Abstract
We report that interleukin (IL)-4 and IL-10 can significantly up- or down-regulate CXC chemokine receptor 4 (CXCR4) expression on CD4+ T lymphocytes, respectively. Stromal cell-derived factor-1α (SDF-1α)-induced CD4+ T-lymphocyte chemotaxis was also correspondingly regulated by IL-4 and IL-10. IL-4 and IL-10 up- or down-regulated CXCR4 mRNA expression in CD4+ T lymphocytes, respectively, as detected by real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR). Scatchard analysis revealed a type of CXCR4 with affinity (Kd ≈ 6·3 nm), and ≈ 70 000 SDF-1α-binding sites per cell, among freshly isolated CD4+ T lymphocytes, and two types of CXCR4 with different affinities (Kd1 ≈ 4·4 nm and Kd2 ≈ 14·6 nm), and a total of ≈ 130 000 SDF-1α-binding sites per cell, among IL-4-stimulated CD4+ T lymphocytes. The regulation of CXCR4 expression in CD4+ T lymphocytes by IL-4 and IL-10 could be blocked by a selective inhibitor of protein kinase (staurosporine) or by a selective inhibitor of cAMP- and cGMP-dependent protein kinase (H-8), indicating that these cytokines regulate CXCR4 on CD4+ T lymphocytes via both cAMP and cGMP signalling pathways. The fact that cyclosporin A or ionomycin were able to independently change the CXCR4 expression and block the effects of IL-4 and IL-10 on CXCR4 expression implied that the capacity of IL-4 and IL-10 to regulate CXCR4 on CD4+ T lymphocytes is not linked to calcium-mobilization stimulation. These results indicate that the effects of IL-4 and IL-10 on the CXCR4–SDF-1 receptor–ligand pair may be of particular importance in the cytokine/chemokine environment concerning the inflammatory processes and in the progression of human immunodeficiency virus (HIV) infection.
Introduction
The CXC chemokine receptor 4 (CXCR4) has been shown to respond to the CXC chemokine stromal-derived factor (SDF-1α) and to be an important co-receptor for human immunodeficiency virus-1 (HIV-1) infection.1 CXCR4 is predominantly expressed on the naive, unactivated CD26low CD45RA+ CD45RO− T-cell subset of peripheral blood lymphocytes2 as well as on B lymphocytes,3 dendritic cells,4 endothelial cells5, 6 and mature polyploid megakaryocytes.7 SDF-1α is a highly efficacious lymphocyte chemoattractant, which induces an increase in intracellular free Ca2+ and chemotaxis in CXCR4-transfected cells, and inhibits infection with CXCR4-dependent HIV.1 Because of the importance of CXCR4 and SDF-1α in immunity and the acquired immune deficiency syndrome (AIDS), many investigators have attempted to develop CXCR4 antagonists or agonists, for instance T228 and T134,9 and to chemically synthesize a SDF-1α analogue, for instance N33A, which can be produced rapidly in large quantities, possesses the same capacity as native SDF-1α to activate CXCR4-expressing cells and is a valuable agent for research on the host immune response and AIDS.10 The investigators have even found that SDF-1-mediated down-regulation of cell-surface CXCR4 expression contributes to chemokine-mediated inhibition of HIV infection.11
In the present study, we report that interleukin (IL)-4 and IL-10 can significantly up- or down-regulate the expression of CXCR4 on CD4+ T lymphocytes, respectively, at both the protein and mRNA level. Correspondingly, IL-4 and IL-10 also up-regulated and down-regulated SDF-1α-induced CD4+ T-lymphocyte chemotaxis. Scatchard analysis revealed one type of CXCR4 with affinity (Kd ≈ 6·3 nm) on freshly isolated CD4+ T lymphocytes, and two types of CXCR4 with different affinities (Kd1 ≈ 4·4 nm and Kd2 ≈ 14·6 nm) on IL-4-stimulated CD4+ T lymphocytes. These results strongly indicate that IL-4 and IL-10, as well as the CXCR4–SDF-1α receptor–ligand pair, may be particularly important elements in the cytokine/chemokine environment, in terms of T-lymphocyte migration, accumulation and recruitment, which occur during the initiation and development of inflammatory processes.
Materials and methods
Preparation of cells
CD4+ T-lymphocyte purification
Positive selection of CD4+ T lymphocytes was performed by adding Dynabeads M-450 (Dynal, Oslo, Norway) into mononuclear cells from healthy donors, according to the manufacturer's instructions. The cell suspension was retained at 4° throughout the entire procedure to prevent attachment of phagocytic cells to Dynabeads M-450. The CD4+ T-lymphocyte populations had a purity of > 97%, as measured by using a fluorescence-activated cell sorter (FACS).
Modulation of CXCR4 on CD4+ T lymphocytes
CD4+ T lymphocytes were preincubated with T helper 1 (Th1)-associated cytokines (interferon-γ [IFN-γ], tumour necrosis factor-α [TNF-α], or IL-2) and T helper 2 (Th2)-associated cytokines (IL-4, IL-5, or IL-10) at 10 ng/ml12 for 24 hr (or the indicated time intervals) at 37° before immunofluorescence staining. For measurement of CXCR4 mRNA, CD4+ T lymphocytes were preincubated with IL-4 (10 ng/ml) or IL-10 (10 ng/ml) for 24 hr at 37°. Then the cells were subjected to mRNA isolation for real time quantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis. All cytokines used were purchased from R & D Systems Europe Ltd (Abingdon, UK). For investigation of the signalling pathway, the cells were preincubated for 30 min at room temperature with tyrphostin 23 (Ty23; 1 µm), staurosporine (Sta; 1 µm), N-(2-(methylamino)ethyl)-5-isoquenolinesulphonamide dihydrochloride (H-8; 30 µm), N-(2-(ρ-bromocinnamylamino)ethyl)-5-isoquenilesulfonamide (H-89; 30 µm), pertussis toxin (PT; 1 µg/ml), or bisindolylmaleimide I (BIM I; 1 µm), respectively, before being used in further experiments. All signalling pathway inhibitors used were purchased from Sigma Chemical Co. (St. Louis, MO).
Flow cytometry
As previously described13 freshly isolated CD4+ T lymphocytes or CD4+ T lymphocytes stimulated with different Th1- and Th2-associated cytokines or signalling pathway inhibitors, as described above, were first incubated with a mouse anti-human CXCR4 monoclonal antibody (mAb) (R & D Systems Europe Ltd, clone number 44708.111 and 12G5) at 5 µg/ml, or with 5 µg/ml of immunoglobulin G2a (IgG2a) isotype-matched mAb (Dako, Glostrup, Denmark) in phosphate-buffered saline (PBS) containing 2% human pooled AB serum and 0·1% sodium azide (staining buffer). After 20 min, the cells were washed twice with staining buffer and resuspended in 50 µl of fluorescein isothiocyanate (FITC)-conjugated affinity-purified F(ab′)2 rabbit anti-mouse mAb (Dako) for 20 min. The cells were then washed twice in staining buffer prior to labelling with phycoerythrin (PE)-conjugated mouse anti-human CD4 mAb (Dako) for 20 min. All procedures were carried out at 4°. The labelled cells were fixed with 1% paraformaldehyde. The analyses were performed using a flow cytometer (COULTER® XL, Coulter Corp., Miami, FL).
Chemotaxis assay
The following human recombinant chemokines were studied: SDF-1α and macrophage inflammatory protein-1α (MIP-1α) (R & D Systems Europe Ltd). The chemotaxis assay was performed in a 48-well microchamber (Neuro Probe, Bethesda, MD), as described previously.13 Briefly, chemokines were diluted in RPMI-1640 containing 0·5% pooled human serum and placed in the lower wells (25 µl). Fifty microlitres of the cell suspension (5 × 106 cells/ml) was added to the upper well of the chamber, which was separated from the lower well by a 5-µm pore-size, mouse collagen IV-coated, polycarbonate, polyvinylpyrolidone-free membrane (Nucleopore, Pleasanton, CA). The chamber was incubated for 120 min at 37° in an atmosphere containing 5% CO2. The membrane was then carefully removed, fixed in 70% methanol and stained for 5 min in Coomassie Brilliant Blue. The cells that had migrated and adhered to the lower surface of the membrane were counted by light microscopy. The results were expressed as chemotactic index (C. I.), which represents the ratio between the number of migrating cells in the sample and in the medium control.13
Real time quantitative RT–PCR assay
All real time quantitative RT–PCR reactions were performed as described previously.14,15 Briefly, total RNA from unstimulated and stimulated cells was prepared by using Quick Prep® Total RNA Extraction Kit (Pharmacia Biotech, Uppsala, Sweden) and was reverse transcribed by using oligo pd(T)12–18 and Superscript II reverse transcriptase (Life Technologies, Grand Island, NY), according to the manufacturer's instructions. The real time quantitative PCR was performed in special optical tubes in a 96-well microtitre plate (Perkin Elmer Applied Biosystems, Foster City, CA) format on an ABI PRISM® 7700 Sequence Detector System (Perkin Elmer Applied Biosystems), according to the manufacturer's instructions. By using SYBR® Green PCR Core Reagents Kit ( P/N 4304886; Perkin Elmer Applied Biosystems), fluorescence signals were generated during each PCR cycle via the 5′ to 3′ endonuclease activity of AmpliTaq Gold14 to provide real time quantitative PCR information. The following sequences of the specific primers (purchased from Amersham Pharmacia Biotech Inc., Buckinghamshire, UK) were used in PCR: CXCR4, sense: 5′-TCCAAAGCCTTCCCTGTGTC-3′; antisense: 5′-AAAACAT CCACTTTCCCCCC-3′.
The concentration of target cDNA was adjusted to an amount equal to that of a housekeeping gene (β-actin) by performing quantitative PCR of β-actin cDNA according to the manufacturer's instructions. These adjusted amounts of cDNA were then used to quantify genes of interest in the presence of specific primers. PCR reaction conditions were optimized for each amplification (according to the manufacturer's instructions) at: 40 cycles for 15 seconds at 95° and 60 seconds at 60°. In order to analyse the PCR products, two terms were used to express the results: ΔRn, representing the normalized reporter signal minus the baseline signal established in the first few cycles of PCR; and CT (threshold cycle), representing the PCR cycle at which an increase in reporter fluorescence signal above the baseline was first detected.
Chemokine binding assay
The chemokine binding assay was performed as described previously.16 Briefly, CD4+ T cells (5 × 105 cells/ml), either freshly isolated or stimulated with IL-4 (10 ng/ml) for 24 hr, were incubated (at 4° for 1 hr) with 125I-labelled SDF-1α (0·2 nm, New England Nuclear, Boston, MA) and varying concentrations of unlabelled SDF-1α. The incubation was stopped by removing aliquots from the cell suspension and separating cells from buffer by centrifugation through a silicone/paraffin oil mixture. Non-specific binding was evaluated in the presence of 1 µm unlabelled SDF-1α. The binding data were used to determine the affinity (Kd) and specific binding (Scatchard analysis).
Results
Expression of CXCR4 on CD4+ T lymphocytes is regulated by IL-4 and IL-10
The results from flow cytometric analyses (Fig. 1) show ≈ 47·3% of CXCR4+ cell fractions in freshly isolated CD4+ T lymphocytes (Fig. 1a). After 24 hr of incubation with cytokine-free medium (Fig. 1b), there was no significant change of CXCR4+ cell fraction (52·6%). IL-2 (Fig. 1c), TNF-α (Fig. 1e), and IL-5 (Fig. 1g) did not regulate the expression of CXCR4 on human peripheral CD4+ T lymphocytes (CXCR4+ cell fractions were 45·3%, 52·8% and 52·0%, respectively). Interestingly, IL-4, a Th2-associated cytokine, significantly up-regulated (to 91·7%) the expression of CXCR4 on CD4+ T cells (Fig. 1f). Moreover, a bright fraction of CXCR4+ cells (≈ 33·4%) appeared in IL-4-stimulated CD4+ T lymphocytes. IFN-γ, a Th1-associated cytokine, also slightly increased the expression of CXCR4 on CD4+ T cells (to ≈ 64·0%) (Fig. 1d). The increased fraction of CXCR4+ T lymphocytes was bright (≈ 10%). In these instances, most CXCR4 negative fractions were reduced and CXCR4 bright fractions appeared. Interestingly, IL-10, a Th2-associated cytokine, showed a robust ability to down-regulate the expression of CXCR4 on human peripheral CD4+ T lymphocytes. After 24 hr of incubation with IL-10 (10 ng/ml), the proportion of CXCR4+ T lymphocytes decreased to 1·4% (Fig. 1h). The CD4+ T lymphocytes that were converted (by either IFN-γ or IL-4) from CXCR4 negative to CXCR bright were presumably those that were low expressors of CD4+ cells.
Figure 1.
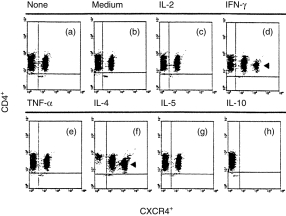
Double colour flow cytometric analysis of the distribution and modulation of CXC chemokine receptor 4 (CXCR4) on CD4+ T lymphocytes. The cells were either freshly isolated (a) or were stimulated for 24 hr with cytokine-free medium (b), interleukin (IL)-2 (c), interferon-γ (IFN-γ) (d), tumour necrosis factor-α (TNF-α) (e), IL-4 (f), IL-5 (g), or IL-10 (h), respectively. The percentages of CXCR4+ cells are indicated in the Results. The arrows indicate the bright fraction of CXCR4+ cells. The data were taken from a single experiment, which was representative of six similar experiments performed.
SDF-1α-induced CD4+ T-lymphocyte chemotaxis is regulated by IL-4 and IL-10
The results in Fig. 2(a) show that SDF-1α induces a chemotactic migration in freshly isolated CD4+ T lymphocytes, yielding a typical bell-shaped dose-dependent chemotaxis response curve. The optimal chemotactic concentration of SDF-1α was 100 ng/ml (C. I. = 2·2). In cultured CD4+ T lymphocytes without stimulus, SDF-1α induced a similar chemotactic migration (C. I. = 2·3). IL-4 was found to significantly increase chemotactic migration of CD4+ T lymphocytes towards 100 ng/ml of SDF-1α (C. I. = 3·5; P < 0·01), compared with untreated or cultured unstimulated cells, whereas IL-10 significantly decreased chemotactic migration of CD4+ T lymphocytes towards 100 ng/ml of SDF-1α (C. I. = 0·93; P < 0·001), compared with untreated cells. However, Fig. 2(b) shows that MIP-1α also induced a chemotactic migration in freshly isolated CD4+ T lymphocytes, yielding a typical bell-shaped dose-dependent chemotaxis response curve (C. I. = 2·3). However, neither IL-4 nor IL-10 changed the migratory activity of CD4+ T lymphocytes towards 100 ng/ml of MIP-1α within 24 hr (C. I. = 2·4, 2·5, respectively; all P > 0·1) compared with untreated cells. In our system, the C. I. values between 1·8 and 2·0 were considered as moderate; 2·1–3·0 as strong; and > 3·1 as very strong.12,13
Figure 2.
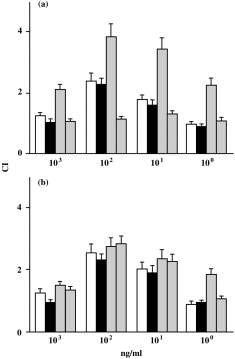
Migration of CD4+ T lymphocytes towards stromal cell-derived factor-1α (SDF-1α) (a), or macrophage inflammatory protein-1α (MIP-1α) (b). CD4+ T lymphocytes were freshly isolated (open bars), incubated in medium only (black bars), or stimulated with IL-4 (grey bars) or IL-10 (half black bars). The data are taken from a single experiment, representative of four performed. All results were determined as described in the Materials and methods, are expressed as chemotactic index (C. I.) and based on triplicate determination of chemotaxis on each concentration of chemoattractant. The chemokine concentrations applied (ng/ml) are indicated.
Kinetics of CXCR4 expression on CD4+ T lymphocytes are regulated by IL-4 and IL-10
The results shown in Fig. 3 indicate that IL-4 is a robust up-regulator of CXCR4 expression. It can significantly up-regulate the expression of CXCR4 on CD4+ T lymphocytes from 55·5% (0 hr) (Fig. 3m) to 61·3% (1 hr) (Fig. 3a), 52·3% (2 hr) (Fig. 3b), 78·2% (4 hr) (Fig. 3c), 77·9% (8 hr) (Fig. 3d), 96·4% (16 hr) (Fig. 3e) and 96·4% (24 hr) (Fig. 3f). Interestingly, a bright fraction of CXCR4+ cells (8·5%) appeared within 2 hr of stimulation with IL-4: this fraction increased to 15·5% within 4 hr, to 15·6% within 8 hr, to 32·3% within 16 hr, and to 32·5% within 24 hr. IL-10 was a strong down-regulator of CXCR4 expression. It significantly down-regulated the expression of CXCR4 on CD4+ T lymphocytes from 55·5% (0 hr) (Fig. 3m) to 40·3% (1 hr) (Fig. 3g), 47·1% (2 hr) (Fig. 3h), 37·1% (4 hr) (Fig. 3i), 22·3% (8 hr) (Fig. 3j), 9·2% (16 hr) (Fig. 3k) and 0·3% (24 hr) (Fig. 3l). Surprisingly, a bright fraction of CXCR4+ cells (7·2%) appeared within 8 hr of stimulation with IL-10; this fraction was still present at 16 hr (at 8·3%), but had disappeared by 24 hr. To exclude the possibility of IL-10 affecting cell viability during stimulation, the cell viability was examined (after 24 hr of stimulation with 10 ng/ml of IL-10) by using the Trypan Blue exclusion test. The cell viability was found not to be affected during 24 hr of stimulation with 10 ng/ml of IL-10.
Figure 3.
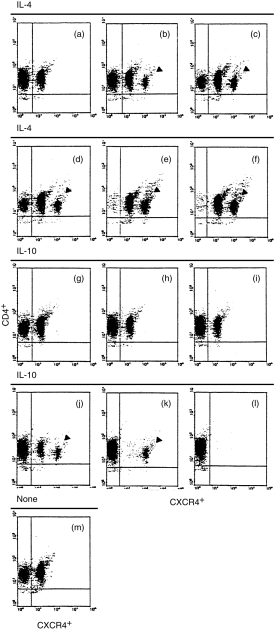
Double colour flow cytometric analysis of the kinetics of CXC chemokine receptor 4 (CXCR4) expression on human peripheral CD4+ T lymphocytes. The cells were stimulated either with IL-4 (10 ng/ml) for different time intervals indicated as (a, 1 hr), (b, 2 hr), (c, 4 hr), (d, 8 hr), (e, 16 hr) and (f, 24 hr), respectively, or with IL-10 (10 ng/ml) for different time intervals indicated as (g, 1 hr), (h, 2 hr), (I, 4 hr), (j, 8 hr), (k, 16 hr) and (l, 24 hr), respectively. (m) Freshly isolated unstimulated CD4+ T cells. The cells were then stained with anti-CXCR4 monoclonal antibody as described in the Materials and methods. The percentages of CXCR4+ cells are given in the Results. Arrows indicate the bright fraction of CXCR4+ cells. The data were taken from a single experiment, which was representative of each of three similar experiments performed.
The mRNA of CXCR4 in CD4+ T lymphocytes is regulated by IL-4 and IL-10
The results presented in Fig. 4(a) show that CXCR4 mRNA was detected in human peripheral resting CD4+ T lymphocytes. Compared with the amplification of standard DNA template (2·0 × 104 copies) of a housekeeping gene (β-actin), there were ≈ 7·2 × 102 copies for CXCR4 in the resting CD4+ T lymphocytes. There were ≈ 8·1 × 103 copies for CXCR4 in the IL-4-stimulated CD4+ T lymphocytes within 4 hr, 2·0 × 104 copies within 16 hr and 1·1 × 103 copies within 24 hr, respectively. The results in Fig. 4(b) show that there were ≈ 9·2 × 102 copies for CXCR4 in the IL-10-stimulated CD4+ T lymphocytes within 4 hr, 6·2 × 100 copies within 16 hr and 7·1 × 102 copies within 24 hr, respectively. The results in Fig. 4(c) show that a linear relationship between CT and log starting quantity of standard DNA template or target cDNA (CXCR4) was detected. In all experiments the correlation coefficients were ≈ 0·936.
Figure 4.
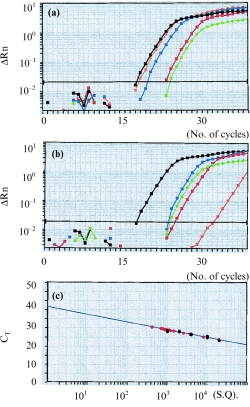
The plots of the real time detection and amplification of mRNA of CXC chemokine receptor 4 (CXCR4) in unstimulated, and in interleukin (IL)-4- and IL-10-stimulated, CD4+ T lymphocytes at different time intervals. (a) Green plots represent the amplification of mRNA of CXCR4 in unstimulated CD4+ T lymphocytes; blue, orange and red plots represent the amplification of mRNA of IL-4-stimulated CD4+ T lymphocytes at 4 hr, 16 hr and 24 hr, respectively; black plots represent the amplification of standard DNA template (2·0 × 104 copies) with a housekeeping gene (β-actin). CT values (representing the polymerase chain reaction cycle at which an increase in reporter fluorescence signal above baseline was first detected) were 18·1 for standard DNA template; 24·7 for CXCR4 mRNA in unstimulated CD4+ T lymphocytes; 22·8 for CXCR4 mRNA in IL-4-stimulated CD4+ T lymphocytes at 4 hr; 18·6 for 16 hr; and 22·8 for 24 hr, respectively. (b) Green plots represent the amplification of CXCR4 mRNA in unstimulated CD4+ T lymphocytes; blue, orange and red plots represent the amplification of mRNA of IL-10-stimulated CD4+ T lymphocytes at 4 hr, 16 hr and 24 hr, respectively; black plots represent the amplification of standard DNA template (2·0 × 104 copies) with a housekeeping gene (β-actin). CT values were 24·2 for CXCR4 mRNA in IL-10-stimulated CD4+ T lymphocytes at 4 hr; 34·9 for 16 hr; and 24·7 for 24 hr, respectively. (c) Demonstration of the linear relationship between CT and log starting quantity (S. Q.) of standard DNA template (black circles) or target (CXCR4) mRNA (red circles). The plots shown are representative of two similar experiments conducted.
Binding characteristics of SDF-1α on CD4+ T lymphocytes
As two distinct fractions of CXCR4+ T cells (bright and dim) were identified after stimulation with IL-4, a binding experiment was therefore performed of SDF-1α on CD4+ T lymphocytes, in terms of comparison between freshly isolated and IL-4-stimulated T cells. The results (Fig. 5a, 5b) showed that IL-4 stimulation significantly increased the amount of bound SDF-1α, indicating up-regulation of the expression of CXCR4. Inhibition of binding, by addition of either unlabelled SDF-1α or anti-CXCR4 mAb, supported the fact that the SDF-1α protein was binding to CXCR4 receptors. Dose–response competitive reactions were generated between unlabelled SDF-1α and 125I-labelled SDF-1α (data not shown). Scatchard analysis of these data (Fig. 5c, 5d) showed that the competition curve yielded one linear plot with Kd ≈ 6·3 nm and ≈ 70 000 SDF-1α-binding sites per cell among freshly isolated CD4+ T lymphocytes, and that the competition curve yielded two linear plots with Kd1 ≈ 4·4 nm (85 000 SDF-1α-binding sites per cell) and Kd2 ≈ 14·6 nm (45 000 SDF-1α-binding sites per cell) on IL-4-stimulated CD4+ T lymphocytes. Thus, there is a type of CXCR4 with almost equal affinity on freshly isolated CD4+ T lymphocytes, and there are two types of CXCR4 with different affinities on IL-4-stimulated CD4+ T lymphocytes. One type of affinity of CXCR4 on IL-4-stimulated CD4+ T lymphocytes was moderately higher than that on freshly isolated CD4+ T lymphocytes, whereas the other type of affinity of CXCR4 on IL-4-stimulated CD4+ T lymphocytes was lower. Thus, IL-4 not only induces different affinity of CXCR4 expression on the cells, but also increases SDF-1α-binding sites (50 000 binding sites per cell).
Figure 5.
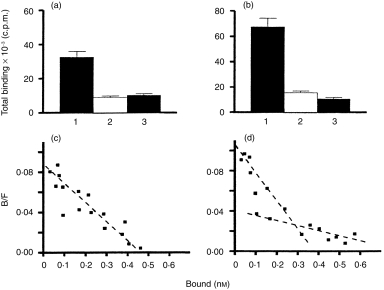
Displacement of 125I-labelled stromal cell-derived factor-1α (SDF-1α) binding to freshly isolated (a) and interleukin (IL)-4-stimulated (b) CD4+ T lymphocytes by unlabelled SDF-1α and a specific monoclonal antibody (mAb) (12G5) to CXC chemokine receptor 4 (CXCR4). CD4+ T lymphocytes (5 × 105/ml) were incubated in phosphate-buffered saline (PBS) with 125I-labelled SDF-1α (0·2 nm) in the absence (indicated as 1) and presence (indicated as 2) of unlabelled SDF-1α (1 µm), or with CXCR4 mAb (500 nm) (indicated as 3), at 4° for 1 hr. The plots of Scatchard analysis on binding characteristics for SDF-1α are shown on freshly isolated (c) and IL-4-stimulated (d) CD4+ T lymphocytes. The dissociation constant (Kd) for each is given in the Results. The results shown in each plot are representative of those found in two similar experiments.
Involvement of signalling pathways in the regulation of CXCR4 expression, in CD4+ T lymphocytes, by IL-4 and IL-10
We pretreated the cells with Ty23, a selective inhibitor of protein tyrosine kinase (PTK),17 Sta, a selective inhibitor of protein kinase,18 H-89, a selective inhibitor of cAMP-dependent protein kinase,19 PT (1 µg/ml), a specific inhibitor of certain G proteins,20 H-8, a selective inhibitor of cAMP- and cGMP-dependent protein kinase A (PKA),21 or BIM I, a selective inhibitor of protein kinase C (PKC),22 respectively. Figure 6 shows that IL-4 and IL-10 were able to significantly regulate the expression of CXCR4 in CD4+ T lymphocytes (Fig. 6b, 6c) compared with freshly isolated CD4+ T lymphocytes (Fig. 6a) in the absence of inhibitors of signal transduction pathways. None of the inhibitors alone changed the expression of CXCR4 in CD4+ T lymphocytes (Fig. 6d, 6g, 6j, 6m, 6p, 6s). The doses of Ty23, H-89, PT or BIM I employed (see the Materials and methods) did not changed the pattern of regulation of the expression of CXCR4 in CD4+ T lymphocytes induced by IL-4 and IL-10 (Fig. 6e, 6f, 6h, 6i, 6k, 6l, 6t, 6u). Sta and H-8 completely blocked the effects of IL-4 and IL-10 on the expression of CXCR4 in CD4+ T lymphocytes (Fig. 6n, 6o, 6q, 6r). As Sta is a selective inhibitor of protein kinase and H-8 is a selective inhibitor of cAMP- and cGMP-dependent PK A, these results strongly indicate that both the cAMP- and cGMP-dependent PK A signalling pathways are involved in the physiological and pathphysiological events in terms of the regulation of CXCR4 expression on CD4+ T lymphocytes caused by IL-4 and IL-10 stimulation. W examined the effect of Sta and H-8 on IL-4-induced cellular proliferation. No difference of cellular proliferation induced by IL-4 was observed between CD4+ T lymphocytes preincubated with and without Sta or H-8 within 24 hr, as detected by [3H]thymidine incorporation into DNA (T. Jinquan et al., unpublished).
Figure 6.

Double colour flow cytometric analysis of effects of different signalling pathway inhibitors on the expression of CXC chemokine receptor 4 (CXCR4) on CD4+ T lymphocytes. The cells were first cultured either with or without different signalling pathway inhibitors, as indicated, for 30 min. The cells were then stimulated either with or without interleukin (IL)-4 (10 ng/ml) or IL-10 (10 ng/ml) for 24 hr, as indicated. The order is untreated, IL-4, IL-10 in each set of three panels. The cells were subsequently stained with anti-CXCR4 monoclonal antibody, as described in the Materials and methods. (/) Indicates that the percentage of CXCR4+ cells varied from 45 to 55%, implying no significant regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. (−) Indicates that the percentage of CXCR4+ cells was < 5%, implying significant down-regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. (+) Indicates that the percentage of CXCR4+ cells was > 90%, implying significant up-regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. The data were taken from a single experiment, which was representative of four similar experiments performed. BIM I, bisindolylmaleimide I; H-8, N-(2-(methylamino)ethyl)-5-isoquenolinesulphonamide dihydrochloride; H-89, N-(2-(ρ-bromocinnamylamino)ethyl)-5-isoquenilesulfonamide; PT, pertussis toxin; Sta, staurosporine; Ty23, tyrphostin 23.
As shown in Fig. 7, cyclosporin A (0·1 µg/ml) significantly down-regulated the expression of CXCR4 in CD4+ T lymphocytes within 60 min (Fig. 7d), as well as blocking the regulatory effects of IL-4 and IL-10 (Fig. 7e, 7f), compared with freshly isolated and untreated CD4+ T lymphocytes (Fig. 7a, 7b, 7c). Ionomycin significantly up-regulated expression of CXCR4 in CD4+ T lymphocytes within 60 min (Fig. 7g), but it blocked the regulatory effects of IL-4 and IL-10 (Fig. 7h, 7i), compared with freshly isolated and untreated CD4+ T lymphocytes.
Figure 7.
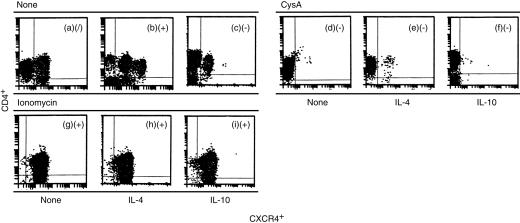
Double colour flow cytometric analysis of the effect of cyclosporin A (CsA) and ionomycin on the expression of CXC chemokine receptor 4 (CXCR4) on CD4+ T lymphocytes. The cells were first stimulated either with or without CsA (0·1 mg/ml) or ionomycin (0·1 mg/ml), as indicated, for 30 min. The cells were then stimulated either with or without interleukin (IL)-4 (10 ng/ml) or IL-10 (10 ng/ml), as indicated, for 24 hr. The cells were subsequently stained with anti-CXCR4 monoclonal antibody as described in the Materials and methods. (/) Indicates that the percentage of CXCR4+ cells varied from 45 to 55%, implying no significant regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. (−) Indicates that the percentage of CXCR4+ cells was <5%, implying significant down-regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. (+) Indicates that the percentage of CXCR4+ cells was >90%, implying significant up-regulation by IL-4 or IL-10 compared with CXCR4 expression on resting CD4+ T lymphocytes. The data were taken from a single experiment, which was representative of six similar experiments performed.
Discussion
Chemokines and their receptors are important in cell migration during inflammation, in the establishment of functional lymphoid microenvironments and in organogenesis. CXCR4 is expressed extensively by cells of the immune1 and central nervous23 systems. Our results demonstrating that IL-4 can up-regulate CXCR4 on CD4+ T lymphocytes are in agreement with those of several independent groups who have also reported that IL-4 specifically enhances cell-surface expression of CXCR4 on resting peripheral and cord blood T cells.24–28 We demonstrated that IL-4 and IL-10 up- or down-regulate, respectively, CXCR4 surface expression both at the protein and at the mRNA level. More interestingly, we observed a bright CXCR4+ fraction after stimulation with cytokines. Scatchard analysis revealed a type of CXCR4 with similar affinity (Kd ≈ 6·3 nm) on freshly isolated CD4+ T lymphocytes, and two types of CXCR4 with different affinities (Kd1 ≈ 4·4 nm and Kd2 ≈ 14·6 nm) on IL-4-stimulated CD4+ T lymphocytes. Based on this phenomenon, we suggest that there are two types of CXCR4 on CD4+ T lymphocytes (according to their affinity to SDF-1α): CXCR4low and CXCR4high. The resting CD4+ T lymphocytes are preferentially CXCR4low, whereas CXCR4 expression on the activated CD4+ T lymphocytes is up-regulated and a proportion of CXCR4-negative become CXCR4high. The active form of CXCR4 may be the CXCR4high form. The functional CXCR4 could comprise both CXCR4low and CXCR4high types, owing to the fact that untreated CD4+ T lymphocytes also respond to SDF-1α although they do not express CXCR4 high.
IL-10, an immunosuppressive and anti-inflammatory cytokine produced by monocytes and T lymphocytes, regulates both inflammatory and immune responses by modulating the activities of T-lymphocyte, B-lymphocyte and mononuclear phagocyte function, and also by modulating polymorphonuclear cell (PMN)-associated chemokine expression.29 IL-10 selectively up-regulates the expression of CC chemokine receptor 1 (CCR1), CCR2 and CCR5 in human monocytes by prolonging their mRNA half-life, increasing number of cell surface receptors and by giving a better chemotactic responsiveness to relevant ligands.30 In contrast, it has also been reported that although IL-10 inhibits HIV-1 replication in macrophages, it does not suppress surface CCR5 expression induced by colony-stimulating factors.31 To our knowledge, this is the first demonstration that IL-10 can dramatically down-regulate CXCR4 expression on CD4+ T lymphocytes in vitro within 24 hr. The cytokine environment determines the susceptibility of macrophages and T lymphocytes to HIV-1 infection by various mechanisms, one of which is the regulation of HIV-1 co-receptor expression including CXCR4. Together with the immunosuppressive and anti-inflammatory functions demonstrated previously, our results again indicate an interesting therapeutic possibility of IL-10.
Th1 and Th2 lymphocytes express different chemokine receptors. For instance, some current opinions are that CCR5 and CXCR3 are markers of Th1 lymphocytes, whereas CCR3 and CXCR4 are predominately expressed on Th2 lymphocytes.32 Our data, that IL-4 can up-regulate CXCR4 expression on CD4+ T lymphocytes, seem to confirm these notions because IL-4 is known to be an important cytokine that aids the differentiation of Th2 lymphocytes.
To delineate the mechanisms of the regulation of CXCR4 expression by cytokines, we investigated a series of specific inhibitors of different signalling pathways in terms of their capacity to regulate CXCR4 expression. Our current results clearly show that the inhibitors (Sta and H-8) of the cAMP- and cGMP-dependent protein kinase signalling pathways inhibit the effects of IL-4 and IL-10 on CXCR4 expression, but themselves cannot regulate CXCR4 expression. In other words, cAMP- and cGMP-dependent protein kinase signalling pathways are required for IL-4 and IL-10 to regulate CXCR4 expression. The calcium-mobilization stimulator (ionomycin) and inhibitor (cyclosporin A), block the effects of IL-4 and IL-10 on CXCR4 expression, and also themselves up- or down-regulate CXCR4 expression. This implies that intercellular calcium mobilization is important for CXCR4 expression, but IL-4 and IL-10 have no effect on CXCR4 expression on cells after incubation with ionomycin or cyclosporin A. The effects of IL-4 and IL-10 on CXCR4 expression are therefore independent of the calcium-mobilization stimulation. Furthermore, we have detected that IL-4 and IL-10 may act via the CD26 molecule to regulate CXCR4 expression on CD4+ T lymphocytes (T. Jinquan, unpublished).
The present study provides useful insights into the novel mechanism of the effects of IL-4 and IL-10 on the expression of CXCR4, which is largely responsible for the progression of inflammation and AIDS.
Acknowledgments
The authors thank Gitte Pedersen, Anne Corfitz and Jannie Neumann for their excellent technical assistance. T. J. and S. Q. are supported by generous grants from Danish Allergy Research Centre, and from the Alfred Benzons Foundation. C.G. is supported by the Danish Environmental Research Programme 1998–2001. This work was partially supported by the Simon Fougner Hartmanns Foundation.
Abbreviations
- C. I.
chemotactic index
- CXCR
CXC chemokine receptor
- CsA
cyclosporin A
- MIP
macrophage inflammatory protein
- PKA
protein kinase A
- PKC
protein kinase C
- PTK
protein tyrosine kinase
- SDF-1α
stromal cell-derived factor-1α
References
- 1.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 2.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sozzani S, Luini W, Borsatti A, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993. [PubMed] [Google Scholar]
- 5.Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247:38. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 6.Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 7.Hamada T, Mohle R, Hesselgesser J, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188:539. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami T, Nakajima T, Koyanagi Y, et al. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arakaki R, Tamamura H, Premanathan M, et al. T134, a small-molecule CXCR4 inhibitor, has no cross-drug resistance with AMD3100, a CXCR4 antagonist with a different structure. J Virol. 1999;73:1719. doi: 10.1128/jvi.73.2.1719-1723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda H, Siani MA, Gong W, Thompson DA, Brown GG, Wang JM. Chemically synthesized SDF-1alpha analogue, N33A, is a potent chemotactic agent for CXCR4/Fusin/LESTR-expressing human leukocytes. J Biol Chem. 1997;272:24966. doi: 10.1074/jbc.272.40.24966. [DOI] [PubMed] [Google Scholar]
- 11.Signoret N, Oldridge J, Pelchen-Matthews A, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinquan T, Deleuran B, Gesser B, et al. Regulation of human T lymphocyte chemotaxis in vitro by T cell-derived cytokines IL-2, IFN-γ, IL-4, IL-10, and IL-13. J Immunol. 1995;154:3742. [PubMed] [Google Scholar]
- 13.Jinquan T, Frydenberg J, Mukaida N, et al. Recombinant human growth regulated oncogene-α induces T lymphocyte chemotaxis; a process regulated via interleukin-8 receptors by IFN-γ, TNF-α, IL-4, IL-10 and IL-13. J Immunol. 1995;155:5359. [PubMed] [Google Scholar]
- 14.Heid CA, Stevens J, Livak KJ, William PM. Real time quantitative PCR. Genome Res. 1996;6:986. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 15.Kruse N, Pette M, Toyka K, Rieckmann P. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Methods. 1997;210:195. doi: 10.1016/s0022-1759(97)00188-9. [DOI] [PubMed] [Google Scholar]
- 16.Hesselgesser J, Liang M, Hoxie J, et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160:877. [PubMed] [Google Scholar]
- 17.Williams EJ, Walsh FS, Doherty P. Tyrosine kinase inhibitors can differentially inhibit integrin-dependent and CAM-stimulated neurite outgrowth. J Cell Biol. 1994;124:1029. doi: 10.1083/jcb.124.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto H, Sasaki Y. Staurosporine, a protein kinase C inhibitor, interferes with proliferation of arterial smooth muscle cells. Biochem Biophys Res Commun. 1989;158:105. doi: 10.1016/s0006-291x(89)80183-4. [DOI] [PubMed] [Google Scholar]
- 19.Chijiwa T, Mishima A, Hagiwara H, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-(2-(ρ-bromocinnamylamino)ethyl)-5-isoquenilesulfonamide (H-89), of PC12D pheocromocitoma cells. J Biol Chem. 1990;265:5267. [PubMed] [Google Scholar]
- 20.Sumi T, Ui M. Potentiation of the adrenergic beta-receptor-mediated insulin secretion in pertussis-sensitized rats. Endocrinology. 1975;97:352. doi: 10.1210/endo-97-2-352. [DOI] [PubMed] [Google Scholar]
- 21.Blaya C, Crespo J, Crespo A, Alino SF. Effect of the protein kinase inhibitors, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine H-7 and N-(2-[methylamino]ethyl)-5-isoquinoline-sulfonamide H-8 on Lewis lung carcinoma tumor progression. Eur J Pharmacol. 1998;354:99. doi: 10.1016/s0014-2999(98)00434-8. [DOI] [PubMed] [Google Scholar]
- 22.Toullec D, Pianetti P, Coste H, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771. [PubMed] [Google Scholar]
- 23.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 24.Carroll RG, Riley JL, Levine BL, et al. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 25.Jourdan P, Abbal C, Nora N, et al. IL-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol. 1998;160:4153. [PubMed] [Google Scholar]
- 26.Valentin A, Lu W, Rosati M, et al. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galli G, Annunziato F, Mavilia C, et al. Enhanced HIV expression during Th2-oriented responses explained by the opposite regulatory effect of IL-4 and IFN-gamma of fusin/CXCR4. Eur J Immunol. 1998;28:3280. doi: 10.1002/(SICI)1521-4141(199810)28:10<3280::AID-IMMU3280>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Harada A, Matsushita S, et al. IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+ T lymphocytes and enhance HIV-1 replication. J Leukoc Biol. 1998;64:642. doi: 10.1002/jlb.64.5.642. [DOI] [PubMed] [Google Scholar]
- 29.Kasama T, Strieter RM, Lukacs NW, Burdick MD, Kunkel SL. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994;152:3559. [PubMed] [Google Scholar]
- 30.Sozzani S, Ghezzi S, Iannolo G, et al. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Roderiquez G, Oravecz T, Norcross MA. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]


