of DC from periphery to secondary lymphoid tissues, which leads to initiation of T-cell-mediated responses. The capacity of CpG to up-regulate surface and co-stimulatory molecules and activate murine DC to produce IL-12 may explain its profound adjuvant effect for Th1-type responses in mice. It has been shown that the efficiency of DNA vaccines in mice is correlated with the presence of CpG motifs in the backbone of plasmids used in DNA vaccines and that methylation abolishes its effectiveness.19,20 Optimal activation of T cells requires TCR occupancy by antigen–MHC complexes and additional signals through engagement of co-stimulatory molecules. The higher T-cell proliferation in allo-MLR assays induced by DC stimulated with CpG is therefore likely to be owing to up-regulation of surface molecules and co-stimulatory molecules.
In contrast, the CpG did not induce maturation of human monocyte-derived DC, presumably owing to species-specific sequence requirements. Interestingly, activation of human B cells1 and NK cells2 by DNA containing this same CpG motif sequence has been reported. This latter is of interest in the context of DNA immunization. Since the immune responses induced by DNA vaccination can be divided conceptually into two distinct units: a transcription unit that directs antigen synthesis, and an adjuvant unit (CpG motif) in the plasmid DNA backbone, it has been suggested that peripheral blood dendritic precursor cells respond to CpG which promotes survival and maturation.21 To explore further the role of the CpG motif in human DNA vaccines, the adjuvanticity of CpG should be assessed.
CpG binds to the surface of murine macrophages and B cells16 and is taken up via endocytosis, which leads to a downstream cellular activation process involving generation of reactive oxygen species and NF-κB activation.10 Surface staining analysis using biotinylated oligonucleotides reveals that oligonucleotides bind to the cell surface of those cell subsets that could be activated by CpG but not to the surface of T cells.16 In this study, it has been shown that biotinylated CpG or control GpG binds to the surface of murine and human DC (data not shown). Therefore, the lack of responses of human DC to CpG is not due to inefficient binding as previously suggested for T cells.16 There might be inefficiency in taking up oligonucleotides by human DC or, more likely downstream cellular activation.
It has been suggested that oligonucleotides bind to Mac-1 (CD11b) and up-regulation of cell surface Mac-1 in polymorphonuclear leucocytes (PMNs) increases cell-surface binding of oligonucleotides and this binding was inhibited by anti-Mac-1 antibodies.22 In our hands monocyte-derived DC and murine DC express CD11b molecules (data not shown). In addition, maturation of murine DC by CpG was not blocked by anti-mouse CD11b antibody (data not shown). This implies that other surface receptors could be involved in binding to oligonucleotides on murine DC. Further experiments are required to clarify why the oligonucleotides used in this study do not induce human monocyte-derived DC maturation. However, it has been recently shown that CpG induce the expression of IL-12 and IL-18 mRNA in human monocyte-derived DC,23 and induce maturation of primary precursor cells isolated from human blood but not monocyte-derived DC.21
It has been shown that CpG induce murine DC maturation in vivo, as assessed by up-regulation of surface co-stimulatory molecules.4,5 These functional changes correlate with a rapid migration of DC to T-cell areas,24 and are followed by the loss of most splenic DC 48 hr after intravenous injection of CpG, as shown by the disappearance of CD11c-positive cells from spleen.18 The disappearance of DC from spleen of mice injected with CpG is conceivably due to apoptosis. This could potentially be induced by TNF which is produced by macrophages upon in vivo administration of CpG.3
In conclusion, the present studies, in accordance with previous data,1–12 suggest that CpG initiate immune responses through activation of innate immune responses. The CpG has profound effects on murine DC, as shown by up-regulation of co-stimulatory molecules, enhancement of acting in functional assays, induction of IL-12 production and migration out of spleen. These effects may be critical in the initiation of immune responses by viral or bacterial DNA and may contribute to the adjuvant activity of CpG in mice. The CpG did not mature human DC in vitro. It will be important to establish which CpGs are stimulating in which human cell types, and what the basis of this specificity is at a molecular level.
References
- 1.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;6522:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;5:1840. [PubMed] [Google Scholar]
- 3.Sparwasser T, Miethke T, Lipford G, et al. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-α-mediated shock. Eur J Immunol. 1997;27:1671. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 4.Sparwasser T, koch ES, Vabules RM, et al. Bacterial DNA immunostimulatory CpG oligonucleotides maturation and activation of murine dendritic cells. Eur J Immunol. 1998;6:2045. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;6:3042. [PubMed] [Google Scholar]
- 6.Lipford GB, Sparwasser T, Bauer M, et al. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;12:3420. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 7.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;7:2879. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi AK, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996;12:5394. [PubMed] [Google Scholar]
- 9.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;12:2335. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755. [PubMed] [Google Scholar]
- 11.Hacker H, Mischak H, Miethke T, et al. CpG-DNA specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO. 1998;21:6230. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induce NK cells to produce IFN-γin vivo and increase the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570. [PubMed] [Google Scholar]
- 13.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligonucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson DA, Raz E. Oligonucleotide adjuvants for T helper 1 (Th1) -specific vaccination. J Exp Med. 1997;186:1621. doi: 10.1084/jem.186.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunisation. Proc Natl Acad Sci USA. 1997;94:10833. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxenius A, Martinic MMA, Hengartner H, Klenerman P. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. J Virol. 1999;73:4120. doi: 10.1128/jvi.73.5.4120-4126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Meth. 1996;196:121. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 18.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;4:1413. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunisation. Science. 1996;273:352. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 20.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;8:3635. [PubMed] [Google Scholar]
- 21.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of dendritic cells. PNAS. 1999;16:9305. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benimetskaya L, Loike JD, Khaled Z, loike G. MAC-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;4:414. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 23.Bohle B, Jahn-schmid B, Maurer D, Kraft D, Ebner C. Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-γ production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur J Immunol. 1999;29:2344. doi: 10.1002/(SICI)1521-4141(199907)29:07<2344::AID-IMMU2344>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Austyn J. Dendritic cells. Curr Opin Hematol. 1998;5:3. doi: 10.1097/00062752-199801000-00002. [DOI] [PubMed] [Google Scholar]


