Abstract
Lymphocyte recruitment into tissues involves interactions between adhesion molecules on vascular endothelial cells and corresponding ligands on the lymphocyte surface. In the present study we investigated the expression of four endothelial addressins in the vagina and their possible up‐regulation by interferon‐γ (IFN‐γ) in immune mice after vaginal challenge with herpes simplex virus type 2. The adhesion molecules intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1) were minimally expressed in the vagina of non‐immune mice with or without vaginal challenge and in immune mice before challenge, but both were up‐regulated by IFN‐γ, directly or indirectly, in immune mice after challenge. Mucosal addressin cell adhesion molecule‐1 (MAdCAM‐1) was detected in most vaginas but was not up‐regulated by IFN‐γ in immune mice after virus challenge. E‐selectin was not detected in any vaginas. The results suggest that ICAM‐1 and VCAM‐1 may be involved in rapid, IFN‐γ‐mediated recruitment of lymphocytes to the vaginal mucosal of immune mice after local virus challenge.
Introduction
Recent studies have demonstrated a memory T‐cell‐dependent secretion of interferon‐γ (IFN‐γ) in the vagina of herpes simplex virus type 2 (HSV‐2)‐immune mice within 8 hr after vaginal challenge with virus.1,2 The IFN‐γ secretion coincided with a rapid increase (within 8 hr) in the number of lymphocytes in the vagina (approximately 20‐fold). Neutralization of the IFN‐γ in vivo with monoclonal antibody eliminated the cytokine from vaginal secretions, increased replication of challenge virus in the vaginal epithelium, blocked recruitment of T lymphocytes to the vagina, and inhibited recruitment of B lymphocytes.1 Rapid recruitment of T and B lymphocytes to a site of antigen challenge in immunized animals is not currently recognized as a function of IFN‐γ.3–7 Nevertheless, our data indicate that IFN‐γ was secreted in the vagina of immune mice after local HSV‐2 challenge and was responsible for rapid recruitment of large numbers of additional T and B lymphocytes to the vagina.
The lymphocytes that were recruited to the vagina appeared to be derived from the blood, because large numbers of lymphocytes were adherent to the endothelium of small veins in the vagina of immune mice after virus challenge, but lymphocytes were virtually absent from such vessels in immune mice without challenge. In immune mice that were pretreated with anti‐IFN‐γ before vaginal challenge with virus, T lymphocytes were virtually absent from the vessels and B‐cell numbers were reduced. Recruitment of leucocytes into tissues is controlled by the vascular endothelium through its expression of adhesion molecules. Leucocyte recruitment from the blood involves multiple steps: an initial contact or rolling step that is mediated by primary adhesion receptors; chemokine or chemoattractant activation of secondary adhesion receptors; firm attachment; and transendothelial migration.5,8 Regulation at any one of these steps can confer selectivity for a particular leucocyte subset. The ability of cytokines to influence leucocyte–endothelial cell interactions and to modulate leucocyte recruitment can be an important mechanism by which cytokines control inflammation and immune responses. In particular, IFN‐γ has been reported to modify endothelial cell morphology in vitro,9 to increase expression of intercellular adhesion molecule‐1 (ICAM‐1)10,11 and E‐selectin12 on cultured endothelial cells, to increase binding of lymphocytes to endothelial cells in vitro,12–14 to stimulate lymphocyte migration into skin,15,16 and to mediate lymphocyte recruitment into sites of delayed‐type hypersensitivity (DTH) reactions.15 Currently, however, little information is available concerning the specific endothelial cell addressins that are regulated in vivo by IFN‐γ to mediate lymphocyte recruitment in particular inflammatory reactions. In the present study we investigated the expression of four endothelial cell addressins in the vagina and their regulation by IFN‐γ.
Materials and methods
Animals and virus
Female BALB/c mice were purchased from Harlan/Sprague‐Dawley (Indianapolis, IN) and were 10–20 weeks old when used. They were housed in compliance with all institutional and federal animal welfare requirements, and all experimental procedures were approved by the institutional Animal Care and Use Committee. The mice were used in a previous study that involved in vivo depletion of IFN‐γ.1 Wild‐type TK+ HSV‐2 and attenuated ΔTK– HSV‐2, a strain that contains a partial deletion of the thymidine kinase gene, were generously provided by Dr Mark McDermott, McMaster University, Hamilton, Canada.17,18
Vaginal immunization and challenge
Mice to be immunized were pretreated with 2·0 mg of Depo‐Provera® (DP) (Upjohn Co., Kalamazoo, MI) in phosphate‐buffered saline (PBS) subcutaneously. Six days later they were immunized by intravaginal (i.vag.) inoculation of 20 µl of attenuated HSV‐2 at 1·5 × 106 plaque‐forming units (PFU)/ml. Five weeks later, the immunized and age‐matched non‐immune mice were treated with DP. Six days later, most of the mice in each group were challenged by i.vag. inoculation of 20 µl of wild‐type HSV‐2 at 3·5 × 106 PFU/ml. The immune/challenged mice were killed at 8, 16, 24, 48 and 96 hr after challenge. Non‐immune/challenged mice were killed at 24, 32, 48, and 96 hr after challenge. The remaining immune and non‐immune mice were not challenged with virus (the 0 hr groups). A total of 49 immune mice and 40 non‐immune mice were used, with 5–10 mice per group.
In vivo depletion of IFN‐γ
The hybridoma cell line R4‐6A2 (rat anti‐mouse IFN‐γ) was purchased from ATCC (Rockville, MD), and ascites fluid containing the monoclonal antibody was produced by TSD BioServices (Germantown, NY). The rat immunoglobulin G (IgG) concentration in the ascites was 2·0 mg/ml. For in vivo depletion of IFN‐γ, 10 additional immunized mice received 0·5 ml of ascites intraperitoneally 17 hr before vaginal challenge with HSV‐2. This treatment has been shown to block recruitment of both CD4+ and CD8+ T cells to the vagina of immune mice after virus challenge, and to block up‐regulation of major histocompatibility complex (MHC) class II antigens in the vaginal epithelium of such mice.1 In contrast, injection of anti‐CD4 ascites had no effect on CD8+ cell recruitment, anti‐CD8 ascites had no effect on CD4+ cell recruitment, and neither ascites was able to block up‐regulation of MHC class II antigens in the epithelium.19 Thus, the effect of anti‐IFN‐γ ascites on T‐cell recruitment appears to be due to antibody neutralization of IFN‐γ rather than to non‐specific effects of ascites fluid, and no non‐immune or irrelevant ascites pretreatment was used in the present study.
Tissues
For immunolabelling of vascular cell adhesion molecule‐1 (VCAM‐1), ICAM‐1, and mucosal addressin cell adhesion molecule‐1 (MAdCAM‐1), vaginas were fixed with 2% paraformaldehyde in 0·1 m phosphate buffer, pH 7·4 (4°, 2 hr), washed with PBS containing 10% sucrose (4°, 2 hr), embedded in optimum cutting temperature (OCT; Tissue‐Tek, Miles Scientific, Naperville, IL), frozen in isopentane cooled with liquid nitrogen, and stored at −70° until needed. Cryostat sections (5 µm) were mounted on silanized slides, air dried, and stored with desiccant at −20° until needed. For immunolabelling of E‐selectin, portions of vaginas were embedded in OCT without paraformaldehyde fixation and frozen as above; cryostat sections were then fixed 10 min in acetone before labelling.
Immunolabelling
For VCAM‐1 labelling, sections of vagina were blocked in 2% fetal calf serum, incubated in monoclonal rat anti‐mouse CD106 (VCAM‐1, PharMingen, San Diego, CA), washed in PBS, treated with 0·5% hydrogen peroxide in methanol, washed in PBS, incubated in biotinylated goat anti‐rat IgG (Vector Labs Inc., Burlingame, CA) followed by streptavidin–peroxidase (Zymed Labs Inc., San Francisco, CA), and exposed to substrate (AEC kit; Zymed Labs). Non‐immune and immune mice without challenge and at all times after challenge were compared using this three‐step staining procedure. Alternately, the effect of in vivo neutralization of IFN‐γ on VCAM‐1 (and MAdCAM‐1) was evaluated by staining with biotinylated antibodies, as rat anti‐mouse IFN‐γ in the interstitial space caused high background staining with the three‐step procedure. Thus, sections were treated as above except that a biotinylated rat anti‐mouse CD106 (PharMingen) was used in place of the primary and secondary antibodies. This staining method was used to compare the ascites‐treated and untreated immune mice at 24 hr after challenge. For ICAM‐1 labelling, sections were immersed in 0·10 m citrate buffer, pH 6·8, and heated in a 780 W microwave oven at 30% power until the buffer temperature reached 60° (approximately 1 min). Sections were then incubated in goat anti‐mouse ICAM‐1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), biotinylated rabbit anti‐goat IgG (Vector Labs Inc.), and streptavidin–peroxidase. The pretreatment in heated citrate buffer increased the intensity of staining. MAdCAM‐1 was labelled using biotin anti‐mouse MAdCAM‐1 (MECA‐89; PharMingen) or rat anti‐mouse MAdCAM‐1 (MECA‐367; PharMingen) that was conjugated in our laboratory to biotin‐X‐NHS (Calbiochem Corp., La Jolla, CA). E‐selectin was labelled as described for VCAM‐1 except that the primary antibody was monoclonal rat anti‐mouse E‐selectin, clone 10E9.6 (PharMingen), shown by the manufacturer to detect E‐selectin on histological sections of inflamed tissues by immunostaining and used on cryostat sections of unfixed tissues as recommended. Specificity of labelling was indicated by the absence of staining when irrelevant rat IgG, normal goat serum, or irrelevant biotinylated antibody was substituted for the primary antibodies. Sections were counterstained with Gill’s haematoxylin and mounted in Gelmount (Biomeda Corp., Foster City, CA).
Results
ICAM‐1
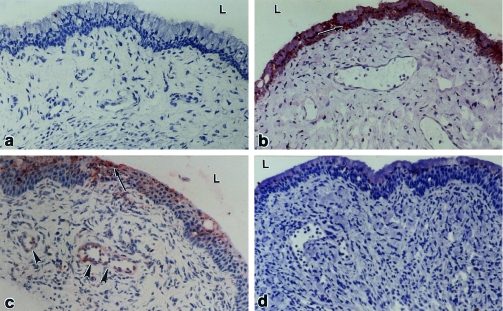
Little, if any, immunostaining of ICAM‐1 was observed in vaginas of non‐immune or immune mice that were not challenged with virus or in vaginas of non‐immune mice at any time after challenge (Table 1; Fig. 1a). In contrast, prominent labelling of ICAM‐1 was present in basal cells of the vaginal epithelium in immune mice at 8 and 16 hr after challenge (Fig. 1b) and in the mucous epithelial cells at 16, 24 (Fig. 1c), and 48 hr. Weak labelling of ICAM‐1 was also observed in the endothelium of a few small venules located close to the vaginal epithelium at 24 hr after challenge (Fig. 1c), but not at any other time. The epithelial and endothelial staining at 24 hr was markedly diminished by in vivo neutralization of IFN‐γ (Fig. 1d).
Table 1.
Expression of adhesion molecules on vascular endothelium in vagina of immune mice after vaginal challenge with HSV‐2
| Time (h) after challenge | |||||||
|---|---|---|---|---|---|---|---|
| Adhesion molecules | 0* | 8 | 16 | 24 | 24† | 48 | 96 |
| ICAM‐1 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| VCAM‐1 | 0/+ | ++/+++ | +++ | +++ | 0/+ | NA | ++ |
| MAdCAM‐1 | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ | ++ | + |
The 0 hr group was not challenged with virus. Labelling of adhesion molecules in this group was similar to that observed in non‐immune mice without challenge and in non‐immune mice at 24, 32, 48, and 96 hr after challenge. A total of 49 immune mice not treated with antibody to IFN‐γ, 10 immune mice pretreated with IFN‐γ antibody, and 40 non‐immune mice were used, with 5–10 mice per group. Data indicate both the intensity and extent of immunolabelling, with 0 being none and +++ the darkest. Staining was consistent within groups, and comparisons were done at least twice for each antibody using sections that were stained at the same time.
Mice were treated with anti‐IFN‐γ 17 hr prior to challenge.
Figure 1.
ICAM‐1 in the vagina. No labelling was detected in vaginas from non‐immune/non‐challenged mice (a). Labelling was apparent in the basal layer of the vaginal epithelium (arrows) in immune mice at 8 hr after challenge (b) and in the mucous layer of the epithelium (arrows) and in blood vessels (arrowheads) in the stroma at 24 hr after challenge (c). In immune mice that were pretreated with anti‐IFN‐γ ascites 17 hr before challenge, no labelling was seen in the vagina at 24 hr after challenge (d). L, lumen. ×250.
VCAM‐1
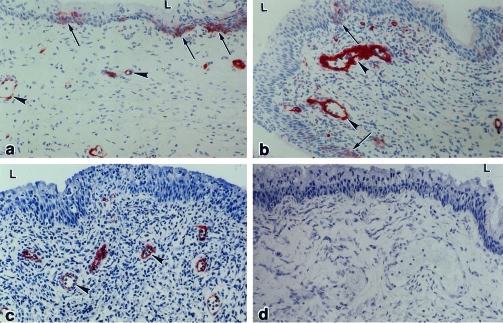
Little, if any, labelling of VCAM‐1 was observed in vaginas of non‐immune or immune mice that were not challenged with virus or in vaginas of non‐immune mice at any time after challenge (Table 1). However, labelling of VCAM‐1 was observed in the endothelial lining of small venules in the vaginal mucosa and in arterioles in the muscle layer and adventitia in immune/challenged mice from 8 to 96 hr after challenge (Fig. 2a–c). All endothelial labelling at 24 hr after challenge was abolished by in vivo neutralization of IFN‐γ (Fig. 2d). Labelling was also present in patches of basal cells in the vaginal epithelium of immune mice at 8 hr after challenge (Fig. 2b), but this staining was diminished by 16 hr (Fig. 2c).
Figure 2.
VCAM‐1 in the vagina of immune mice after challenge with wild‐type virus. At 8 hr (a) and 16 hr (b) after challenge, labelling was detected in patches of vaginal epithelium (arrows) and in the endothelium of blood vessels in the stroma (arrowheads). At 24 hr (c) after challenge, labelling was present in blood vessels but not in the vaginal epithelium. In immune mice that were pretreated with anti‐IFN‐γ ascites before challenge, no labelling was seen in the vagina at 24 hr after challenge (d). L, lumen. ×250.
MAdCAM‐1
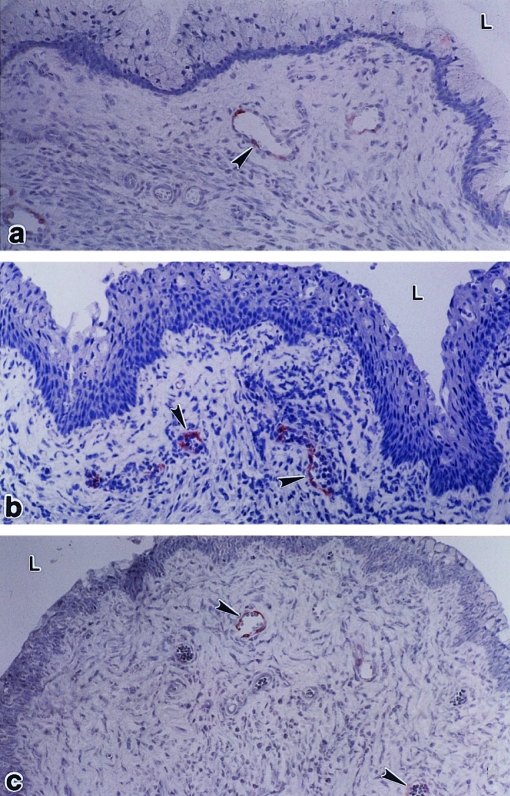
Using biotin anti‐mouse MAdCAM‐1 (clone MECA‐367), weak immunolabelling was observed in a few small venules in the vaginal stroma of non‐immune and immune mice without challenge (Fig. 3a) and in non‐immune and immune mice at several times after challenge (Table 1; Fig. 3b). The intensity and frequency of labelling were greatest in immune mice at 48 hr after challenge. Labelling was restricted to the endothelial lining of blood vessels in the stroma close to the vaginal epithelium. Treatment with anti‐IFN‐γ did not reduce staining of MAdCAM‐1 in immune mice at 24 hr after challenge (Fig. 3b–c). No labelling was found in any vagina using biotinylated anti‐mouse MAdCAM‐1 (clone MECA 89).
Figure 3.
MAdCAM‐1 in the vagina. Faint labelling was seen in blood vessels (arrowheads) in the vaginas of immune mice without challenge (a), in immune mice at 24 hr after challenge (b), and in immune/challenged mice that were pretreated with anti‐IFN‐γ ascites (c). L, lumen. ×250.
E‐selectin
No labelling was found in any vagina using anti‐mouse E‐selectin.
Discussion
Our results indicate that vaginal challenge with HSV‐2 in immune mice triggered local memory T cells to secrete IFN‐γ that in turn, directly or indirectly, up‐regulated ICAM‐1 and VCAM‐1 expression on vascular endothelium in the vagina. This appears to be the first direct evidence that IFN‐γ can up‐regulate VCAM‐1 expression in vascular endothelium. Expression of both adhesion molecules was observed on the endothelium of postcapillary venules, the vessels in which lymphocyte adherence was observed in a previous study.1 Either or both of the adhesion molecules might thus be involved in the rapid, IFN‐γ‐mediated recruitment of T and B lymphocytes to the vagina that was demonstrated in these same mice.1 Studies using in vivo blocking of the adhesion molecules with monoclonal antibodies are currently in progress to determine whether they are in fact responsible for the lymphocyte recruitment.
The suggestion that ICAM‐1 and/or VCAM‐1 may be involved in lymphocyte recruitment in the vagina of immune/challenged mice is consistent with previous observations involving different inflammatory reactions. Thus, IFN‐γ up‐regulated ICAM‐1 expression on vascular endothelium when injected directly into the dermis in humans20 and when added to the medium supporting skin organ cultures.21 Antigen challenge in the skin of immune sheep induced rapid expression of VCAM‐1 on vascular endothelium.22 In systemically immunized and aerosol‐challenged guinea‐pigs, T‐cell numbers in the bronchial wall were increased about twofold at 24 hr after challenge, and in vivo administration of antibody to very late antigen‐4 (VLA‐4), the lymphocyte ligand for endothelial VCAM‐1, prevented the increase.23 Similar studies in mice showed that antibodies to either VCAM‐1 or VLA‐4 inhibited T‐cell recruitment to lungs of immune mice after antigen challenge, but antibodies to ICAM‐1 or its ligand, lymphocyte function‐associated antigen‐1 (LFA‐1), had less effect.24 Neutralization of IFN‐γ in vivo caused a 50–90% reduction in lymphocyte recruitment to the site of a DTH reaction,15 and in vivo treatment with antibodies to VCAM‐1, LFA‐1, or VLA‐4 inhibited DTH reactions.25–27
E‐selectin is an inducible adhesion molecule that mediates selective homing of lymphocytes to inflamed skin but not to other inflamed tissues.28 It is not expressed on the endothelial cells of normal tissues,29–31 but it is rapidly induced in skin by inflammatory cytokines including interleukin‐1 (IL‐1), tumour necrosis factor‐α (TNF‐α), and lipopolysaccharide (LPS), and this expression is enhanced and prolonged by IFN‐γ.30,31 E‐selectin is prominently expressed in skin during DTH reactions and dermatoses of immunological aetiology, and it is expressed in lymph node high endothelial venules during acute granulomatous lymphadenitis.29,32 E‐selectin has been shown to mediate neutrophil adhesion, leucocyte migration into DTH reactions, binding of a subset of memory T cells to dermal endothelium, and recruitment of T helper 1 (Th1) but not Th2 cells into inflamed skin.12,27,32−34 We did not detect E‐selectin in any of the vaginal tissues we examined using a sensitive immunostaining procedure, indicating that its expression on non‐inflamed vaginal endothelial cells was nil or very weak and that it was not up‐regulated during the period of rapid lymphocyte recruitment in the immune/challenged mice. This suggests that E‐selectin probably does not play a significant role in the IFN‐γ‐mediated recruitment of lymphocytes to the vagina.
MAdCAM‐1 is an endothelial adhesion molecule that is expressed in the normal intestine and its associated lymphoid tissues35–37 but not in muscle, brain, lung, salivary glands, uterus or heart.36,37 In vivo administration of monoclonal antibody to integrin alpha 4 beta 7, the lymphocyte ligand that binds MAdCAM‐1, blocked lymphocyte adhesion to mesenteric lymph nodes and T cell migration to Peyer’s patches.38 The present study revealed constitutive expression of MAdCAM‐1 in the endothelium of vaginal blood vessels, followed by up‐regulation in immune mice at 48 hr after challenge. The in vivo administration of anti‐IFN‐γ did not alter immunostaining of MAdCAM‐1 at 24 hr, indicating that this adhesion molecule probably is not directly involved in the IFN‐γ‐mediated recruitment of lymphocytes in immune/challenged mice. This leaves open the possibility that MAdCAM‐1 may participate in the continuous low level of lymphocyte migration to the vagina that is seen in non‐immune mice and in immune mice without challenge.39
Early studies of adhesion molecules in skin indicated that ICAM‐1 was induced on epidermal keratinocytes during DTH reactions,40 and studies in vitro demonstrated adhesion of T lymphoblasts to keratinocytes that was mediated by ICAM‐1 and up‐regulated by IFN‐γ.41 These observations suggested that epidermal cells may actively participate in the trafficking of lymphocytes to the skin. In the present study we observed that IFN‐γ up‐regulated ICAM‐1 and VCAM‐1 not only in the vascular endothelium but also in the vaginal epithelium. We have previously shown that lymphocytes continuously recirculate through the vaginal epithelium and that the number of such cells was markedly increased when immune mice were challenged in the vagina with HSV‐2.39 It is thus possible that ICAM‐1 and/or VCAM‐1 may be involved in the migration of lymphocytes not only in the epidermis but also through the vaginal epithelium.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HD 17337). The authors thank Dr Mark McDermott for supplying attenuated and wild‐type HSV‐2 and Sheila Scillufo and Maureen Doran for their excellent technical assistance.
Glossary
Abbreviations
- HSV‐2
herpes simplex virus‐type 2
- IFN‐γ
interferon‐γ
- ICAM‐1
intercellular cell adhesion molecule‐1
- VCAM‐1
vascular cell adhesion molecule‐1
- MAdCAM‐1
mucosal addressin cell adhesion molecule‐1
- DP
Depo‐Provera®
- PFU
plaque‐forming units
- PBS
phosphate‐buffered saline
- VLA‐4
very late antigen‐4
- LFA‐1
lymphocyte function‐associated antigen‐1
- ELISA
enzyme‐linked immunosorbent assay
- DTH
delayed‐type hypersensitivity
- and OCT
optimum cutting temperature
References
- 1.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 2.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093. [PubMed] [Google Scholar]
- 3.Nathan C. Interferon and inflammation. In: Callin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. New York: Raven Press; 1992. p. 265. [Google Scholar]
- 4.Farrar MA, Schreiber RD. The molecular cell biology of interferon‐γ and its receptor. Annu Rev Immunol. 1993;11:571. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration. The multi‐step paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Billiau A. Interferon‐γ: biology and role in pathogenesis. In: Dixon FJ, editor. Advances in Immunology. New York: Academic Press; 1996. p. 61. [DOI] [PubMed] [Google Scholar]
- 7.Dijkmans R, Billiau A. Interferon γ: a master key in the immune system. Curr Opin Immunol. 1988;1:269. doi: 10.1016/0952-7915(88)90013-1. [DOI] [PubMed] [Google Scholar]
- 8.Butcher EC. Leukocyte–endothelial cell recognition – three (or more) steps to specificity and diversity. Cell. 1991;67:1033. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 9.Ruszczak Z, Detmar M, Imcke E, Orfanos CE. Effects of rIFN alpha, beta, and gamma on the morphology, proliferation and cell surface antigen expression of human dermal microvascular endothelial cells in vitro. J Invest Dermatol. 1990;95:693. doi: 10.1111/1523-1747.ep12514496. [DOI] [PubMed] [Google Scholar]
- 10.Wellicome SM, Thornhill MH, Pitzalis C, et al. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL‐1 or lipopolysaccharide. J Immunol. 1990;144:2558. [PubMed] [Google Scholar]
- 11.Pober JS, Gimbrone MA, Lapierre LA, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893. [PubMed] [Google Scholar]
- 12.Lee K‐H, Chung K‐Y, Koh Y‐J. Memory T lymphocytes’ adherence to interferon gamma‐activated human dermal microvascular endothelial cells via E‐selectin. J Dermatol Sci. 1995;10:166. doi: 10.1016/0923-1811(95)00434-t. [DOI] [PubMed] [Google Scholar]
- 13.Yu C‐L, Haskard DO, Cavender D, Johnson AR, Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985;62:554. [PMC free article] [PubMed] [Google Scholar]
- 14.Issekutz TB. Effects of six different cytokines on lymphocyte adherence to microvascular endothelium and in vivo lymphocyte migration in the rat. J Immunol. 1990;144:2140. [PubMed] [Google Scholar]
- 15.Issekutz TB, Stoltz JM, Meide PV&d. Lymphocyte recruitment in delayed‐type hypersensitivity. J Immunol. 1988;140:2989. [PubMed] [Google Scholar]
- 16.Munro JM, Pober JS, Cotran RS. Tumor necrosis factor and interferon‐γ induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989;135:121. [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MR, Brais PLJ, Goettsche GC, Evelegh MJ, Goldsmith CH. Expression of immunity to intravaginal herpes simplex virus type 2 infection in the genital tract and associated lymph nodes. Arch Virol. 1987;93:51. doi: 10.1007/BF01313893. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MR, Smiley BJ, Brais PLJ, Rudzroga H, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:247. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JNWN, Allen MH, MacDonald DM. The effect of in vivo interferon‐gamma on the distribution of LFA‐1 and ICAM‐1 in normal human skin. J Invest Dermatol. 1989;93:439. doi: 10.1111/1523-1747.ep12284016. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths CEM, Voorhees JJ, Nickoloff BJ. Characterization of intercellular adhesion molecule‐1 and HLA‐DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989;20:617. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- 22.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue‐specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 23.Pretolani M, Ruffe C, Lape e Silva J‐R, Joseph D, Lobb RR, Vargaftig BB. Antibody to very late activation antigen 4 prevents antigen‐induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med. 1994;180:795. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function‐associated antigen 1 interacts in antigen‐induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issekutz TB. Dual inhibition of VLA‐4 and LFA‐1 maximally inhibits cutaneous delayed type hypersensitivity induced inflammation. Am J Pathol. 1993;143:1285. [PMC free article] [PubMed] [Google Scholar]
- 26.Chisholm PL, Williams CA, Lobb RR. Monoclonal antibodies to the integrin α‐4 subunit inhibit the murine contact hypersensitivity response. Eur J Immunol. 1993;23:682. doi: 10.1002/eji.1830230317. [DOI] [PubMed] [Google Scholar]
- 27.Silber A, Newman W, Reimann KA, Hendricks E, Walsh D, Ringler DJ. Kinetic expression of endothelial adhesion molecules and relationship to leukocyte recruitment in two cutaneous models of inflammation. Lab Invest. 1994;70:163. [PubMed] [Google Scholar]
- 28.Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocye homing receptor for the vascular lectin endothelial cell‐leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pober JS. Cytokine‐mediated activation of vascular endothelium; physiology and pathology. Am J Pathol. 1988;133:426. [PMC free article] [PubMed] [Google Scholar]
- 30.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenberg JFM, von Asmuth EJU, Jeunhomme TMAA, Buurman WA. IFN‐γ regulates the expression of the adhesion molecule ELAM‐1 and IL‐6 production by human endothelial cells in vitro. J Immunol. 1990;145:2110. [PubMed] [Google Scholar]
- 32.Silber A, Newman W, Sasseville VG, et al. Recruitment of lymphocytes during cutaneous delayed hypersensitivity in nonhuman primates is dependent on E‐selectin and vascular cell adhesion molecule 1. J Clin Invest. 1994;93:1554. doi: 10.1172/JCI117134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tietz W, Allemand Y, Borges E, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E‐ and P‐ selectin. J Immunol. 1998;161:963. [PubMed] [Google Scholar]
- 34.Austrup F, Vestweber D, Borges E, et al. P‐ and E‐selectin mediate recruitment of T‐helper‐1 but not T‐helper‐2 cells into inflamed tissues. Nature. 1997;385:81. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 35.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule‐1: comparison with vascular cell adhesion molecule‐1 and correlation with B7 integrins and memory differentiation. J Immunol. 1996;156:3727. [PubMed] [Google Scholar]
- 36.Briskin M, Winsor‐hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule‐1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97. [PMC free article] [PubMed] [Google Scholar]
- 37.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule‐1 (MAdCAM‐1) in acute and chronic inflammation. J Leukocyte Biol. 1999;65:349. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 38.Palecanda A, Marshall JS, Li X, Briskin MJ, Issekutz TB. Selective antibody blockade of lymphocyte migration to mucosal sites and mast cell adhesion. J Leukocyte Biol. 1999;65:649. doi: 10.1002/jlb.65.5.649. [DOI] [PubMed] [Google Scholar]
- 39.King NJC, Parr EL, Parr MB. Migration of lymphoid cells from vaginal epithelium to the iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173. [PubMed] [Google Scholar]
- 40.Dustin ML, Singer KH, Tuck DT, Springer TA. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM‐1) J Exp Med. 1988;167:1323. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickoloff BJ. Role of interferon‐γ in cutaneous trafficking of lymphocytes with emphasis on molecular and cellular adhesion events. Arch Dermatol. 1988;124:1835. [PubMed] [Google Scholar]