Abstract
A non-cognate mechanism of protection against human immunodeficiency virus-1 (HIV-1) infection involves up-regulation of β-chemokines, which bind and may down-modulate the CCR5 co-receptors, thereby preventing transmission of M-tropic HIV-1. The objective of this investigation was to evaluate this mechanism in vivo in non-human primates. Rhesus macaques were immunized by a modified targeted lymph nodes (TLN) route with recombinant simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and p27 in alum, and adsorbed recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) with either interleukin (IL)-2 or IL-4. Immunization induced significant increases in the concentrations of CD8 cell-derived suppressor factor (CD8-SF), regulated on activation normal T cells expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α and MIP-1β, and down-modulation of the proportion of cells expressing CCR5 (r = 0·737, P < 0·05). The macaques were then challenged with SIVmac 220 by the rectal mucosal route. The plasma SIVmac RNA showed a significant inverse correlation with the CD8-SF or the concentration of the three β-chemokines (r = 0·831 and 0·824, P < 0·01), but a positive correlation between the proportion of CCR5+ cells and SIVmac RNA (r = 0·613, P = 0·05). These results demonstrate for the first time in vivo that immunization up-regulates β-chemokines, which may down-modulate CCR5 co-receptors, and both functions are significantly correlated with the viral load. Hence, the non-cognate β-chemokine–CCR5 mechanism should be considered as complementary to specific immunity in vaccination against HIV.
Introduction
Mucosal human immunodeficiency virus (HIV) infection has been responsible for the predominantly heterosexual transmission in developing countries and homosexual transmission in developed countries. The genital or rectal mucosa and the draining regional lymph nodes are the primary and secondary barriers that the virus has to breach.1–3 One approach to the prevention of mucosal infection has been direct vaginal4,5 or rectal6,7 immunization with simian immunodefiency virus (SIV) antigens, but this has not achieved consistent protection. An alternative approach has been to target the iliac lymph nodes, which function as an inductive immune site for the genital and rectal mucosa.3 Immunization by the latter route with the recombinant SIV subunit envelope glycoprotein 120 (gp120) and core p27 antigens in alum resulted in either total protection or a significant decrease in viral load after challenge with a pathogenic SIV.8 The mechanism of protection was not clarified, but in addition to SIV-specific serum immunoglobulin G (IgG) and secretory immunoglobulin A (IgA) antibodies, and CD4+ T-cell proliferative responses, IgA antibody-forming B cells were demonstrated in the regional iliac lymph nodes. The novel and significant finding was an increase in secretion of the CD8-suppressor factor (CD8-SF) and the three β-chemokines – regulated on activation normal T cells expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α and MIP-1β – derived from the regional lymph nodes and peripheral blood CD8+ cells, as compared with immunized controls.8 These results suggested that in addition to cognate SIV-specific immunity, immunization can elicit CD8-SF (or CAF)9 and three β-chemokines,10 which may inhibit SIV or HIV replication by blocking the CCR5 co-receptors or inhibiting SIV transcription. Furthermore, there is in vitro evidence that RANTES or stromal-derived factor (SDF-1) chemokine down-regulates the corresponding CCR5 or CXCR4 co-receptors, respectively.11,12 These receptors are internalized within 20 min but are recycled to the cell surface during the next 20 min. If immunization up-regulated the concentration of β-chemokines, cell surface expression of CCR5 might be down-modulated in vivo. Indeed, alloimmunization in humans up-regulated the three β-chemokines and down-modulated the CCR5 co-receptors and, interestingly, only the latter was maintained for up to 1 year.13
This investigation was based on the hypothesis that immunization with SIV antigens, with or without cytokines, in vivo may, in addition to specific immunity to SIV, up-regulate CD8-SF and elicit innate immune responses by generating β-chemokines that block and down-modulate CCR5, thereby decreasing SIV transmission. In this experiment we targeted for the first time the readily accessible subcutaneous (s.c.) inguinal and external iliac lymph nodes, instead of the deep internal iliac lymph nodes, in an attempt to avoid the deep injection that may not be acceptable for use in humans. The rationale was to induce immune responses at the mucosal site of entry of SIV, in the draining lymph nodes and the circulation, in order to generate three immune barriers to the virus.
Materials and methods
Immunization of macaques
Nine mature macaques were immunized by a modified targeted lymph node (TLN) route, which involved conventional s.c. injection, but given in the inguinal region. The vaccine was administered s.c. three times at two sites, near the inguinal and external iliac lymph nodes on both sides. Immunization was carried out at approximately monthly intervals, and a fourth booster injection was given into the gluteal muscles. A group of three macaques was immunized with recombinant SIVgp120 and p27 (200 µg of each) in alum (AluGel; Uniscience, London, UK). Recombinant SIVmac 251 gp120 was expressed in Baculovirus-infected cells,14 and rSIVp27 was expressed in pGEX-3X as a glutathione-S-transferase fusion protein. A second group of three macaques was given the same vaccine but mixed with recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 µg/kg; Sandoz Pharmaceuticals, Surrey, UK) and simian interleukin (IL)-2 (5 µg/kg of each).15 The third group was given the vaccine with GM-CSF and simian IL-4 (4 µg/kg). All experimental procedures were performed under sedation with ketamine hydrochloride (10 mg/kg) and Domitor (1 mg/kg).
Serum and rectal fluid antibody assays
Samples of fluid were collected before and at monthly intervals after each immunization. Blood collected from the femoral vessels was defibrinated and the serum separated. A constant volume of ≈ 6·0 ml of rectal washings was collected without trauma, with the aid of flexible, lubricated paediatric naso-gastric tubes, as described previously.6 IgA and IgG antibodies to gp120 and p27 were determined by enzyme-linked immunosorbent assay (ELISA), as described previously.6 Briefly, plates were coated with a predetermined optimal concentration of gp120 or p27 (at 1 µg/ml) and with a random peptide 20mer (R20) as a control antigen; they were incubated with doubling dilutions of the test samples. Bound antibody was detected by incubation with rabbit IgG anti-monkey IgA (8 µg/ml; Nordic Immunological Labs, Tilbury, The Netherlands) or IgG (2 µg; Sigma Fine Chemicals Ltd, Poole, Dorset, UK), followed by incubation with affinity-purified goat anti-rabbit IgG–alkaline phosphatase conjugate (Sigma Fine Chemicals Ltd). For rectal washings (concentrated ×4), the starting samples were used neat and then diluted 1 : 2, 1 : 4, etc., whereas serum samples were diluted 1 : 100, 1 : 200, etc. Rectal washings were concentrated by lyophilization and the concentration step was taken into account when the results were calculated.
T-cell proliferative responses
T-cell cultures were set up by separating mononuclear cells from defibrinated blood, using Lymphoprep (NYCOMED, Oslo, Norway) density-gradient centrifugation, before and after immunization of all macaques.3 The cells were cultured without antigen and with 1 and 10 µg/ml of gp120, p27, a control peptide (R20) or concanavalin A (Con A) in 96-well round-bottomed plates (Costar, Cambridge, MA), containing RPMI-1640 (Gibco, Paisley, Strathclyde, UK). The results were expressed as stimulation indices (ratio of counts with and without antigen), for cultures stimulated with the optimum (10 µg/ml) gp120 or p27 concentration. All cultures yielded high stimulation indices and counts with Con A, and no significant increases in counts were seen with the control peptide (data not shown).
Assay of CD8-SF and β-chemokines in stimulated culture supernatant
Peripheral blood mononuclear cells (PBMC) were separated and CD8+ cells were enriched by depletion of CD4+ cells, B cells and monocytes by panning with CD4 (OKT4 hybridoma culture supernatant) and antibodies to human immunoglobulin (Serotec, Oxford, UK). The enriched CD8+ cells were then cultured with 10 µg/ml of phytohaemagglutinin (PHA) for 3 days. Preparation of the CD8 culture supernatant was then carried out according to a method described previously.8,9 PHA-stimulated CD8+ cells were cultured at a concentration of 3 × 106 cells/ml in RPMI-1640 containing 10% fetal calf serum (FCS) and 10% human IL-2 preparation (TLF; Biotest, Solihull, UK). After 2 days of incubation (at 37°, in 5% CO2), the culture supernatant was collected, and the cells were reconstituted with fresh medium; this was repeated up to three times. At each passage the cellular density was adjusted to 3 × 106 cells/ml. The collected supernatants were filtered through a 0·45-µm filter and stored at − 70° for CD8-SF and the β-chemokine assay. SIVmac acutely infected CD4+ cells were cultured in the presence of CD8-SF at 1 : 2 and 1 : 5 dilutions in 96-well plates. The CD8-SF were replenished every 2–3 days and on day 7 the culture supernatant was used to determine the reverse transcription (RT) activity by using the Quant RT kit (Amersham, UK). RANTES, MIP-1α, MIP-1β and macrophage chemoattractant protein-1 (MCP-1) were assayed in the CD8+ cell culture supernatant using a specific enzyme immunoassay (R & D Systems Europe Ltd, Abingdon, Oxfordshire, UK).
Cell surface expression of CCR5 by flow cytometry
Freshly isolated PBMC were incubated with monoclonal antibodies (mAb) to CCR5 (227), kindly supplied by Dr P. Gray (ICOS Corporation, Seattle, WA). The cells were incubated with fluorescein isothiocyanate (FITC)-labelled rabbit anti-mouse IgG (Dako, Glostrup, Denmark), or with the latter alone as a control, and flow cytometry was performed using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Franklin Lakes, NJ) running lysis II software for both acquisition and analysis.
Rectal mucosal challenge with SIV
Viral challenge was carried out by topical application to the rectal mucosa of 25MID50 (median monkey infectious dose) of SIVmac 220, which is a cell-free virus stock prepared from the spleen of a rhesus monkey infected with the J5 molecular clone of SIVmac 251 (32H).16
Determination of plasma SIVmac RNA and cell-associated SIV load
The plasma concentration of SIVmac RNA was determined using the SIVmac branched DNA assay of Chiron Diagnostics (Amsterdam, the Netherlands) and expressed in equivalents/ml (Eq/ml) (one equivalent is approximately one molecule of SIVmac RNA). PBMC-associated virus loads were determined by limiting-dilution analysis. Briefly, simian PBMCs were separated from whole blood by centrifugation on Ficoll Paque (Pharmacia Biotech, Bucks, UK). Cells were diluted from 106 to 4 × 105 and subsequently in fivefold steps to 130 cells, and duplicate cultures were co-cultured with the human T-cell line C8166 in 25-cm2 flasks. Medium and C8166 cells were replenished every 3–4 days, and the total culture volume was maintained at ≈ 15 ml. All cultures were kept for 30 days or until a cytopathic effect was apparent. Virus isolation was confirmed by indirect immunofluorescence using polyclonal anti-SIV serum. Fifty per cent end-points were calculated using the Karber formula, and the results were expressed as the number of infected cells per 106 PBMCs. In addition, the polymerase chain reaction (PCR) was carried out in all macaques, as described previously.17
Statistical analysis
Data from the three groups of macaques were expressed as mean ± SEM. Any relationship between the β-chemokines and CCR5 or plasma viral load, or between CCR5 or CD8-SF and the plasma viral load, was determined by calculating the correlation coefficient. The Student’s t-test was used to analyse the differences between the percentage and median fluorescence intensity (MFI) of CCR5 in immunized and unimmunized macaques.
Results
Serum antibodies to SIVgp120 and p27
Serum IgG antibody titre, and to a lesser extent IgA and secretory IgA (sIgA) antibody titres, to SIVgp120 and p27 were higher after the s.c. inguinal and external iliac immunization than after the deep internal iliac TLN route of immunization, which was added for comparison but was published previously8 (Table 1). Immunomodulation with GM-CSF and either IL-2 or IL-4 elicited an increase in serum IgG antibody titres to both SIV antigens, and surprisingly no significant variation was observed between the macaques, compared with those given the vaccine alone. However, higher IgA antibody titres to gp120 and p27 were induced with the vaccine containing GM-CSF + IL-2, compared with GM-CSF + IL-4. These results suggest that targeting the s.c. inguinal and external iliac lymph nodes with SIVgp120 and p27 in alum induces higher titres of serum antibodies than those achieved by the deep injection targeting the internal iliac lymph nodes. The antibody titres could be further enhanced by adding GM-CSF with either IL-2 or IL-4. Correlations between the plasma SIVmac RNA and serum IgG or IgA antibodies to SIVgp120 and p27 were calculated but none of the correlation coefficients reached the 5% level of significance (r = 0·476, 0·411, 0·500, 0·478, respectively). However, the variation in titres between the outbred macaques was considerable, and this was found especially with IgG antibodies to SIVp27. Neutralizing antibodies were examined by Dr D. Montefiore (Duke University, Medical Centre, Durham, NC) but no significant antibodies were detected in these macaques.
Table 1.
The modulating effect of granulocyte–macrophage colony-stimulating factor (GM-CSF) with interleukin (IL)-2 or IL-4 on subcutaneous (s.c.) inguinal and external iliac targeted lymph node (TLN) immunization with simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and protein 27 (p27) in alum (×4)
| Targeted inguinal+external iliac LN | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine (SIV gp120+p27) | Vaccine+GM-CSF+IL-4 | Vaccine+GM-CSF+IL-2 | *Targeted iliac LN vaccine | |||||
| Immune response | Pre† | Post† | Pre | Post | Pre | Post | Pre | Post |
| Serum IgG to gp120 | <50 | 147 200 (131 317) | <50 | 409 600 (0) | <50 | 409 600 (0) | <50 | 7467 (2822) |
| Serum IgA to gp120 | <50 | 8553 (2153) | <50 | 68 267 (17 067) | <50 | 341 333 (68 267) | <50 | 4800 (1600) |
| Serum IgG to p27 | <50 | 55 467 (25 953) | <50 | 546 133 (136 533) | <50 | 682 667 (136 533) | <50 | 12 800 (6400) |
| Serum IgA to p27 | <50 | 8533 (2133) | <50 | 136 533 (34 133) | <50 | 341 333 (68 267) | <50 | 3200 (1600) |
| Rectal sIgA to gp120 | <0.5 | 24.7 (19.7) | <0.5 | 0.5 (0.3) | <0.5 | 53.3 (37.3) | <0.5 | 4 (0) |
| Rectal IgG to gp120 | <0.5 | 6.0 (2.0) | <0.5 | 0.5 (0.3) | <0.5 | 6.7 (1.3) | <0.5 | 4 (0) |
| Rectal sIgA to p27 | <0.5 | 66.7 (34.7) | <0.5 | 0.5 (0.3) | <0.5 | 96 (80) | <0.5 | 4 (0) |
| Rectal IgG to p27 | <0.5 | 6.7 (1.3) | <0.5 | 0.5 (0.3) | <0.5 | 21.3 (5.3) | <0.5 | 3 (1) |
No serum (< 1 : 50) or rectal (< 1 : 0·5) antibodies were detectable before immunization. Mean titres (± SEM) of serum and rectal antibodies are given for the four groups, each comprising three macaques.
The data of macaques immunized by the deep internal and external iliac TLN route are presented, for comparative purposes, from a previous publication.8
Pre and post refer to pre- and postimmunization titres, respectively.
IgA, immunoglobulin A; IgG, immunoglobulin G; sIgA, secretory immunoglobulin A.
Rectal mucosal antibodies to SIVgp120 and p27
sIgA and IgG antibodies to SIVgp120 and p27 were raised after immunization with SIVgp120 and p27 in alum (Table 1). A further increase in titres of these antibodies was found when GM-CSF and IL-2 were adsorbed to alum and added to the vaccine, but the variation in sIgA antibody titres between individual macaques was considerable. Surprisingly, adsorption of GM-CSF and IL-4 to alum inhibited both sIgA and IgG rectal antibodies, unlike the increase in serum antibodies (Table 1). However, SC inguinal and external iliac TLN immunization with SIVgp120 and p27 in alum, with or without GM-CSF and IL-2 elicited at least as high sIgA and IgG antibody levels as those found after the deep internal iliac TLN route of immunization. Calculations of the correlation coefficients between the rectal sIgA or IgG antibodies to SIVgp120 or p27 and the plasma SIV RNA failed to yield significant correlation (r = 0·191 and 0·163).
T-cell proliferation to SIVgp120 and p27
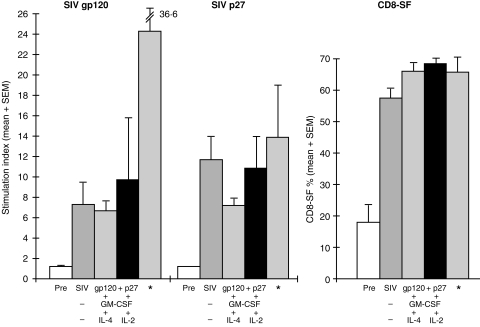
The T-cell proliferative responses to SIVgp120 and p27 were raised in all nine macaques from a stimulatory index of < 2·0 before to a mean (± SEM) of between 6·7 ± 0·9 and 11·8 ± 2·7 after immunization, but there was no significant difference between the three groups of SC-TLN-immunized macaques (Fig. 1). However, the T-cell proliferative response stimulated by SIVgp120 was much lower than that elicited by the deep iliac TLN immunization (24·4 ± 12·2).
Figure 1.
T-cell proliferative responses to simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and protein 27 (p27) and CD8-suppressor factor (CD8-SF) before (Pre) and after subcutaneous (s.c.) inguinal and external iliac* targeted lymph node (TLN) immunization (× 4) with SIVgp120 and p27 in alum, with or without granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 or IL-2. The results are presented as mean (+ SEM) of the stimulation indices. The data of three macaques immunized by the deep targeted iliac lymph node route are presented for comparison.8
CD8 cell-generated suppressor factor
The CD8 cell-generated SF showed an increase from 17·7 ± 5·8% before immunization to 57·5 ± 3·1% after immunization with SIVgp120 and p27 (Fig. 1). There was a further slight increase with the addition of either M-CSF + IL-4 (65·9 ± 2·9%) or GM-CSF + IL-2 (68·9 ± 2·0%). These results were similar to those induced by targeting the internal and external iliac lymph nodes with the same vaccine but without any cytokines (Fig. 1).
CD8-cell generated β-chemokines
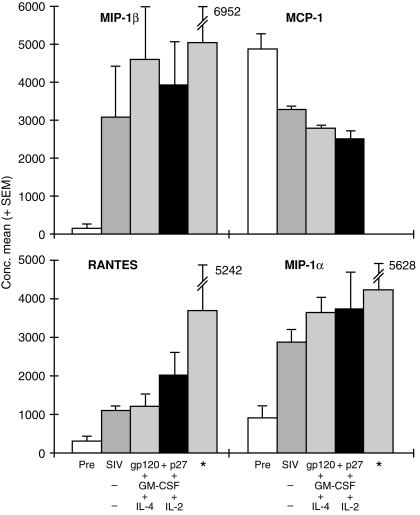
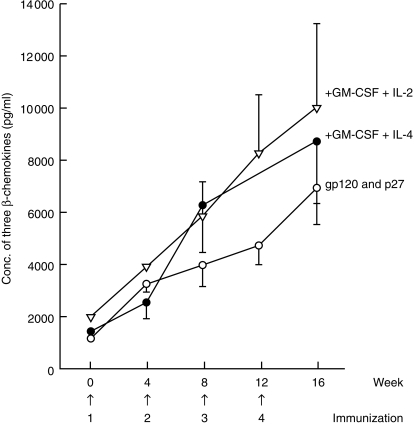
The CD8+ T-cell generated factors showed a significant increase in the concentrations of RANTES, MIP-1α and MIP-1β after immunization with SIVgp120 and p27 in alum (Fig. 2). Addition of GM-CSF with either IL-2 or IL-4 to the vaccine increased slightly the concentration of each of the three β-chemokines, without any obvious differential effect on these chemokines, except for an increase in RANTES of the GM-CSF and IL-2-treated animals. These results were consistent with those obtained previously by the deep TLN immunization, except for RANTES, the concentration of which was greater (6·2×), although the level of unimmunized macaques for that series was higher at 602 ± 339 pg/ml. Surprisingly, there was a decrease in the concentration of MCP-1 after immunization, which was slightly more marked with the cytokine immunomodulators. The combined concentration of RANTES, MIP-1α and MIP-1β showed a progressive increase in concentration after each of the four immunizations (Fig. 3). The concentration was highest (9997 ± 3303 pg/ml) when the vaccine was administered by the s.c. TLN route with GM-CSF + IL-2 but, owing to variation in concentrations of the chemokines and the small number of macaques used, these differences failed to reach the 5% level of significance. The concentration of the three β-chemokines generated by the deep iliac TLN immunization was higher (13 096 ± 5768) than that resulting from the s.c. inguinal TLN immunization with GM-CSF + IL-2 (9997 ± 3003).
Figure 2.
Concentrations of RANTES, MIP-1α, MIP-1β and MCP-1 (mean ± SEM pg/ml), before and after subcutaneous (s.c.) inguinal and external iliac* targeted lymph node (TLN) immunization (× 4) with simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and protein 27 (p27) in alum alone or with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-2 or IL-4. The chemokines were assayed in phytohaemagglutinin (PHA)-stimulated CD8+ cell culture supernatants ≈ 1 month after the fourth immunization. The data of three macaques immunized by the deep TLN iliac route are presented for comparison.8 Pre, chemokine concentration prior to immunization.
Figure 3.
Sequential changes of the combined concentrations of RANTES, macrophage inflammatory protein (MIP)-1α and MIP-1β after subcutaneous (s.c.) inguinal and external iliac targeted lymph node (TLN) immunization with simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and protein 27 (p27) alone in alum or with granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 or IL-2. The results are presented as mean (± SEM) before and 4 weeks after each of the four immunizations.
Cell-surface expression of CCR5
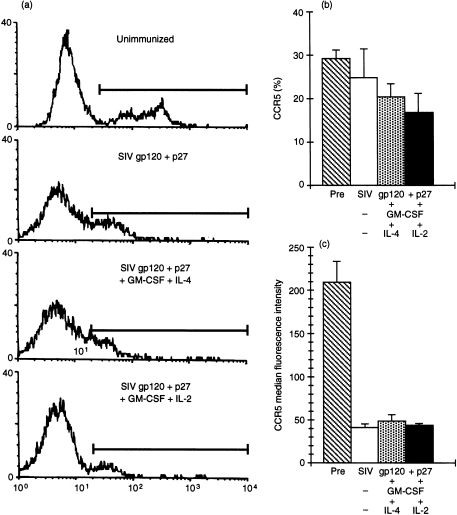
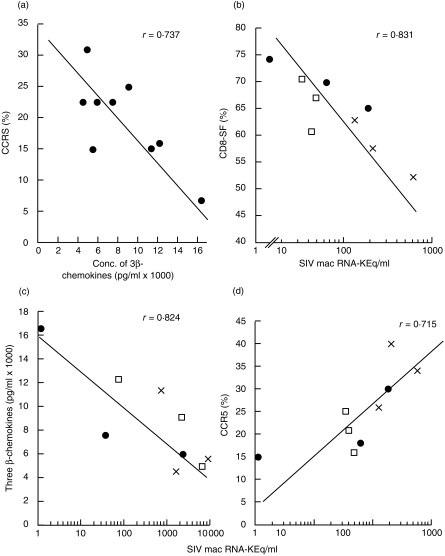
Flow cytometry examination of CCR5 directly on freshly isolated PBMC showed a decreased proportion and cell surface expression of CCR5 in PBMC of immunized macaques, which reached the lowest levels in those given SIVgp120 and p27 with GM-CSF + IL-2 (Fig. 4a). Unimmunized macaques showed 29·0 ± 2·3% CCR5+ cells and MFI of 209 ± 25·1, which decreased to 25 ± 6·7% and MFI 39·9 ± 4·8 after immunization with gp120 and p27 (Fig. 4b). A further decrease to 20·6 ± 2·8% and 17·1 ± 4·2% of CCR5+ cells was found in the PBMC of macaques immunized with the vaccine and GM-CSF + IL-4 or IL-2, respectively, although the MFI showed little change (47·6 ± 7·6 and 42·8 ± 1·0, respectively). The proportion (t = 3·72, P < 0·05) and especially MFI (t = 6·20, P < 0·001) of CCR5 was significantly lower after, than before, immunization. Furthermore, a significant inverse correlation was found (Fig. 5a) between the total concentration of the three CD8 cell-generated β-chemokines (RANTES, MIP-1α and MIP-1β) and that of the percentage, but not the MFI, of CCR5 in PBMC after the last immunization (r = 0·737, P < 0·05, d.f. 7). Thus, the higher the concentration of the three β-chemokines, the lower the proportion of CCR5+ cells, suggesting that the increased concentration of β-chemokines in vivo down-modulates the CCR5 cell-surface expression, as found previously in vitro.11,12
Figure 4.
(a) Flow cytometry of representative results of the proportion and cell surface expression of CCR5 in an unimmunized macaque (32·1%, median fluorescence intensity [MFI] 298), and in macaques immunized with simian immunodeficiency virus (SIV) glycoprotein 120 (gp120) and protein 27 (p27) (25·3%, MFI 66·4), SIVgp120 and p27 + granulocyte–macrophage colony-stimulating factor (GM-CSF) + interleukin (IL)-4 (20·6%, MFI 103), and SIVgp120 and p27 + GM-CSF + IL-2 (11·4%, MFI 36). The results of the mean (± SEM) of each group of three macaques is given in (b) as the proportion of cells (%) and in (c) as the MFI of cell surface-expressed CCR5. Maximum decrease in percentage and MFI of CCR5 was found with macaques immunized with the vaccine containing GM-CSF + IL-2. Pre, chemokine concentration prior to immunization.
Figure 5.
(a) Inverse correlation between the concentration of CD8-cell generated RANTES, macrophage inflammatory protein (MIP)-1α and MIP-1β, and the percentage of CCR5 co-receptors in peripheral blood mononuclear cells (PBMC) after the fourth immunization (before challenge) (r = − 0·737, d.f. 7, P < 0·05). (b) Inverse correlation between CD8 cell-generated suppressor factor and the simian immunodeficiency virus (SIV)mac RNA 4 weeks after challenge with SIVmac 220 (r = − 0·831, d.f. 7, P < 0·01). Correlation between the plasma SIVmac RNA 2 weeks after challenge with SIV (c) against the combined concentrations of the three β-chemokines (r = − 0·824, d.f. 7, P < 0·01) and (d) against the percentage of CCR5+ cells (r = 0·715, d.f. 7, P = 0·05), both after the last immunization. (×) glycoprotein 120 (gp120) and protein 27 (p27) alone; (•) gp120 + p27 + granulocyte–macrophage colony-stimulating factor (GM-CSF) + interleukin (IL)-2; (□) gp120 + p27 + GM-CSF + IL-4. The proportion of CCR5+ cells was determined by flow cytometry, β-chemokines by enzyme-linked immunosorbent assay (ELISA) and plasma SIVmac RNA by the SIVmac bDNA assay (Chiron Diagnostics). Polymerase chain reaction analyses were also carried out and these were positive in all but one macaque, in which SIVmac RNA was not detectable in the plasma. SIV co-culture was significantly correlated with the plasma SIVmac RNA results (r = 0·71, d.f. 7, P < 0·05).
Plasma SIVmac RNA
Comparison of the plasma RNA load with the cell-associated SIV assay, 2 weeks after mucosal challenge, showed a significant correlation between the two assays (r = 0·71, d.f. 7, P < 0·05; data not presented). One of the three macaques immunized with SIVgp120, p27 and GM-CSF with IL-2 adsorbed to alum was not infected after rectal mucosal challenge with SIVmac 220 and was also negative by the co-culture technique and by PCR. Although the remaining eight macaques were infected, a significant, inverse correlation (Fig. 5b) was found between the plasma SIVmac RNA and the CD8-SF (r = 0·831, d.f. 7, P < 0·01), as well as the concentration of the CD8 cell-generated three β-chemokines (r = 0·824, P < 0·01; Fig. 5c). The control β-chemokine (MCP-1) and five cytokines (IL-2, interferon-γ [IFN-γ], IL-12, IL-4 and IL-10) showed no correlation with the plasma SIV RNA (data not presented). In contrast, the percentage of CCR5+ cells showed a positive correlation with the plasma SIVmac RNA (r = 0·715, P = 0·05; Fig. 5d). Thus, the higher the β-chemokine concentration, the lower the cell-surface expression of CCR5 and the lower the plasma concentration of SIVmac RNA. There was no significant difference between the nine macaques immunized with or without cytokines, although the group given GM-CSF + IL-2 yielded the lowest plasma SIV RNA load.
Discussion
In view of the consistency of the immune responses and significant protection elicited by TLN immunization with recombinant SIVgp120 and p27 in alum,8 we immunized nine macaques with the same vaccine. However, in order to avoid the deep injection used previously for the TLN immunization, which is unlikely to be suitable for use in humans, we modified the administration of the vaccine, giving it by s.c. injection into the region of the inguinal and external iliac lymph nodes. In addition, we attempted to modulate the immune responses by incorporating, in the SIVgp120 and p27 vaccine, GM-CSF with either IL-4 or IL-2, which were co-adsorbed to alum, in order to up-regulate T helper (Th)1 or Th2 responses.18–20
Whereas the deep TLN immunization elicited the highest CD4+ T-cell stimulation and CD8 cell-derived β-chemokines, the s.c. inguinal and external iliac TLN immunization elicited higher serum and possibly rectal fluid antibody titres than the previous deep TLN immunization. There is no obvious explanation to account for this remarkable difference, but differential activation of the two groups of lymph nodes should be considered, corresponding to the predominantly mucosal drainage of a presumably high load of resident micro-organisms to the internal iliac, and that of cutaneous and mucosal drainage probably with a lower load of resident bacteria to the inguinal and external iliac lymph nodes. Surprisingly, immunomodulation with GM-CSF + IL-2 (a Th1 cytokine) up-regulated serum and sIgA to gp120 and p27, whereas GM-CSF + IL-4 (a Th2 cytokine) inhibited secretory IgA antibodies in the rectal fluid (Table 1). The mechanism of this inhibitory effect of IL-4 on sIgA is not understood and requires further investigation. The CD4+ T-cell proliferative responses showed no significant differences in the macaques co-immunized with the cytokines but were lower than those elicited by the deep TLN route of immunization.
A decrease in the proportion (%) of PBMC expressing cell-surface CCR5 was noted in the immunized macaques (P < 0·05) but a much more significant decrease was found in the cell-surface expression (MFI) of CCR5 (P < 0·001). As GM-CSF is capable of down-modulating CCR5 and decreasing the capacity to support HIV entry,21 this cytokine might have been involved in the two groups of macaques, treated with GM-CSF, which also showed the lowest proportion of CCR5+ cells. However this is debatable, as it was administered only at the time of each immunization, the last of which was ≈ 2 weeks before challenge with SIV. We have also not excluded the possibility that SIVgp120 might bind and down-modulate CCR5, although there is no published evidence of this.
A further finding of this investigation was the significant inverse correlation between the concentration of three CD8 cell-generated β-chemokines (RANTES, MIP-1α and MIP-1β) and the proportion of CCR5 in PBMC after the last immunization (P < 0·01). These in vivo results suggest that immunization of macaques with SIVgp120 and p27 in alum up-regulates the three β-chemokines, which may down-modulate the cell-surface expression of CCR5 receptors – this is consistent with the in vitro data.11,12 Furthermore, a significant, inverse correlation between the plasma SIVmac RNA and the three β-chemokines and a positive correlation between the SIVmac RNA and the proportion of CCR5+ cells is the first in vivo evidence to support the β-chemokine–CCR5 interaction protecting SIV mucosal infection. We suggest that up-regulation of the three β-chemokines in vivo down-modulates the cell-surface expression (MFI) of CCR5 and decreases the proportion (%) of CCR5-detectable cells, both of which affect SIV transmission, resulting in an inverse correlation between the viral load and proportion of cells that carry a lower cell surface expression of CCR5. It should be noted that in vitro inhibition studies of SIV replication suggest that the concentration of the three relevant β-chemokines must be between 2·5 and 25 ng/ml to achieve 90–95% inhibition of SIV replication.22 It is unlikely that these high concentrations will be required in vivo, in view of the proximity of CD8 and CD4 cells; nevertheless, concentrations of the three β-chemokines up to 13 ng/ml were reached. Indeed, high concentrations of RANTES (> 1000 ng/ml), but not MIP-1α or MIP-1β, can enhance the infectivity of M-tropic HIV.23
The possibility has to be considered that the redundancy in chemokine receptors to which SIV can bind, such as Bonzo (STRL), Bob (GPR15) or GPR1,24–27 may enable the virus to adapt under the immune and chemokine pressures and infect the cells via an alternative co-receptor. However, subjects having the homozygous 32 bp deletion of CCR5, with a few exceptions, do not show evidence of co-receptor switch, as they remain free of HIV infection.28–30 It is noteworthy that vaccination per se does not up-regulate the β-chemokines, as was found by immunization with measles virus in macaques22 or influenza virus in humans.31 Similarly, the adjuvant used (MDP, alum or ISCOM) did not affect the level of the β-chemokines.22 However, using the same vaccine (SIVgp120 and p27) administered by different routes of immunization appears to affect the level of β-chemokine concentration.8,22 Indeed, conventional intramuscular (i.m.) immunization up-regulates the concentration of the three β-chemokines, but the levels are five to eight times lower, and the CD8-SF is also lower, than those reached by the deep TLN route of immunization.32 Furthermore, protection from rectal SIV challenge was found only in the deep TLN-immunized macaques, which was significantly associated with the levels of the three β-chemokines and CD8-SF, but not neutralizing antibodies.8 We cannot exclude another possibility, that a decrease in the concentration of MCP-1 might also be involved, by attracting fewer macrophages and thereby decreasing the number of cells available for virus infection.
We suggest a dual objective in immunization against HIV or SIV: to elicit both cognate (specific) immunity and innate or non-specific up-regulation of β-chemokines. The vaccine and route of immunization can be designed to elicit cellular and humoral immunity against HIV and to up-regulate the β-chemokines that bind and down-modulate the co-receptors, exerting a complementary effect in preventing or decreasing HIV infection. This dual strategy is aimed at the three SIV or HIV barriers: the mucosal site of entry, the draining lymph nodes and circulating blood, as reviewed recently.33
Acknowledgments
This work was supported by grants from the National Institute of Acquired Immunodeficiency Disease, Washington DC, USA and the European Community Biomed II Grant (no: BMH4 CT97-2345). We wish to thank Dr P. Gray (ICOS Corporation, Seattle, WA, USA) for the monoclonal antibody to CCR5 and Dr D. Montefiori (Duke University) for performing the neutralization assays.
Glossary
Abbreviations
- MFI
median fluorescence intensity
- s.c.
subcutaneous
- SEM
standard error of the mean
- SF
suppressor factor
- sIgA
secretory immunoglobulin A
- SIV
simian immunodeficiency virus
- TLN
targeted lymph node
References
- 1.Miller CJ, Alexander NJ, Vogel P, Anderson J, Marx PA. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol. 1992;21:64. [PubMed] [Google Scholar]
- 2.Spira AI, Marx PA, Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehner T, Bergmeier LA, Tao L, et al. Targeted lymph node immunization with simian immunodeficiency virus p27 antigen to elicit genital, rectal and urinary immune responses in nonhuman primates. J Immunol. 1994;153:1858. [PubMed] [Google Scholar]
- 4.Marx PA, Compans RW, Gettie A, et al. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 5.Lehner T, Bergmeier LA, Panagiotidi C, et al. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science. 1992;258:1365. doi: 10.1126/science.1360702. [DOI] [PubMed] [Google Scholar]
- 6.Lehner T, Brookes R, Panagiotidi C, et al. T and B cell functions and epitope expression in non-human primates immunized with SIV by the rectal as compared with the systemic route. Proc Natl Acad Sci USA. 1993;90:8638. doi: 10.1073/pnas.90.18.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauza CD, Mau E, Salvato MS. Pathogenesis of SIVmac251 after atraumatic inoculation of the rectal mucosa in rhesus monkeys. J Med Primatol. 1993;22:154. [PubMed] [Google Scholar]
- 8.Lehner T, Wang Y, Cranage M, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 9.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, Devico AL, Garzino-demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α and MIP-1β as the major HIV suppressive factors produced by CD8+ cells. Science. 1995;270:1811. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Amara A, Gall SL, Schwartz O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aramori I, Zhang J, Ferguson SSG, Bieniasz PD, Cullen BR, Caron MG. Molecular mechanism of desensitization of the chemokine receptor CCR: receptor signaling and internalization are dissociable from its role as an HIV co-receptor. EMBO J. 1997;16:4606. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Tao L, Mitchell E, et al. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat Med. 1999;5:1004. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 14.Doyle CB, Bhattacharyya U, Kent KA, Stott EJ, Jones IM. Regions required for CD4 binding the external glycoprotein gp120 of simian immunodeficiency virus. J Virol. 1995;69:1256. doi: 10.1128/jvi.69.2.1256-1260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villinger F, Brar SS, Mayne A, Chikkala N, Ansari AA. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946. [PubMed] [Google Scholar]
- 16.Polyanskaya N, Sharpe S, Cook N, et al. Anti-major histocompatibility complex antibody responses to simian B cells do not protect macaques against SIVmac infection. AIDS Res Hum Retroviruses. 1997;13:923. doi: 10.1089/aid.1997.13.923. [DOI] [PubMed] [Google Scholar]
- 17.Rose J, Silvera P, Flanagan B, Kitchin P, Almond N. The development of PCR based assays for the detection and differentiation of simian immunodeficiency virus in vivo. J Virol Methods. 1995;51:229. doi: 10.1016/0166-0934(94)00109-t. [DOI] [PubMed] [Google Scholar]
- 18.Vogel FR. The role of adjuvants in retroviral vaccines. Int J Immunopharmacol. 1995;17:85. doi: 10.1016/0192-0561(94)00095-6. [DOI] [PubMed] [Google Scholar]
- 19.Jones TC. Future uses of granulocyte-macrophage colony-stimulating factor (GM-CSF) Stem Cells. 1994;12:229. doi: 10.1002/stem.5530120719. [DOI] [PubMed] [Google Scholar]
- 20.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte–macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytokine T lymphocytes. J Immunol. 1997;158:3947. [PubMed] [Google Scholar]
- 21.Di Marzio PD, Tse J, Landau NR. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Tao L, Mitchell I, et al. Generation of CD8 suppressor factor and β chemokines induced by xenogeneic immunization, in the prevention of simian immunodeficiency virus infection in macaques. Proc Natl Acad Sci USA. 1998;95:5223. doi: 10.1073/pnas.95.9.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon CJ, Muesing MA, Proudfoot AEI, Power CA, Moore JP, Trkola A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokines RANTES is independent of the mechanism of virus-cell fusion. J Virol. 1999;73:684. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng H, Unutmaz D, Kewal Ramani VN, Littman DR. Expression cloning of nef receptors used by simian and human immunodeficiency virus. Nature. 1997;388:296. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 25.Akhatib G, Liao F, Berger EA, Peden CWC. A new SIV co-receptor STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 26.Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;11:2015. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farzan M, Choe H, Martin K, et al. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 co-receptor accounts for resistance of some multiply-exposed individuals to HIV infection. Cell. 1996;86:367. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 29.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR5 chemokine receptor gene. Nature (Lond) 1996;382:722. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 30.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 31.Moss RB, Wallace MR, Lanxa P, et al. In vitro p24 antigen-stimulated lymphocyte proliferation and Β-chemokine production in human immunodeficiency virus type 1 (HIV-1) -seropositive subjects after immunization with an inactivated gp120-depleted HIV-1 immunogen (Remune) Clin Diag Lab Immunol. 1998;5:308. doi: 10.1128/cdli.5.3.308-312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Tao L, Mitchell E, Bergmeier L, Doyle C, Lehner T. The effect of immunization on chemokines and CCR5 and CXCR4 coreceptor functions in SIV binding and chemotaxis. Vaccine. 1999;17:1826. doi: 10.1016/s0264-410x(98)00482-4. [DOI] [PubMed] [Google Scholar]
- 33.Lehner T, Bergmeier L, Wang Y, Tao L, Mitchell E. A rational basis for mucosal vaccination against HIV infection. Immunol Rev. 1999;170:183. doi: 10.1111/j.1600-065x.1999.tb01338.x. [DOI] [PubMed] [Google Scholar]