Abstract
The distribution and function of connexins (integral membrane proteins assembled into gap junction intercellular communication channels) were studied in human lymphocyte subpopulations. The expression of mRNA encoding connexins in peripheral blood and tonsil‐derived T, B and natural killer (NK) lymphocytes was examined. Connexin43 (Cx43) mRNA was expressed in peripheral blood and tonsil lymphocytes, but Cx40 mRNA expression was confined to tonsil‐derived T and B lymphocytes; Cx26, Cx32, Cx37 and Cx45 were not detected by reverse transcription–polymerase chain reaction (RT–PCR). Western blot analysis also demonstrated the presence of Cx40 and Cx43 proteins in T and B lymphocytes in a manner coincidental to the mRNA detection. Stimulation in vitro of T and B lymphocytes with phytohaemagglutinin (PHA) and lipopolysaccharide (LPS), respectively, increased Cx40 and Cx43 protein expression. Flow cytometric analysis, using antibodies to extracellular loop amino acid sequences of connexins, confirmed the surface expression of connexins in all lymphocyte subpopulations. Assembly of connexins into gap junctions providing direct intercellular channels linking attached lymphocytes was demonstrated by using a dye transfer technique. The exchange of dye between lymphocytes was inhibited by a connexin extracellular loop mimetic peptide and α‐glycyrrhetinic acid, two reagents that restrict intercellular communication across gap junctions. Dye coupling occurred between homologous and heterologous co‐cultures of T and B lymphocytes, and was not influenced by their stimulation with PHA and LPS. The connexin mimetic peptide caused a significant decrease in the in vitro synthesis of immunoglobulin M (IgM) by T‐ and B‐lymphocyte co‐cultured populations in the presence or absence of stimulation by PHA. The results identify connexins as important cell surface components that modulate immune processes.
Introduction
Circulating lymphocytes respond to a broad spectrum of stimuli. During migration from the blood into tissues, lymphocytes interact with endothelial cells, a process involving a range of adhesion molecules, e.g. cadherins, integrins and selectins located on cell surfaces.1–3 These interactions trigger signal transduction cascades that allow lymphocytes to proceed through maturation steps, characterized by the expression of new molecules implicated in transit across tissues.4,5
Among the main categories of intercellular junctions, gap junctions comprise an important group of surface specializations that facilitate, in tissues and organs, cell‐to‐cell adhesion and also provide pathways that can allow direct intercellular communication, with signalling and developmental consequences. Gap junctions are clusters of intercellular channels in the plasma membrane that allow direct cross‐talk between attached cells. Each channel consists of a pair of interacting connexon hemichannels, contributed by the co‐operating cells. These connexon hemichannels are assembled from six polypeptide subunits, termed connexins.6,7 Connexins comprise a family of proteins with extensive sequence homology and a conserved topographic arrangement in the membrane. Connexins traverse the plasma membrane four times with the amino and carboxyl termini located at the cytoplasmic aspect, thus generating two ‘gap’ facing extracellular loops and a single intracellular loop.8 Connexin proteins are widely distributed, being found in all tissues and organs except striated muscle. It is now well established that cells express more than one connexin type,9 thus making probable the formation of heteromeric connexons and heterotypic gap junctions. Connexins have short half‐lives, and gap junctions are subject to developmental or pathological changes.10–14
The distribution of connexins in cells of the immune system has not been explored in detail. Lymphocytes, during maturation, interact continuously with many other cells that influence their behaviour.15,16 Peripheral blood mononuclear cells (PBMC) stimulated in vitro with phytohamagglutinin (PHA)17 exhibit putative surface junctions and electrophysiological characteristics which suggested that the cells were capable of communicating directly.18,19 A key advance was the demonstration that thymic epithelial cells and thymocytes derived from human and murine thymi communicated via gap junctions that were constructed from connexin43 (Cx43),20 one of the most widely distributed proteins in the connexin family. Gap junctions occur in the lymphoreticular system20–22 and Cx43 has also been identified in human and mouse bone marrow preparations,23,24 especially in follicular dendritic cells within the light zone of germinal centres where lymphocyte maturation occurs.25 Cx43 was also detected immunocytochemically in follicular dendritic cells of secondary lymphoid follicles, in the lymphoendothelial network including afferent lymphatics and sinus lining cells inside organs, and in vascular endothelium, including the high endothelial venule.26,27
The present work addresses the expression and role of connexin proteins in purified human lymphocyte subpopulations (T, B and natural killer [NK] lymphocytes). We provide evidence that human lymphocyte subpopulations express Cx40 and Cx43, and show the presence of intercellular channels directly linking these cells. The consequences of exposure of lymphocytes to lipopolysaccharide (LPS) or PHA‐L on the expression levels of Cx43 and Cx40 were explored. Paradoxically, dye transfer across gap junctions was not significantly affected by the stimulation of lymphocytes with PHA‐L and LPS. However, addition of two independent inhibitors of gap junctional communication blocked dye transfer, especially when T lymphocytes were participating as dye donor cells in homotypic and heterotypic cultures of lymphocytes. Finally, we demonstrate that communication via the gap junction between lymphocytes will probably play a crucial role in eliciting an immune response, as the gap junction inhibitors significantly decreased immunoglobulin synthesis by B lymphocytes in the presence of T cells.
Materials and methods
Statistical analysis
Experiments, unless stated otherwise, were performed at least three times. Differences between the samples were analysed by using the Student’s t‐test. A P‐value of ≤ 0·05 was considered significant.
Cells
Lymphocytes from heparinized blood of healthy human donors, and surgically removed tonsils from children with recurrent tonsillitis, were purified by using Histopaque‐1077® (Sigma Chemical Co., St Louis, MO). Cell counting was performed in a haemocytometer chamber, and viability was assessed by Trypan Blue staining. Cell suspensions were stained with antibodies relevant to each lymphocyte subset (see below) using standard procedures, and sorted on a fluorescence‐activated cell sorter (FACS) flow cytometer (FACS‐440, Becton‐Dickinson, Oxford, UK). T and B lymphocytes were also purified using a human T‐cell enrichment column (R & D Systems, Minneapolis, MN) or a human B‐cell Accessory kit (Biotecx Labs, Houston, TX), respectively, according to the manufacturer’s instructions. The purity of isolated cells prepared by both methods was between 95 and 99%, as determined by flow cytometric analysis. NK lymphocytes were sorted by FACS using a mouse anti‐human CD56 monoclonal antibody (mAb) that was fluorescein isothiocyanate (FITC) conjugated. In order to avoid an allogeneic mixed lymphocyte reaction, lymphocyte cultures were performed only with cells isolated and purified from the same donor.
Antibodies and peptides
Table 1 gives details of the connexin40 (Cx40) and Cx43 peptide sequences used to generate rabbit antisera for Western blotting, fluorescence microscopy or cell surface staining by flow cytometry analysis. Peptides were conjugated to limpet haemocyanin or prepared as multiple antigenic peptide (MAP) derivatives.28,29 A mAb against Cx43 (Chemicon, Temecula, CA) was also used. The Cx43 antibodies used have been extensively characterized.30–32
Table 1.
Peptides/antibodies used for connexin40 (Cx40) and Cx43 protein expression analysis
| Peptide /antibody | Cx | Amino acids | Peptide sequence | Topographical position | Experimental use |
|---|---|---|---|---|---|
| GAP18 | 43 | 1–16 | MGDWSALGKLLDKVQAC | Intracellular N‐terminal tail | ELISA |
| GAP35 | 43 | 44–62 | AWGDEQSAFRCNTQQPGC | Extracellular loop 1 | FM, FACS |
| GAP36 | 43 | 191–209 | KRDPCPHQVDCFLSRPTEK | Extracellular loop 2 | FM, FACS |
| GAP38 | 40 | 285–296 | NMASQQNTDNLV | C terminus | FM, WB, FACS |
| GAP27 | 43 | 204–214 | SRPTEKTIFII | Extracellular loop 2 | ELISA |
| mAbCx43* | 43 | 252–270 | GPLSPSKDCGSPKYAYFNG | C terminus | WB |
Cx, connexin; ELISA, enzyme‐linked immunosorbent assay; FM, fluorescence microscopy; mAb, monclonal antibody; WB, Western blotting.
Commercial mAb (Chemicon).
The anti‐human lymphocyte cell marker antibodies used were: mouse anti‐CD3 conjugated to FITC; mouse anti‐CD19 conjugated to FITC (DAKO, Cambridge, UK); anti‐CD4 and anti‐CD8, both FITC conjugated, (DAKO) and mouse anti‐CD56 FITC (Sigma). A mouse immunoglobulin G1 (IgG1)‐FITC conjugate (DAKO) was used as a control. In the affinity purification of GAP35 (Cx43), GAP36 (Cx43) and GAP38 (Cx40) (see Table 1) peptides were used in the affinity purification, IgG was prepared from rabbit antisera using a Protein A–Sepharose column, and affinity purification then carried out by elution with a glycine/HCl pH 2·0 buffer from a column containing the respective peptides coupled to cyanogen bromide (CnBr)‐activated Sepharose CL‐4B (Sigma).33 These antibodies were detected by using an anti‐rabbit IgG conjugated to phycoerythrin (PE) (Sigma) as a secondary antibody. Another secondary antibody, goat anti‐rabbit Cy3‐conjugate (Amersham, Little Chalfont, Bucks, UK) was used for fluorescence microscopy.
RNA extraction and reverse transcription–polymerase chain reaction (RT–PCR) analysis
RNA was obtained by using the ULTRASPEC™ RNA isolation system according to the manufacturer’s instructions (Biotecx Labs). First‐strand cDNA synthesis was carried out using 1 µg of total RNA purified from 7 × 106 T cells, 1 × 106 B cells or 0·5 × 106 NK cells derived from peripheral blood or from tonsils. Reverse transcription was performed in a 20‐µl final volume containing 50 ng of pd(N)6 (Amersham, Phamacia Biotec, Herts, UK), 0·2 mm of dATP, dCTP, dGTP and dTTP, and 10 mm dithiothreitol (DTT). Each reaction mix was incubated for 10 min at 25° with 40 U of rRNasin® (RNase inhibitor; Promega, Madison, WI) followed by incubation with 200 U of Moloney murine leukaemia virus reverse transcriptase (Gibco‐BRL, Paisley, UK) for 40 min at 42° followed by 2 min at 99°. PCR analysis was performed, in a final volume of 50 µl, by using 2 µl of cDNA solution in a mix containing 0·2 mm deoxynucleotide triphosphates, 20 pmol of both sense and antisense oligonucleotide primers according to the connexin type to be detected, 1·5 mm MgCl2, 1 U of Taq DNA polymerase (Promega) and 5 µl of 10× Taq DNA polymerase reaction buffer (500 mm KCl, 100 mm Tris‐HCl [pH 9·0], 1% Triton®‐X‐100). PCR primer sequences, direction, size and reaction conditions are shown in Table 2. To eliminate any possibility of genomic DNA contamination, the PCR amplification reaction was carried out on each sample of the RNA extraction. As another internal contamination control, PCR amplification was also carried out on a reaction mix sample in the absence of cDNA.
Table 2.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis for connexin mRNA expression in lymphocyte subpopulations
| Name | Sequence | Direction | Expected product size | PCR conditions for pair of primers |
|---|---|---|---|---|
| HmCx26Up | CCG AAG TTC ATG AAG GGA GAG AT | Sense | 390 bp | 1 cycle: 94°, 4 min |
| HmCx26Lo | GGT CTT TTG GAC TTC CCT GAG CA | Antisense | 35 cycles: 94°, 1 min; 61°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min | |
| HmCx32Up | CTG CTC TAC CCG GGC TAT GC | Sense | 386 bp | 1 cycle: 94°, 4 min |
| HmCx32Lo | CAG GCT GAG CAT CGG TCG CTC TT | Antisense | ||
| 35 cycles: 94°, 1 min; 60°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min | ||||
| RatCx37P1 | GAC TGG GGC TTC CTG GAG AAG | Sense | 463 bp | 1 cycle: 94°, 4 min |
| RatCx37P2 | GCC ACC GAG ATC TTG GCC ATC | Antisense | ||
| 35 cycles: 94°, 1 min; 65°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min | ||||
| RatCx40P1 | ATG CAC TGT GCG CAT GCA GGA | Sense | 399 bp | 1 cycle: 94°, 4 min |
| RatCx37P2 | CAG GTG GTA GTG TTC AGC CAG | Antisense | ||
| 35 cycles: 94°, 1 min; 58°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min | ||||
| HmCx43Up | TAC CAT GCG ACC AGT GGT GCG CT | Sense | 294 bp | 1 cycle: 94°, 4 min |
| HmCx43Lo | GAA TTC TGG TTA TCA TCG TCG GGG AA | Antisense | ||
| 35 cycles: 94°, 1 min; 60°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min | ||||
| HmCx45Up | CTA TGC AAT GCG CTG GAA ACA ACA | Sense | 815 bp | 1 cycle: 94°, 4 min |
| HmCx45Lo | CCC TGA TTT GCT ACT GGC AGT | Antisense | 35 cycles: 94°, 1 min; 55°, 1 min; 72°, 1 min 1 cycle: 72°, 8 min |
Sequencing of PCR products
The PCR products from Cx40 and Cx43 cDNA synthesis reactions were purified using a QIAquick PCR Purification kit (Qiagen, West Sussex). Each sequencing reaction used 8 µl of Terminator Ready Reaction Mix, from an ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA), 60 ng of PCR product, 3·2 pmol of the sense or antisense primers and deionized water up to 20 µl, and overlaid with 40 µl of light mineral oil. The amplification was carried out on a Perkin‐Elmer Cetus DNA Thermal Cycler (Perkin‐Elmer, Norwalk, CT) using the following programme: 25 cycles commencing with a rapid thermal ramp to 96°; 96° for 30 seconds; rapid thermal cycle to 50°; 50° for 15 seconds; rapid thermal cycle to 60° and 60° for 4 min. The reaction products were purified by ethanol precipitation and analysed in a Perkin‐Elmer Applied Biosystems ABI Prism 377 DNA Sequencer.
In vitro lymphocyte activation
Purified and sorted T lymphocytes (10 × 106 cells) and B lymphocytes (5 × 106 cells) from peripheral blood and tonsils were incubated for 3, 6, 9, 12, 24 and/or 48 hr with 1 µg/ml of PHA‐L (Boehringer Mannheim, Mannheim, Germany) or 5 µg/ml of LPS (from Escherichia coli, serotype 055:B5; Sigma), respectively, in RPMI‐1640 culture medium (Gibco‐BRL) supplemented with 10% FCS (for LPS stimulation 10% human male AB serum [Sigma] was used), 1 × 105 U penicillin, 1 × 105 µg streptomycin, 250 µg/ml of amphotericin B, 25 mm HEPES and 1 mm l‐glutamine (antibiotics and HEPES were purchased from Gibco‐BRL). A control group of lymphocytes was cultured under the same conditions without PHA‐L or LPS. All treatments were carried out in triplicate.
Western blotting
Cells resuspended in phosphate‐buffered saline (PBS: 100 mm NaCl, 100 mm Na2HPO4/NaH2PO4, pH 7·5) were sedimented and lysed by vortexing and after resuspension in a pH 7·4 buffer containing 100 mm Tris‐HCl, 20 mm EDTA, protease inhibitors (0·3 µm aprotinin, 130 µm bestatin, 100 µm chymostatin, 1 µm pepstatin [Boehringer Mannheim], 2 mm phenylmethylsulphonyl fluoride (PMSF; Aldrich, Dorset, UK) and 1% sodium dodecyl sulphate (SDS). The lysates were centrifuged at 3000 g for 10 min and supernatants were collected and then mixed with 2× SDS loading buffer. Protein concentrations were measured using a BioRad Assay kit (BioRad, Hercules, CA). Equivalent amounts of total protein were loaded onto 12·5% SDS polyacrylamide gels (SDS–PAGE). After electrophoresis, proteins were transferred to nitrocellulose membranes and blocked in 5% (w/v) skimmed milk, Tris‐buffered saline [pH 7·6] containing 0·1% Tween‐20 (TBST). Membranes were incubated overnight with GAP38 (Cx40) polyclonal antibody (Table 1) and a Cx43 mAb, respectively (see Table 1), before washing three times with TBST. Membranes were incubated with horseradish peroxidase‐conjugated anti‐rabbit or anti‐mouse IgG (DAKO) for 1 hr at room temperature and then washed three times in TBST. Antigen–antibody recognition was visualized using enhanced chemiluminescence (ECL; Amersham). Protein was measured in each trace using a BioRad GS‐700 Imaging Densitometer controlled by Quantity One software (version 4.0.3) and the amount is expressed in arbitrary units.
Surface staining for fluorescence microscopy and flow cytometric analysis
One million cells (for fluorescence microscopy, cells were previously stimulated in vitro with PHA‐L for 3, 6 and 12 hr as described above) were washed in PBS containing 1% bovine serum albumin (BSA), 0·02% sodium azide, 10 mm glucose and 2·5 mm EDTA, and stained using mouse anti‐CD3‐FITC, anti‐CD19‐FITC, anti‐CD4‐FITC, anti‐CD8‐FITC (DAKO), anti‐CD56‐FITC (Sigma) and equal amounts of affinity‐purified GAP35 and GAP36 antibodies (Table 1). Mouse IgG1‐FITC conjugate was used as an internal control, and it showed no reactivity with human lymphocytes. After incubation for 30 min at 4°, cells were washed three times in PBS/1% BSA and then incubated for 30 min at 4° with the mouse anti‐rabbit IgG‐PE conjugate (for flow cytometric analysis) or goat anti‐rabbit Cy3 (for fluorescence microscopy) as described above. For flow cytometric analysis, cells were fixed in 2% formaldehyde in PBS. Data were collected using a FACScan analyser (Becton‐Dickinson) and processed using WinMDI software, version 2·5. For fluorescence microscopy, cells were washed twice with PBS before mounting on slides and visualized using a Zeiss Axiovert‐10 fluorescence microscope (Zeiss, Herts, UK). Controls using preimmune sera did not stain.
Analysis of dye transfer by flow cytometry
A preloading dye transfer method34 was used to assess gap junction functionality. Lymphocyte subpopulations were loaded with DiIC18 (1,1′‐dioctadecyl‐3,3,3′,3′‐tetra‐methylindodicarbocyanine perchlorate) and Calcein AM (Calcein acetoxymethyl ester) (Molecular Probes, Eugene, OR) and then plated with unlabelled cells. DiIC18 is a lipophilic dye that labels cell membranes and appears red when viewed through the appropriate filters. This dye attaches to the plasma membrane and is unable to diffuse from cell to cell, thereby allowing identification of donor cells. Calcein AM (MW 0·955) is non‐fluorescent, electrically neutral and membrane permeable. After uptake into cells, Calcein AM is converted into calcein by endogenous esterase activity, becoming a fluorescent and negatively charged molecule (MW 0·623, charge − 4) that is unable to diffuse out of the cells across the plasma membrane but is able to pass between cells via gap junction channels. Preloaded cells are plated with unlabelled cells and gap junction communication is measured by flow cytometry as the amount of calcein diffusing from the preloaded cells to unlabelled cells after they settle on the plate. The following procedure was use to label donor cells: lymphocyte subpopulations were dye‐loaded for 30 min at 37° with 10 µm DiIC18 and 2·5 µm Calcein AM (dyes were first diluted in PBS containing 1 g/l of glucose from 1 mm stock solutions made in dimethylsulphoxide [DMSO]; dye concentrations were adjusted and possible dye leakage controlled using FACS analysis) in RPMI‐1640 tissue culture medium supplemented with 10% fetal calf serum (FCS), 1 × 105 U penicillin, 1 × 105 µg streptomycin, 250 µg/ml of amphotericin B, 25 mm HEPES and 1 mm l‐glutamine. The cells were washed three times with PBS, and seeded in tissue culture multiwell plates (ICN Biomedicals, Irvine, CA). Unlabelled cells were washed in PBS and then seeded with the dye‐loaded cells at a ratio of 1 : 100 (loaded : non‐loaded cells). Separate replicate groups of co‐cultured labelled/unlabelled lymphocytes were cultured in the presence of PHA‐L (1 µg/ml) or LPS (5 µg/ml) added to cells 30 min after seeding. An additional control, using lymphocytes cultured with the same final concentrations of DMSO used for the dye preparation, was also included. After incubation for 3 hr, cells in each group were harvested by centrifugation, washed three times after vortexing vigorously (to disrupt aggregates) with a solution containing PBS and 10 mm EDTA. Calcein and DiIC18 fluorescence were detected by flow cytometry as described above. Samples were analysed for calcein (excitation at 488 nm and emission at 535 nm) and for DiIC18 (excitation at 488 nm and recorded at 585 nm). Data from 20 000 cells were acquired from each sample. Results are expressed as the mean green fluorescence intensity (caused by dye transfer) above an arbitrary value set on negative cells. To avoid any problems associated with dye leakage, only samples with a high percentage of cell viability (above 98%) were used in these experiments. Dead cells were excluded by Trypan Blue exclusion and by gating based on cellular light scatter. In experiments to assess the specificity of dye transfer across gap junctions, control reactions were carried out by incubation of cells for 3 hr as described above but in the presence of 150 µm of 18‐α‐glycyrrhetinic acid35,36 or 300 µg/ml of GAP27 peptide37–39 and acquired with the same flow cytometric settings.
In vitro antibody production and ELISA
Human B lymphocytes were cultured with or without purified T lymphocytes in tissue culture plates at 5 × 106 cells/well in a final volume of 2 ml of RPMI‐1640 tissue culture medium supplemented with 10% heat‐inactivated FCS, 1 × 105 U penicillin, 1 × 105 µg streptomycin, 250 µg/ml of amphotericin B, 25 mm HEPES and 1 mm l‐glutamine. Five dishes containing co‐cultured T and B lymphocytes were stimulated with PHA‐L (10 µg/ml) and five control dishes contained no mitogen. PHA‐L was used to stimulate T lymphocytes and to evaluate any T‐cell‐dependent effects on the B‐cell function affected by the gap junction communication blockers. A further five dishes of purified B lymphocytes were cultured in parallel in the absence of the mitogen. Supernatants were collected at 0, 6, 12, 24, 36 and 48 hr and immunoglobulin M (IgM) was measured by using enzyme‐linked immunosorbent assay (ELISA) as follows. Data were collected up to 48 hr, but no longer, because cell viability was compromised after longer exposure under the experimental conditions used. Microtitre plates were coated overnight at 4° with 3 µg/ml of Protein‐L (ACTIgen, Cambridge, UK), as a capture molecule, in 0·01 m Na2CO3/NaHCO3 buffer, pH 9·6. After washing with PBS (pH 7·4), containing 0·05% Tween‐20 (PBS‐T), plates were incubated for 2 hr with PBS‐T containing 1% BSA at room temperature. Plates were then incubated with test samples and duplicate serial dilutions of standard human IgM (Sigma). After washing twice with PBS‐T, plates were incubated for a further 1 hr at room temperature with a 1 : 5000 dilution of biotin‐conjugated goat anti‐human µ‐chain Fc‐specific antibody (Sigma). Plates were then rewashed with PBS‐T and incubated with a 1 : 10 000 dilution of horseradish peroxidase‐labelled avidin (Sigma) for 1 hr. Plates were washed again with PBS‐T and developed using o‐phenylenediamine dihydrochloride (OPD) peroxidase substrate (Sigma). The reactions were stopped by the addition of 50 µl of 3 m HCl. Absorbance (A) at 492 nm was determined using a microtitre plate reader.
Results
Cx40 and Cx43 mRNA expression by human T, B and NK lymphocytes
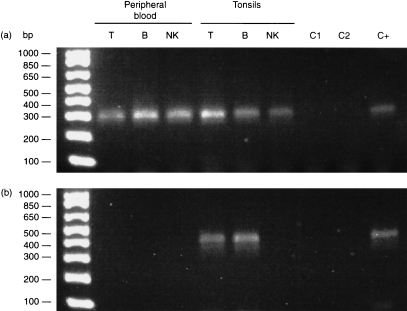
Connexin mRNA levels were too low to allow measurement by Northern blotting and thus RT–PCR was carried out with cDNA isolated from purified T, B and NK lymphocytes freshly isolated from peripheral blood or from tonsils. Cx43 cDNA was amplified from the three cell subpopulations. The expected 294‐bp product was also present when the same Cx43 primers were used to amplify the cloned human full‐length cDNA (Fig. 1a). Cx40 cDNA was amplified from T and B lymphocytes purified from tonsils but no amplification was obtained from T, B or NK lymphocytes purified from peripheral blood (Fig. 1b). No mRNA encoding Cx26, Cx32, Cx37 and Cx45 was observed (data not shown). The presence of mRNA encoding Cx40 and Cx43 was corroborated when the amplified RT–PCR products were sequenced and compared with the reported human cDNA sequences. A sequence similarity of 98–99% was found.
Figure 1.
Expression of connexin40 (Cx40) and Cx43 in human lymphocyte subpopulations. T, B and natural killer (NK) lymphocytes from peripheral blood and tonsils were purified and the mRNA was isolated to produce cDNA. (a) Cx43 cDNA was observed in peripheral blood and tonsil‐derived T, B and NK lymphocytes at the expected electrophoretic mobility. (b) Cx40 cDNA was detected only in T and B lymphocytes isolated from tonsils. In each case, the polymerase chain reaction (PCR) was also carried out on the corresponding human‐cloned Cx40 and Cx43 cDNA, used as a positive control (line C+) showing a band with the same mobility. Lines C1 and C2 in each panel represent DNA‐contamination controls, the first for mRNA isolation and the second for PCR amplification.
Cx40 and 43 proteins are expressed by T and B lymphocytes
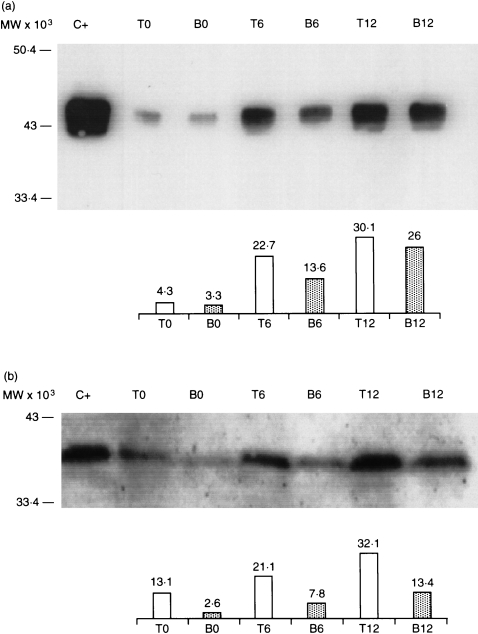
Analysis of Cx43 expression by Western blotting using freshly isolated T and B lymphocytes from peripheral blood and tonsils showed a predominant Cx43 band pattern with the characteristic electrophoretic mobility as compared with the positive control band of Cx43 extracted from heart.40 Stimulation of T and B lymphocytes with PHA‐L and LPS, respectively, for 6 or 12 hr, further increased by two‐ to fourfold the expression of Cx43 (Fig. 2a). Cx40 was also detected by Western blotting but, in contrast to Cx43, Cx40 was only detected in T and B lymphocytes prepared from tonsils. Again, a two‐ to threefold increase in the expression of Cx40 bands in a time‐dependent manner after in vitro stimulation of purified lymphocytes, especially with Tlymphocytes, was observed (Fig. 2b).
Figure 2.
Western blot analysis of connexin40 (Cx40) (a) and Cx43 (b) protein expression in human T and B lymphocytes isolated from tonsils. T and B lymphocytes were stimulated in vitro with phytohaemagglutinin‐L (PHA‐L) and lipopolysaccharide (LPS), respectively, for 6 and 12 hr, as described in the Materials and methods, and the results were compared with non‐stimulated cells. Connexins were detected using a monoclonal antibody (mAb) against Cx43 and GAP38 affinity‐purified antipeptide antibody raised against the carboxyl terminal tail of Cx40 (Table 1). Line C+ in panel (a) is a heart tissue extract used as a positive control. Line C+ in panel (b) is a human Cx40 sample obtained by in vitro transcription/translation. Approximately 100 µg of protein extract was applied to each lane. The bar charts represent the amount of protein measured in each trace by using densitometric analysis and values are expressed in arbitrary units.
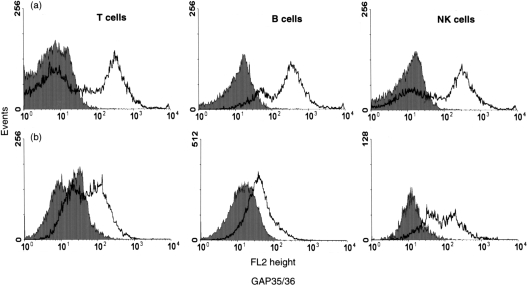
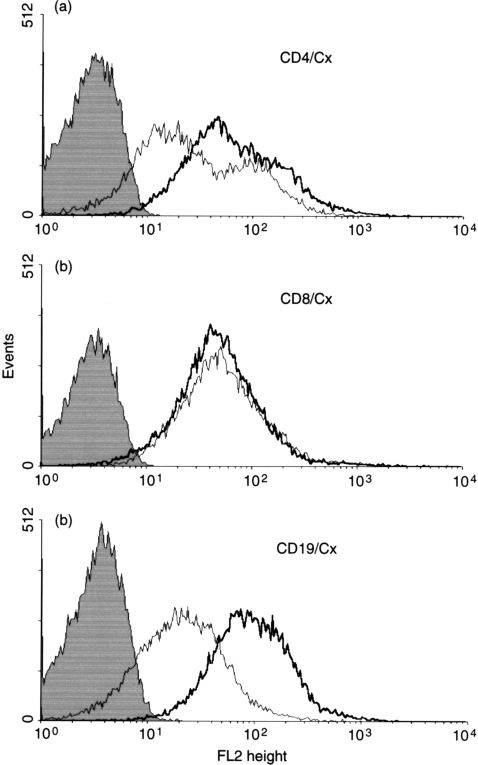
Surface expression of connexins was studied by flow cytometric analysis of lymphocytes derived from peripheral blood and tonsils, using antibodies specific to sequences in the two extracellular loops of Cx43 (Fig. 3). These experiments showed differences in expression levels of connexins detected at the external aspect of the plasma membrane of the various lymphocyte subsets. Expression of connexins was higher in all the subpopulations isolated from peripheral blood (Fig. 3a) as compared with cells derived from tonsils (Fig. 3b). In view of similarities in the extracellular loop sequences of Cx40 and Cx43,6,7,9 the antibodies employed are unlikely to distinguish between the connexins. We also examined, by flow cytometry, changes induced by mitogens in connexin surface expression on T CD4+ helper and CD8+ cytotoxic phenotypes (Fig. 4a, 4b), and B cells (Fig. 4c). Connexin expression increased after 6 hr in all the subpopulations but to a greater extent in B cells. Stimulation with PHA‐L increased the expression level of connexin between 6 and 12 hr in CD4+ and B lymphocytes but had no effect on the level of connexin expressed by CD8+ cells.
Figure 3.
Expression of connexin proteins on lymphocyte subsets in blood and tonsils compartments assessed by flow cytometry analysis. For detection of connexins on the different lymphocyte subsets, lymphocytes were labelled as described in the Materials and methods. Surface expression of connexin was detected in T (CD3+), B (CD19+) and natural killer (NK) (CD56+) lymphocytes derived from peripheral blood (a) and tonsils (b). Solid curves represent isotype control while open curves represent specific staining.
Figure 4.
Expression of activation‐induced cell surface connexin detected by connexin extracellular loop antibodies in T‐cell subsets and B lymphocytes. Cells isolated from tonsils were stimulated with phytohaemagglutinin‐L (PHA‐L) (T cells) and lipopolysaccharide (LPS) (B cells) for 6 and 12 hr, as described in the Materials and methods, before harvesting for flow cytometry analysis. Connexin expression was detected on T helper cells gated with anti‐CD4 fluorescein isothiocyanate (FITC) (a), T‐cytotoxic cells gated with anti‐CD8 FITC (b) and B lymphocytes with anti‐CD19 FITC (c). Rabbit GAP35/36 affinity‐purified anticonnexin antibodies were detected with goat anti‐rabbit immunoglobulin E‐conjugated phycoerythrin (IgG‐PE) after 6 and 12 hr in vitro stimulation (thin and thick lines, respectively). Binding of goat anti‐rabbit IgG‐PE is shown as the solid histogram.
Binding specificity of GAP35/GAP36 antibodies to cells was verified by peptide‐competition experiments. The number of connexin‐positive cells was considerably reduced in the presence of 100 µg/ml of each GAP35/36 peptide, and was almost abolished by using 300 µg/ml of each GAP35/36 peptide (data not shown).
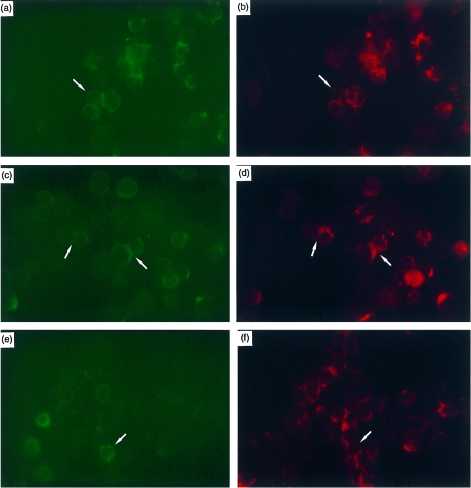
Fluorescence microscopy was also used to study the presence and distribution of gap junction proteins on lymphocyte cell surfaces. Using non‐permeabilized cells, connexins on cell surfaces were studied using the affinity‐purified antibodies generated to the extracellular loop of the protein described above. Characteristic punctate staining was evident around the periphery of the cells, with more intense staining at sites of intercellular contact (Fig. 5). Connexin immunoreactivity was more extensively distributed in CD3‐ and CD19‐stained cells than in CD56‐reactive cells. In spite of the extensive co‐localization of connexins and CD markers, some variability in connexin immunoreactivity was noted. A comparison of homotypic and heterotypic cultures of purified T and B lymphocytes stimulated with PHA‐L and LPS, respectively, for 3–12 hr, showed that fluorescence staining for connexin increased in heterocellular cultures of lymphocytes stimulated for more than 6 hr (data not shown).
Figure 5.
Detection and distribution of connexin protein in T, B and natural killer (NK) lymphocyte subpopulations by fluorescence microscopy. Detection at the plasma membrane level was studied in a total mixed population of non‐permeabilized lymphocytes isolated from tonsils and activated with mitogen for 12 hr. Cells were double labelled, as described in the Materials and methods, with the following markers: mouse anti‐CD3 (T cells), CD19 (B cells), CD56 (NK cells) antibodies, all fluorescein isothiocyanate (FITC) conjugated (panels (a), (c) and (e), respectively), and with affinity‐purified rabbit GAP35/GAP36 antipeptide connexin43 (Cx43) antibodies developed with a goat antirabbit Cy3 conjugate (panels (b), (d) and (f)). Connexins appear as largely red bright, punctuate regions, located particularly at cellular contact regions. Arrows indicate pairs or groups of double‐labelled cells. No surface staining was evident using the mouse‐IgG1 control antibody or the connexin preimmune sera (data not shown).
Functionality of lymphocyte gap junctions
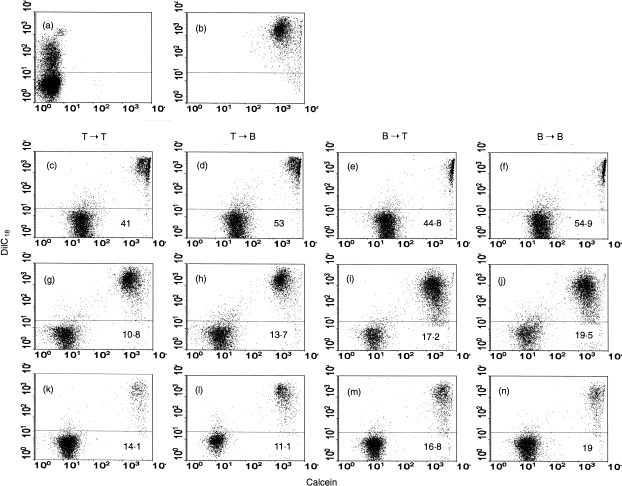
Dye transfer between lymphocytes was evaluated by flow cytometry using different combinations of loaded to unloaded cells (T→T cells, T→B cells, B→T cells, B→B cells). Intercellular calcein transfer occurred in all lymphocyte subpopulation combinations. Several kinetic experiments (data not shown) demonstrated that dye transfer between lymphocytes, under the experimental conditions used, peaked at 3 hr and remained constant after that period of time. No significant difference in the extent of dye transfer and directionality between the groups of co‐cultured cells was observed (Fig. 6c, 6d, 6e, 6f). The specificity of the dye transfer between lymphocytes was examined using two established and independent inhibitors of gap junctional communication. An extracellular connexin mimetic peptide (GAP27) blocked communication in both homotypic and heterotypic co‐cultures (Fig. 6g, 6h, 6i, 6j, and Fig. 7). Similar results were also obtained using α‐glycyrrhetinic acid but, with this inhibitor, the effect was more dramatic when T lymphocytes acted as dye donor cells (Fig. 6k, 6l, 6m, 6n, and Fig. 7). Dye transfer in each of the combinations of T‐ and B‐cell populations (see above) was drastically reduced by the two gap junction communication inhibitors (Fig. 7).
Figure 6.
Intercellular dye transfer studied using flow cytometry. Donor cells were double labelled with Calcein AM and DiIC18 and co‐cultured as described in the Materials and methods. Panel (a) represents a control reaction of donor cells labelled with DiIC18 and incubated with non‐labelled cells. Panel (b) shows double‐labelled cells before being co‐cultured with non‐labelled cells. Panels (c), (d), (e) and (f) are co‐cultured double‐labelled cells with unlabelled lymphocytes. The letters at the top of each panel indicate the directionality between T and B lymphocytes of the transfer assessed. The effect of peptide GAP27 [300 µg/ml] (g), (h), (i) and (j) and 18‐α‐glycyrrhetinic acid [150 µm] (k), (l), (m) and (n) on the co‐cultured combination was studied. Values represent the mean fluorescence values for green fluorescence above the arbitrary marker shown. The experiment was repeated six times with similar results.
Figure 7.
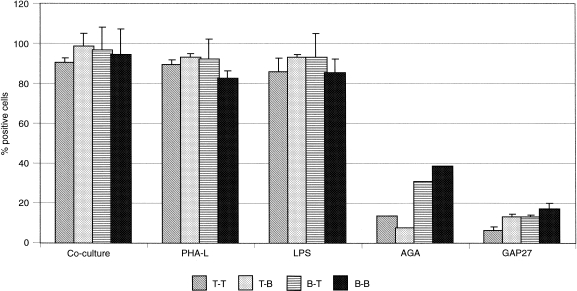
Effect of phytohaemagglutinin‐L (PHA‐L), lipopolysaccharide (LPS) and gap junction communication inhibitors on dye transfer between T and B lymphocytes co‐cultured in different combinations. After loaded and non‐loaded cells were allowed to seed for 30 min, PHA‐L (1 µg/ml), LPS (5 µg/ml), 18‐α‐glycyrrhetinic acid (AGA) (150 µm) and GAP27 peptide (300 µg/ml) were added to the different combination groups, respectively, and cells were incubated for 6 hr. Fluorescence was measured by flow cytometry and the percentage of positive green fluorescent cells above the arbitrary marker was analysed in each case. A control group of non‐treated cells was examined in parallel (co‐cultured cells). Results represent the mean ± standard deviation of six experiments for each combination.
Using the same co‐culture conditions, we also assessed, by flow cytometry, the in vitro effect of PHA‐L and LPS on dye transfer (Fig. 7). No significant differences in the extent of dye transfer were noted among unstimulated and cells stimulated with PHA‐L and LPS under the conditions described above, a result that contrasted with the effects of these reagents on the endogenous synthesis of Cx43.
Effects of gap junction inhibitors on the production of IgM by T‐ and B‐lymphocyte co‐cultured populations
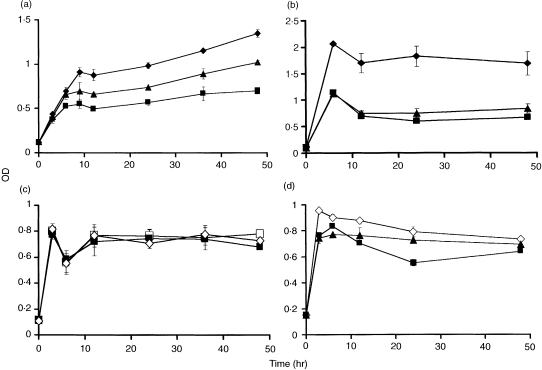
Co‐cultured T‐ and B‐lymphocyte populations producing IgM over 48 hr were treated with the two inhibitors of gap junction communication described above. Figure 8(a) shows that cells incubated in the presence of GAP27, the extracellular connexin mimetic peptide or α‐glycyrrhetinic acid,38,41 decreased IgM production by ≈ 30%. Mixed subsets of lymphocytes stimulated with PHA‐L increased IgM production, and an ≈ twofold increase in inhibition relative to non‐stimulated cells was observed in the presence of GAP27 connexin mimetic peptide or α‐glycyrrhetinic acid (Fig. 8b). In control experiments, two intracellular connexin peptides, which had no effects on gap junctional communication,38 were without effect on IgM production (Fig. 8c). The GAP27 connexin mimetic peptide showed little effect on IgM production by purified B lymphocytes (Fig. 8d). α‐Glycyrrhetinic acid showed some inhibition, although this was lower than observed in mixed‐cell populations.
Figure 8.
Immunoglobulin M (IgM) synthesis by lymphocytes and the effects of two reagents that block intercellular communication across gap junctions. In (a), a mixed population of lymphocytes was used (♦) and the effects of GAP27 (300 µg/ml) (▴) or α‐glycyrrhetinic acid (150 µm) (▪) were determined over 48 hr. In (b), a phytohaemagglutinin‐L (PHA‐L)‐stimulated mixed population of lymphocytes was used (♦) and the effects of the two gap junction inhibitors, as described in (a), were determined. In (c), the effects on IgM production by mixed lymphocyte populations (▪) of two connexin intracellular peptides, viz GAP18 (□) or GAP20 (◊) were examined. In (d), a B‐cell population was used (◊) and the effects of GAP27 (▴) and α‐glycyrrhetinic acid (▪) were examined. Error bars show the level of variability among individual cultures (n = 16). The experiment was repeated eight times with similar results obtained each time.
Discussion
The present studies describe the distribution of connexins in the major human lymphocyte subpopulations and identify new roles for these surface proteins that, when assembled into gap junctions, facilitate intercellular adhesion and communication during the immune response. We demonstrated mRNA encoding Cx43 in systemic and tonsil‐derived T, B and NK lymphocytes and showed that protein expression occurred in T and B lymphocytes. Lymphocytes stimulated with the mitogens PHA and LPS up‐regulated the expression of connexins. However, it appears that connexin expression is differentially regulated in the peripheral blood and secondary lymphoid tissue because, in the latter, Cx40 was also expressed. It is concluded that the connexins detected were assembled into connexon hemichannels and gap junctions that provide pathways for the dye transfer demonstrated to occur between lymphocytes. However, the extent of intercellular dye transfer was not influenced by PHA‐L and LPS stimulation under the experimental conditions used, although the same mitogens increased the overall amount of connexins expressed.
Direct communication between lymphocytes was first shown by electrophysiological measurements and metabolic co‐operation studies in PHA‐stimulated peripheral blood mononuclear cells.17,42 Subsequently, several reports pointed to the existence of gap junction communication in primary and secondary lymphoid tissues,20,23,25,43,44 and gap junction plaques were detected by conventional and freeze‐fracture electron microscopy, especially when mitogen‐stimulated lymphocytes were used.20,22,25,44 A role for gap junctional intercellular communication during inflammation, and its regulation by proinflammatory mediators, has been proposed.45–47 All these observations indicate that cell‐to‐cell contact, direct communication and co‐operation may play a mandatory role in lymphocyte biology.
Analysis of lymphocyte subsets indicated that Cx43 protein expression at the plasma membrane level was higher in T, B and NK lymphocytes purified from the systemic circulation than from cells obtained from secondary lymphoid tissue although, as described above, Cx40 expression was only detected in lymphocytes isolated from tonsils. This differential regulation of connexin expression may be one of the consequences of the microenvironment, because gap junction communication between contacting cells is regulated by cell density and proliferation.48 Heterotypic gap junction channels constructed from Cx43 and Cx40 are non‐functional, as demonstrated in Xenopus oocytes and HeLa cells.9 However, the formation of heterotypic channels by these two connexins has recently been demonstrated in smooth muscle cells, with unique gating and conductance properties raising further physiological possibilities for information exchange across such channels.49
T and B lymphocytes up‐regulated the in vitro expression of Cx40 and Cx43 proteins in a time‐dependent manner when stimulated with PHA‐L and LPS, respectively, a procedure known to promote mitosis as well as the increased exchange of metabolites between cells.42 Western blot analysis showed that the increase in Cx43 protein expression was mainly as the phosphorylated isoforms and therefore progressive changes in Cx43 expression and phosphorylation may be related to the in vitro activity of these mitogens, as described for other proteins.50–54
Up‐regulation by mitogens of connexin protein synthesis in whole‐cell extracts may not be proportional to the amount of protein assembled into functioning connexon hemichannels at the plasma membrane level, especially under mitogen stimulation, because cells maintain a balance between intracellular stores of connexins and those assembled into gap junctions.30,31 A number of other studies have shown a reduction in gap junction‐mediated communication between mitotic and non‐mitotic cells,55,56 and a mitosis‐specific species of Cx43 was described in human umbilical vein endothelial cells and smooth muscle cells.57
The present results indicate that connexin synthesis and turnover are further component processes that reflect the functional fate of lymphocytes, especially during the polarization of the immune response. An increase in Cx43 in lymph node secondary follicles has been described in mice repeatedly challenged with lysozyme.44 In these mice, the secondary follicles contained enlarged follicular dendritic cells with prominent Cx43 gap junctions. A similar high density of gap junctions was found in human tonsils, especially in the light zone of the germinal centres where B lymphocytes undergo functional maturation.44 In the present work it was noted that tonsil‐isolated CD4+ T helper phenotypic cells modified their surface expression of connexin during blastic transformation, whereas CD8+ T‐cytotoxic phenotypic lymphocytes showed no change in connexin expression after 12 hr of stimulation. As B lymphocytes also modified their connexin surface expression under the same conditions, these results suggest that connexins participate in regulatory mechanisms that underwrite T‐cell‐dependent B‐cell interaction and maturation in the context of the immune response. The possibility thus arises that connexin synthesis and turnover may regulate T‐ and B‐lymphocyte co‐operation in secondary lymphoid organs, possibly in conjunction with the cellular activation involving specific lymphocyte cell co‐operation mechanisms.
Gap junctions appear to function in lymphocytes to provide a mechanism for basal cell‐to‐cell ‘cross‐talk’ because no effect, at least at the level of the directionality of dye transfer, was observed between heterotypic or homotypic cultures of T and B lymphocytes. Also, the extent of dye transfer was largely unaffected by mitogens despite the fact that mitogens stimulated the overall connexin composition of lymphocytes. Relationships between dye transfer and plaque size, as well as the ratio of internal connexin stores to gap junctions, are complex and unresolved issues. A significant decrease in dye transfer across gap junction channels was observed in the presence of a peptide with a sequence homologous to a region of the second extracellular loop of Cx43, thereby providing strong evidence for the implication of gap junctions. This connexin mimetic peptide has been shown to inhibit gap junction‐dependent artery oscillations,38 intercellular propagation of calcium waves across gap junctions32 as well as dye coupling in HeLa cells expressing various recombinant connexins.58 These connexin mimetic peptides have been proposed to function either by inhibiting the assembly of new gap junctions by impairing the docking of the extracellular loops of connexons accreting into plaques involved in this process, or more directly by induction of a conformational change in formed gap junctions that results in channel closure.37–39 A further gap junction inhibitor, α‐glycyrrhetinic acid,34 also inhibited dye transfer. Although its specific mechanism of action on gap junction functionality is less clear, it may influence Cx43 phosphorylation status.59 The fact that the most pronounced effects of the gap junction inhibitors on dye transfer communication between lymphocytes occurred when T lymphocytes acted as dye donor cells further supports the hypothesis that T cells regulate direct lymphocyte intercellular communication through gap junctions. As lymphocyte activation triggers important metabolic signalling pathways, the possible involvement of direct cell communication through gap junctions, thereby allowing the intercellular transfer of second messengers,60–62 such as IP3, cAMP and Ca2+, is a further implication of the present work.
The significant reduction of the in vitro synthesis of IgM, by gap junction inhibitors, especially in co‐cultures of T and B lymphocytes stimulated with mitogens, has immunological and inflammatory disease consequences. These results argue strongly for the unforeseen importance of gap junctional communication in lymphocyte co‐operativity underlying immunoglobulin production. The inhibitory effect of the connexin mimetic peptides and α‐glycyrrhetinic acid on IgM secretion mirrored their effects on dye transfer discussed above.
Important questions remain, such as the nature of maturation events or microenvironmental factors that trigger the expression, for example, of Cx40 in the secondary lymphoid organ and the contribution to lymphocyte function of each of the connexins described. Studies on the role of lymphocyte gap junction communication during inflammatory cell recruitment and antigen‐driven effector mechanisms will also contribute to the hypothesis that connexins and gap junctional communication are key mechanisms in the operation of immune networks.
In summary, the results of this work represent the first detailed characterization of connexins that are involved in cell–cell communication in lymphocyte subpopulations. Two independent lines of functional evidence for gap junction communication in purified human T, B and NK lymphocytes, derived from peripheral blood and tonsils, are presented. The differential regulation of Cx40 and Cx43 expression may be related to requirements for cell cooperation in various lymphatic microenvironments. The results suggest that connexins will now require consideration as a component of the molecular mechanisms underlying lymphocyte differentiation and function in the immune response.
Acknowledgments
We thank Dr Mario Labetta (Department of Medicine, UWCM, Cardiff, UK), Dr Graciela Sala‐Newby (Bristol Heart Institute, University of Bristol, Bristol, UK) and Dr Philippe Gasque (Department of Medical Biochemistry, UWCM) for helpful discussions. We also thank Janet Fisher and Dr John Giddings (Department of Haematology, UWCM) for their invaluable technical assistance and discussions. We thank the Wales Office of Research and Development for Health and Social Care for their grant support (grant WS/97/1/013).
Glossary
Abbreviations
- Cx
connexins
- LPS
lipopolysaccharide
- PHA
phytohaemagglutinin
References
- 1.Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins – a family of cell‐surface receptors. Cell. 1987;48:549. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Adhesion receptors of the immune‐system. Nature. 1990;346:425. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 4.Dianzani U, Malavasi F. Lymphocyte adhesion to endothelium. Crit Rev Immunol. 1995;15:167. doi: 10.1615/critrevimmunol.v15.i2.40. [DOI] [PubMed] [Google Scholar]
- 5.Crocketttorabi E, Fantone JC. The selectins: insights into selectin‐induced intracellular signaling in leukocytes. Immunol Res. 1995;14:237. doi: 10.1007/BF02935622. [DOI] [PubMed] [Google Scholar]
- 6.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 7.Evans WH. Intercellular communication. the roles and structure of gap junctions. In: Bittar EE, Bittar N, editors. Principles of Medical Biology. 7B. Greenwich, CT: JAI Press Inc.; 1997. p. 591. [Google Scholar]
- 8.Sosinsky GE. Molecular organization of gap junction membrane channels. J Bioenerg Biomembr. 1996;28:297. doi: 10.1007/BF02110106. [DOI] [PubMed] [Google Scholar]
- 9.White TW, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembr. 1996;28:339. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- 10.Brissette JL, Kumar NM, Gilula NB, Hall JE, Dotto GP. Switch in gap junction protein expression is associated with selective changes in junctional permeability during keratinocyte differentiation. Proc Natl Acad Sci USA. 1994;91:6453. doi: 10.1073/pnas.91.14.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba H, Sawada N, Oyamada M, et al. Relationship between the expression of the gap junction protein and osteoblast phenotype in a human osteoblastic cell‐line during cell‐proliferation. Cell Struct Funct. 1993;18:419. doi: 10.1247/csf.18.419. [DOI] [PubMed] [Google Scholar]
- 12.Veenstra RD, Wang HZ, Westphale EM, Beyer EC. Multiple connexins confer distinct regulatory and conductance properties of gap‐junctions in developing heart. Circ Res. 1992;71:1277. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- 13.Lowenstein WR, Rose B. The cell–cell channel in the control of growth. Semin Cell Dev Biol. 1992;3:59. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- 14.Tomasetto C, Neveu MJ, Daley J, Horan PK, Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol. 1993;122:157. doi: 10.1083/jcb.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 16.Kupfer H, Monks CRF, Kupfer A. Small splenic B‐cells that bind to antigen‐specific T‐helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate – immunofluorescence microscopic studies of Th–B antigen‐presenting cell‐interactions. J Exp Med. 1994;179:1507. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulser DF, Petters JH. Contact cooperation in stimulated lymphocytes. Exp Cell Res. 1972;74:319. doi: 10.1016/0014-4827(72)90383-7. [DOI] [PubMed] [Google Scholar]
- 18.Levy JA, Weiss RM, Dirksen ER, Rosen MR. Possible communication between murine macrophages oriented in linear chains in tissue culture. Exp Cell Res. 1976;103:375. doi: 10.1016/0014-4827(76)90273-1. [DOI] [PubMed] [Google Scholar]
- 19.Porvaznik M, MacVittie TJ. Detection of gap junctions between the progeny of a canine colony‐forming cell in vitro. J Cell Biol. 1979;82:555. doi: 10.1083/jcb.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves LA, Decarvalho ACC, Lima EOC, et al. Functional gap‐junctions in thymic epithelial‐cells are formed by connexin‐43. Eur J Immunol. 1995;25:431. doi: 10.1002/eji.1830250219. [DOI] [PubMed] [Google Scholar]
- 21.Neumark T, Huynh DC. Gap junctions between human T‐colony cells. Acta Morph Hungary. 1989;37:147. [PubMed] [Google Scholar]
- 22.Kapsenberg ML, Leene W. Formation of B type gap junctions between PHA‐stimulated rabbit lymphocytes. Exp Cell Res. 1979;120:211. doi: 10.1016/0014-4827(79)90551-2. [DOI] [PubMed] [Google Scholar]
- 23.Dorshkind K, Green L, Godwin A, Fletcher WH. Connexin‐43 type gap‐junctions mediate communication between bone‐marrow stromal cells. Blood. 1993;82:38. [PubMed] [Google Scholar]
- 24.Rosendaal M, Green CR, Rahman A, Morgan D. Up‐regulation of the connexin43 (+) gap junction network in hematopoietic‐tissue before the growth of stem‐cells. J Cell Sci. 1994;107:29. doi: 10.1242/jcs.107.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Krenacs T, Rosendaal M. Immunohistological detection of gap‐junctions in human lymphoid‐ tissue – connexin43 in follicular dendritic and lymphoendothelial cells. J Histochem Cytochem. 1995;43:1125. doi: 10.1177/43.11.7560895. [DOI] [PubMed] [Google Scholar]
- 26.Jara PI, Boric MP, Saez JC. Leukocytes express connexin‐43 after activation with lipopolysaccharide and appear to form gap‐junctions with endothelial‐ cells after ischemia‐reperfusion. Proc Natl Acad Sci USA. 1995;92:7011. doi: 10.1073/pnas.92.15.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res. 1998;83:1248. doi: 10.1161/01.res.83.12.1248. [DOI] [PubMed] [Google Scholar]
- 28.Posnett DN, McGrath H, Tam JP. A novel method for producing anti‐peptide antibodies – production of site‐specific antibodies to the T‐cell antigen receptor beta‐chain. J Biol Chem. 1988;263:1719. [PubMed] [Google Scholar]
- 29.Tam JP. Synthetic peptide vaccine design – synthesis and properties of a high‐density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin PEM, George CH, Castro C, et al. Assembly of chimeric connexin–aequorin proteins into functional gap junction channels – reporting intracellular and plasma membrane calcium environments. J Biol Chem. 1998;273:1719. doi: 10.1074/jbc.273.3.1719. [DOI] [PubMed] [Google Scholar]
- 31.Nadarajah B, Makarenkova H, Becker DL, Evans WH, Parnavelas JG. Basic FGF increases communication between cells of the developing neocortex. J Neurosci. 1998;18:7881. doi: 10.1523/JNEUROSCI.18-19-07881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boitano S, Evans WH. Inhibition of intercellular Ca2+ signalling with sequence‐specific antibodies and peptides made from gao junctions. Mol Biol Cell. 1998;9:324a. [Google Scholar]
- 33.Rahman S, Evans WH. Topography of connexin32 in rat‐liver gap‐junctions – evidence for an intramolecular disulfide linkage connecting the 2 extracellular peptide loops. J Cell Sci. 1991;100:567. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg GS, Bechberger JF, Naus CCG. A pre‐loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490. [PubMed] [Google Scholar]
- 35.Davison JS, Baumgarten IM. Glycyrrhetinic acid derivates: a novel class of inhibitors of gap‐junctional intercellular communication. Structure–activity relations. J Pharmacol Exp Ther. 1988;246:1104. [PubMed] [Google Scholar]
- 36.Davison JS, Baumgarten IM, Harley EH. Reversible inhibition of intracellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun. 1986;134:29. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- 37.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium‐dependent relaxations of rabbit arteries. J Physiol (London) 1998;508:561. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries (Vol. 503, p. 99, 1997) J Physiol (London) 1997;503:699. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutcheson IR, Chaytor AT, Evans WH, Griffith TM. Nitric oxide independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication – role of gap junctions and phospholipase A(2) Circ Res. 1999;84:53. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- 40.Haefliger JA, Castillo E, Waeber G, et al. Hypertension increases connexin43 in a tissue‐specific manner. Circulation. 1997;95:1007. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HJ, Chaytor AT, Evans WH, Griffith TM. Inhibition of the gap junctional component of endothelium‐dependent relaxations in rabbit iliac artery by 18‐alpha glycyrrhetinic acid. Br J Pharmacol. 1998;125:1. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveracastro GM, Barcinski MA, Cukierman S. Intercellular communication in stimulated human lymphocytes. J Immunol. 1973;111:1616. [PubMed] [Google Scholar]
- 43.Rosendaal M, Mayen A, dekoning A, Duninabarkovskaya T, Krenacs T, Ploemacher R. Does transmembrane communication through gap junctions enable stem cells to overcome stromal inhibition? Leukemia. 1997;11:1281. doi: 10.1038/sj.leu.2400744. [DOI] [PubMed] [Google Scholar]
- 44.Krenacs T, Vandartel M, Lindhout E, Rosendaal M. Direct cell/cell communication in the lymphoid germinal center: connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur J Immunol. 1997;27:1489. doi: 10.1002/eji.1830270627. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, Cotgreave IA. Differential regulation of gap junctions by proinflammatory mediators in vitro. J Clin Invest. 1997;99:2312. doi: 10.1172/JCI119410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hillis GS, Duthie LA, Brown PAJ, Simpson JG, MacLeod AM, Haites NE. Upregulation and co‐localization of connexin43 and cellular adhesion molecules in inflammatory renal disease. J Pathol. 1997;182:373. doi: 10.1002/(SICI)1096-9896(199708)182:4<373::AID-PATH858>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 47.Hillis GS, Duthie LA, Mlynski R, et al. The expression of connexin 43 in human kidney and cultured renal cells. Nephron. 1997;75:458. doi: 10.1159/000189585. [DOI] [PubMed] [Google Scholar]
- 48.Larson DM, Wrobleski MJ, Sagar GDV, Westphale EM, Beyer EC. Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF‐beta 1. Am J Physiol‐Cell Physiol. 1997;41:C405. doi: 10.1152/ajpcell.1997.272.2.C405. [DOI] [PubMed] [Google Scholar]
- 49.He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1999;96:6495. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juan G, Gruenwald S, Darzynkiewicz Z. Phosphorylation of retinoblastoma susceptibility gene protein assayed in individual lymphocytes during their mitogenic stimulation [Full text delivery] Exp Cell Res. 1998;239:104. doi: 10.1006/excr.1997.3885. [DOI] [PubMed] [Google Scholar]
- 51.Gao JJ, Fultz MJ, Vogel SN, Russell SW, Murphy WJ. LPS stimulates Stat1 alpha phosphorylation in mouse macrophages without autocrine feedback by interferon‐gamma. FASEB J. 1996;10:892. [Google Scholar]
- 52.Aramaki Y, Matsuno R, Nitta F, Arima H, Tsuchiya S. Negatively charged liposomes inhibit tyrosine phosphorylation of 41‐kDa protein in murine macrophages stimulated with LPS [Full text delivery] Biochem Biophys Res Commun. 1997;231:827. doi: 10.1006/bbrc.1996.5999. [DOI] [PubMed] [Google Scholar]
- 53.Tanigawa S, Rees R, Welsh M, Gilmont R. Differential tissue expression and phosphorylation of hsp 27 in LPS treated rats. Mol Biol Cell. 1997;8:2133. [Google Scholar]
- 54.Kirken RA, Malabarba MG, Xu J, et al. Prolactin stimulates serine/tyrosine phosphorylation and formation of heterocomplexes. J Biol Chem. 1997;272:14 098. doi: 10.1074/jbc.272.22.14098. [DOI] [PubMed] [Google Scholar]
- 55.Goodall H, Maro B. Major loss of junctional coupling during mitosis in early mouse embryos. J Cell Biol. 1986;102:568. doi: 10.1083/jcb.102.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stein LS, Boonstra J, Burghardt RC. Reduced cell–cell communication between mitotic and nonmitotic coupled cells. Exp Cell Res. 1992;198:1. doi: 10.1016/0014-4827(92)90141-t. [DOI] [PubMed] [Google Scholar]
- 57.Xie HQ, Laird DW, Chang TH, Hu VW. A mitosis‐specific phosphorylation of the gap junction protein connexin43 in human vascular cells: biochemical characterization and localization. J Cell Biol. 1997;137:203. doi: 10.1083/jcb.137.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.George CH, Martin PEM, Evans WH. Rapid determination of gap junction formation using HeLa cells microinjected with cDNAs encoding wild‐type and chimeric connexins. Biochem Biophys Res Commun. 1998;247:785. doi: 10.1006/bbrc.1998.8835. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg GS, Moreno AP, Bechberger JF, et al. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res. 1996;222:48. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- 60.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 61.Kam Y, Kim DY, Koo SK, Joe CO. Transfer of second messengers through gap junction connexin 43 channels reconstituted in liposomes. Biochim Biophys Acta‐Biomembr. 1998;1372:384. doi: 10.1016/s0005-2736(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki H. Cellular and molecular methods to study the role of gap junctional intercellular communication in toxicology. Toxicol Vitro. 1997;11:535. doi: 10.1016/s0887-2333(97)00052-0. [DOI] [PubMed] [Google Scholar]