Abstract
The laminin α2-chain is a component of merosin, a member of the laminin family molecules, which is mainly expressed in the basement membranes of striated muscle. It is known that laminin α2 gene (lama2) null mutant mice (dy3k/dy3k) exhibit congenital muscular dystrophy (CMD). Because the laminin α2-chain is also expressed in the thymus, the role of merosin in the thymus was examined. In association with the onset of muscular dystrophy, CD4+ CD8+ double-positive (DP) thymocytes disappear by apoptotic cell death, while CD4+ CD8– or CD4– CD8+ thymocytes remain. In order to study the mechanisms leading to the selective death of DP cells in the absence of merosin, the role of the interaction between very late activation antigen-6 (VLA-6), a candidate merosin ligand in the thymus, and merosin was examined. The in vitro survival of thymocytes from normal mice was maintained by the addition of either anti-VLA-6 monoclonal antibodies (mAbs) or merosin. Furthermore, when the normal thymocytes were cultured on thymic epithelial cell lines, viable DP cell recoveries on wild-type epithelial cells were better than on cells from null mutant mice. The results suggest that DP cells are more sensitive to an uncharacterized apoptotic death signal, and that survival is supported by the interaction between VLA-6 and merosin.
Introduction
The thymus is the organ in which most T cells develop. T-cell precursors originate from fetal liver during the embryonic stage and from bone marrow in an adult. In the fetal thymus, prothymocytes enter the non-vascularized thymic rudiment by encapsulation. The thymus is composed of thymic lymphocytes (thymocytes) and non-lymphoid stroma. The thymic stroma consists largely of epithelial cells derived from the pharyngeal pouch during development, and monocytes and dendritic cells derived from bone marrow. Furthermore, fibroblasts and various extracellular matrix (ECM) molecules permeate the whole framework. Cellular interactions between stromal cells and thymocytes play crucial roles in T-lymphocyte development.1–4 There are two types of cellular interaction between these cell types: direct cell-to-cell interactions through major histocompatibility complex (MHC)/T-cell receptor (TCR), intracellular adhesion molecule-1 (ICAM-1)/lymphocyte function-associated antigen-1 (LFA-1), and LFA-3/CD2, and bridging by ECM molecules. In the thymus, laminins, fibronectin and type IV collagen interact with thymocytes through their respective ligand.
Laminins are components of basal laminae throughout the body, and play essential roles in the organization of molecular networks of basal laminae, the interaction with cell-surface components and signal transduction into the cells. Laminin consists of three subunits, α-, β- and γ-chains (nomenclature for laminins by Burgeson et al.5). To date, five α-chains (α1–5) have been shown to assemble into at least 11 heterotrimers.6 Merosin (laminin 2) is a 700-MW glycoprotein composed of α2- (300 MW), β1- (200 MW), and γ1- (200 MW) chains. Merosin is found in the basement membranes of striated muscle, Schwann cells, trophoblasts,7,8 kidney, heart, skin,9,10 central nervous system11 and intestine.12 Merosin expression has also been found in the thymus following analysis using the reverse transcription–polymerase chain reaction (RT–PCR),13,14 and possible roles of the merosin–integrin interaction in thymocyte development have been suggested.
Merosin is a specific ligand for dystroglycan in the muscle, and the interaction produces an axis connecting the ECM and subsarcolemmal network.15 The specific absence of merosin results in a congenital muscular dystrophy (CMD) and an autosomal recessive muscle disorder.16 Researchers have identified two naturally occurring mouse models for this disease, dy/dy17–20 and dy2j/dy2j.21,22 Very recently we established laminin α2-chain null mutant mice (dy3k/dy3k) by targeted disruption of the lama2 gene.23 The homozygous mice are characterized by growth retardation and severe muscular dystrophy symptoms and succumb to undetermined causes by 5–6 weeks of age. In the degenerating muscles, considerable amounts of apoptotic cell death are detected.23 We then examined the thymus of dy3k/dy3k mice to investigate the role of merosin in T-cell development. We describe here severe thymic atrophy in dy3k/dy3k mice, and report this atrophy to be associated with the selective apoptotic cell death of CD4+ CD8+ double-positive (DP) thymocytes. The possible role of merosin in the maintenance of DP cells in the thymus is discussed.
Materials and methods
Mice
Heterozygous lama2 gene-targeted mice23 were maintained in our animal facility by mating with normal BALB/c mice. Heterozygous mice were interbred to obtain homozygous mice. Specific pathogen-free BALB/c mice aged 5–6 weeks were purchased from Charles River Japan (Tokyo, Japan).
Genotyping of the lama2 deficiency was performed by PCR on tail genomic DNA. The PCR primers for the wild-type (WT) lama2 allele were: 5′-CCAGATTGCCTACGTAATTG-3′ and 5′-CCTCTCCATTTTCTAAAG-3′. The primer pairs for the mutant allele were: 5′-CTTGGGTGGAGAGGCTATTC-3′ and 5′-AGGTGAGATGACAGGAGATC-3′, which are present in the neo gene. Mice showing WT lama2-negative and neo-positive PCR products were defined as lama2 homodeficient (dy3k/dy3k). Lama2 homodeficient mice are hereafter referred to as mer–/–.
Establishment of thymic epithelial cell lines
Thymi of mer–/– or WT mice were obtained, and thymic epithelial cell (TEC) lines were established according to previously published methods.24 TEC lines derived from mer–/–, WT and normal BALB/c mice were termed S7HoE1, S7wtE1 and S1Bc, respectively. The cell lines were maintained in Dulbecco’s modified Eagle’s medium (Nissui, Tokyo, Japan) containing 10% fetal calf serum (FCS; Boehringer Mannheim, Castle Hill, Australia) with kanamycin (100 mg/l; Meiji Pharmaceutical Co., Tokyo, Japan).
Antibodies
Biotinylated anti-CD4 (GK1.5) and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (53-6.7) (from the American Type Culture Collection [ATCC], Rockville, MD) were prepared in our laboratory. Hamster monoclonal antibodies (mAbs) to mouse integrin α6 (HMα6), α2 (HMα2), and β1 (HMβ1-1),25,26 and rat mAb to α4 (CAS-9)27 (a gift from Dr T. Kina, Kyoto University, Kyoto, Japan) were used. Phycoerythrin (PE)-conjugated anti-CD4 (RM4-5), biotinyl anti-TCRβ (H57) and biotinyl-anti-Vβ8 (F23.1) antibodies were obtained from PharMingen (San Diego, CA). PE-conjugated streptavidin, PE-Cy5-conjugated streptavidin and FITC-conjugated goat anti-hamster immunoglobulin G (IgG) were purchased from DAKO Japan (Tokyo, Japan), Cedarlane (Ontario, Canada), and Organon Teknika (West Chester, PA), respectively. mAb to mouse anti-human laminin α2-chain (2D9) was kindly provided by Dr H. Hori (Tokyo Medical and Dental University, Tokyo, Japan).28 Horseradish peroxidase-conjugated goat anti-mouse IgG was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Other reagents
Human merosin, bovine gelatin, Sephadex G-10 and 7-amino actinomycin D (7-AAD) were purchased from Chemicon (Temecula, CA), Wako Pure Chemicals (Osaka, Japan), Pharmacia Biotech (Uppsala, Sweden) and Sigma Chemical Co. (St. Louis, MO), respectively.
Flow cytometry
Single-cell suspensions were prepared from thymi. Thymocytes (500 000) were stained with each antibody and flow cytometric profiles were analysed using an EPICS ELITE (Coulter Electronics, Hialeah, FL) or a FACSCalibur analyser and cellquest software (Becton-Dickinson Immunocytometry Systems, Mountain View, CA).
Thymocyte survival assay
Freshly isolated normal BALB/c thymocytes were passed through a Sephadex G-10 column to remove adherent cells, and cultured in RPMI-1640 medium, with or without purified human merosin or various mAbs, at 2 × 105 cells/400 µl/well in 48-well culture plates (Sumitomo Bakelite, Tokyo, Japan) at 37° in 5% CO2. Eight hours later, the cells were harvested and stained with 7-AAD (used at 1 µg/ml). The 7-AAD-negative cells were then counted by using flow cytometry.
Co-culture of thymocytes on a TEC line
FCS was passed through a gelatin-conjugated affinity column to remove serum ECM molecules (such as fibronectin) and the eluate is hereafter referred to as FCS′. TECs were cultured in RPMI-1640 supplemented with 2·5% FCS′ (FCS′/RPMI) at 2 × 104 cells/ml/well in 24-well culture plates (Sumitomo Bakelite). Forty-eight hours later, monolayers of TECs were overlaid with normal thymocytes (that had been passed through a Sephadex G-10 column), in FCS′/RPMI, at 5 × 105 cells/ml/well. Forty-two hours later, the thymocytes were harvested and stained with biotinyl-anti-CD4 (GK1.5) plus PE-streptavidin, FITC-anti-CD8 (53-6.7) and 7-AAD, then analysed by flow cytometry.
DNA-fragmentation assay
Two million freshly isolated thymocytes were fixed in 70% ethanol overnight at 4°, washed with phosphate-buffered saline (PBS) and resuspended in 100 µl of phosphate-citrate buffer (pH 7·6) containing 192 mm Na2HPO4 and 4 mm citric acid, and incubated for 30 min at room temperature. The cells were incubated with RNase A (0·2 mg/ml; Sigma) at 37° for 30 min, then with propidium iodide (PI) (100 µg/ml; Sigma) for 30 min in the dark. The DNA content of the cells was analysed by flow cytometry.
RT–PCR
Total RNA was isolated from thymus, muscle, thymocytes or TEC lines using TRIzol (Gibco BRL, Grand Island, NY), and treated with DNase I (Perkin-Elmer, Branchburg, NJ). RNA was transcribed to cDNA using an AMV Reverse Transcriptase First-Strand cDNA Synthesis Kit (Life Sciences, St. Petersburg, FL). cDNA was amplified by using AmpliTaq (Perkin Elmer) in buffer containing 1·5 mm MgCl2. Primers for the laminin α2-chain were the same as used for genotyping. The primer pairs for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were: 5′-CCATCACCATCTTCCAGGAG-3′ and 5′-CCTGCTTCACCACCTTCTTG-3′.
Western blot analysis
Thymus (10 mg, wet weight), spleen (10 mg, wet weight), and muscle (2 mg, wet weight) were homogenized in 40 µl of buffer containing 15% sodium dodecyl sulphate (SDS), 70 mm Tris-HCl (pH 6·7), 10 mm EDTA and 5% 2-ME, and boiled for 5 min. The supernatants were separated by electophoresis (5% SDS–PAGE) and electrotransferred to Protran Nitrocellulose filters (Schleicher & Schuell, Dassel, Germany). The membranes were reacted with 2D9, a mAb to human laminin α2, then with horseradish peroxidase-conjugated goat anti-mouse IgG. The enhanced chemiluminescence method (Luminescence Reagent Set, Wako, Japan) was applied for the visualization of laminin α2 bands.
Results
Reduction of DP thymocytes in mer–/– mice
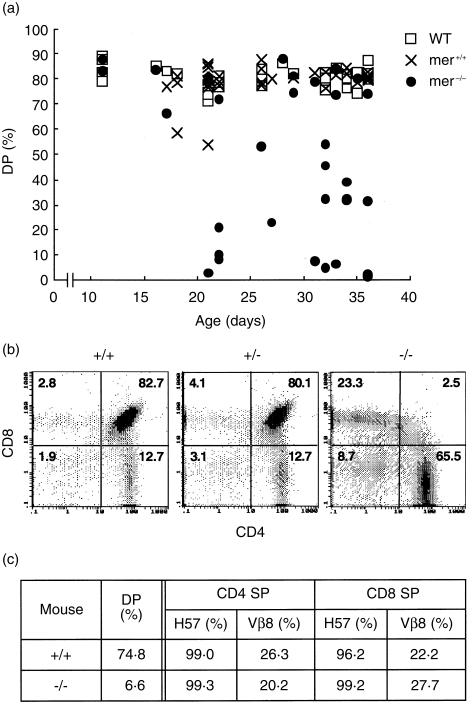
Mer–/– mice were indistinguishable from their heterozygous or WT littermates up to postnatal week 2–3, then showed growth retardation and overt dystrophic symptoms.23 Because merosin is also expressed in the thymus, thymocyte development in mer–/– mice was examined. In association with the onset of dystrophic symptoms, the number of DP thymocytes decreased (Fig. 1a), but CD4+ CD8– and CD4– CD8+ single-positive (SP) cells were less affected (Fig. 1b). Total thymocyte number in WT, mer+/–, or mer–/– mice during this experiment was 260, 108, or 3·0 (× 106 cells/mouse), respectively. The numbers were different in each individual, and mer–/– mice had fewer cells at age 2–3 weeks after birth than WT or mer+/– mice, but there was no significant difference between WT and mer+/– mice in replicate experiments. The expression of SP cell TCR-β and Vβ8 were similar to the WT littermates (Fig. 1c), but the total thymocyte number was reduced, as indicated in the figure legend. Although the data are not shown in this report, splenic cell number in mer–/– mice also decreased to 10–33% of the level observed in WT littermates at age 4–5 weeks. However, the relative compositions of the B220+, CD4+ and CD8+ fractions did not differ from WT littermates.
Figure 1.
Selective cell death in the thymus of mer–/– mice. (a) Thymocytes from wild-type (WT), mer+/– and mer–/– mice of various ages were stained with biotinyl-anti-CD4 (GK1.5) plus phycoerythrin (PE)-streptavidin and fluorescein isothiocyanate (FITC)-anti-CD8 (53-6.7), and the percentages of double-positive (DP) cells are shown. (b) Typical flow cytometric patterns of each thymus from the same littermates are shown (36-day-old mice). (c) Frequencies of T-cell receptor (TCR)-positive cells in single-positive (SP) thymocytes are shown (from 33-day-old mice). Cells were stained with PE-anti-CD4 (RM4-5), FITC-anti-CD8 (53-6.7), and biotinyl-anti-TCR plus PE-Cy5-streptavidin. Total cell numbers of WT and mer–/– mice were 310 and 9·5 (× 106 cells/mouse), respectively.
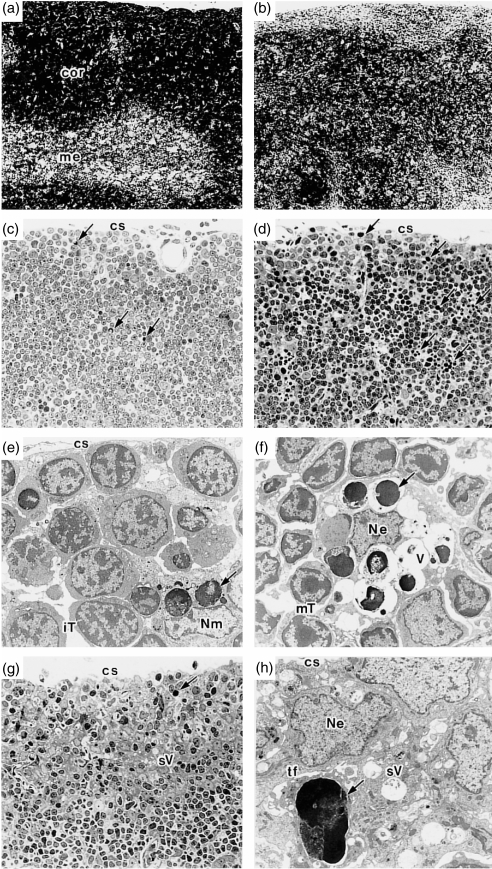
In order to study the mechanisms for the selective decrease in DP cell number in the absence of merosin, the thymi of mer–/– mice and their littermates were examined histologically. Haematoxylin and eosin staining of the thymus of a mer–/– mouse, compared with that of a WT mouse (Fig. 2a), showed a very thin outer cortex with thymocytes scattered in the inner cortex and medullary area (Fig. 2b). The cortico-medullary junction of the mer–/– thymus was obscure. In the thymus of a WT mouse, a few apoptotic thymocytes were seen (Fig. 2c). On the other hand, a large number of apoptotic cells were observed in the subcapsular and outer cortical areas of a mer–/– mouse (Fig. 2d). An electron micrograph (Fig. 2e) of the outer cortex of a WT mouse thymus (the same as Fig. 2c) showed apoptotic thymocytes phagocytosed by a macrophage and many viable immature thymocytes. Figure 2(f) (the same as Fig. 2d) showed apoptotic cells at various stages of degeneration, mainly in the vacuoles of the epithelial thymic nurse cell-like structure, as described previously,29 and mature thymocytes were conspicuous instead of immature thymocytes, as seen in Fig. 2(e). A small number of apoptotic thymocyte-containing macrophages were also observed (data not shown). The subcapsular and outer cortex was rich in epithelial cells, as shown in a section from another mer–/– mouse thymus (Fig. 2g). These results indicate that the thymus in Fig. 2(g) shows a more progressive stage of DP cell depletion than that of Fig. 2(d). An electron micrograph (Fig. 2h) showed the subcapsular cortex of a thymus (the same as Fig. 2g) in which three epithelial cell nuclei and one apoptotic cell are seen. Many small vacuoles with small amounts of non-digestible electron-dense materials derived from apoptotic thymocytes, as described previously,29 were observed within the epithelium.
Figure 2.
Histological studies of the thymus of mer–/– mice. The thymus of a wild-type (WT) (a) or mer–/– (b) mouse (22 days old) was obtained and a paraffin-embedded section of one lobe from each was stained with haematoxylin and eosin (× 60). The frequencies of double-positive (DP) thymocytes were determined, using another lobe, to be 77·9% (a) and 21·1% (b). Epoxy resin-embedded thin sections of the thymi of a WT mouse (c) (× 240) or mer–/– mice (22 days old) (d) and (g) (× 240) were stained with 1% toluidine blue. Electron micrographs of (c), (d) and (g) are shown in (e) (× 1900), (f) (× 1900) and (h) (× 2600), respectively. Ultrathin sections were stained with 1% uranyl acetate and lead citrate. →, apoptotic thymocyte; cor, cortex; cs, capsule; iT, immature thymocyte; me, medulla; mT, mature thymocyte; Ne, nucleus of thymic epithelial cell; Nm, nucleus of macrophage; sV, small vacuole; tf, tonofilament; V, vacuole.
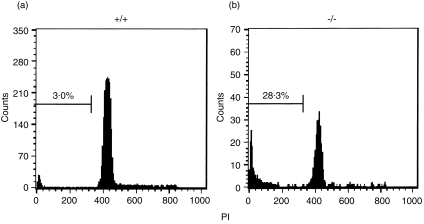
DNA fragmentation of the thymocytes was then examined by PI staining. As shown in Fig. 3, a flow cytometric study revealed that apoptotic thymocytes in WT and mer–/– mice comprise 3·0% (Fig. 3a) and 28·3% (Fig. 3b), respectively, of total thymocytes. Although the data are not shown in this report, we then examined cell death by the TUNEL assay. In contrast to WT mice, in which some apoptotic cells appeared to be randomly scattered throughout the thymus, thymocyte apoptosis was observed intensively in limited regions in the mer–/– thymus. These data suggest that DP thymocytes in the mer–/– thymus are removed by apoptotic cell death.
Figure 3.
DNA fragmentation of double-positive (DP) thymocytes from mer–/– mice. Freshly isolated thymocytes from wild-type (WT) and mer–/– mice (34 days old) were fixed in 70% ethanol, stained with propidium iodide (PI) and analysed by using flow cytometry. The frequencies of DP cells in WT and mer–/– mice were 79·0% and 31·2%, respectively.
Expression of laminin α2 and integrin α6 in the thymus
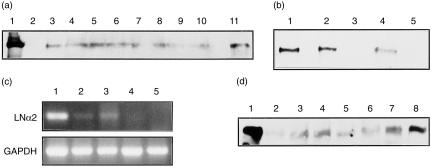
Expression of the laminin α2-chain in the thymus was examined. Thymi from BALB/c mice of various ages were obtained and Western blotting studies were performed. Expression of the laminin α2-chain was observed in thymi from mice at various stages of development (from neonates to adults), as well as in adult mouse skeletal muscle (Fig. 4a, 4b). However, the Sephadex G-10 pass-through fraction of thymocytes, which is depleted in adherent thymic cells, showed no laminin α2-band (Fig. 4a, lane 2). These results suggest that merosin is expressed on the adherent fraction of thymic cells, namely thymic stromal cells. To confirm this, TEC lines from WT and mer–/– mice were examined, by using RT–PCR, for their expression of the mRNA for the laminin α2-chain. As shown in Fig. 4(c), neither purified thymocytes nor TECs from mer–/– mice showed laminin α2-chain bands, while TECs and skeletal muscle from WT mice did. Laminin α2 expression was also observed in the spleen of normal mice of various ages (Fig. 4d).
Figure 4.
Expression of the laminin α2-chain in the thymus. (a) Western blotting pattern of laminin α2-chain expression in the normal BALB/c thymus. Human merosin (lane 1 as a positive control), the Sephadex G-10 pass-through fraction of thymocytes (lane 2), thymi (lane 3–10, from 54-, 35-, 25-, 21-, 18-, 12-, 7- and 0-day-old mice, respectively), or muscle (lane 11, 54-day-old mice). (b) Western blotting pattern of laminin α2-chain expression in muscle and thymus. Muscle of normal BALB/c (lane 1), mer+/– (lane 2), or mer–/– (lane 3), and thymus of mer+/– (lane 4), or mer–/– (lane 5) mice. (c) Reverse transcription–polymerase chain reaction (RT–PCR) analysis of laminin α2-chain expression in thymic epithelial cell (TEC) lines. RNA from the muscle of normal BALB/c mice (lane 1), TEC line S1Bc (from normal BALB/c mice) (lane 2), TEC line S7wtE1 (from WT mice) (lane 3), S7HoE1 (from mer–/– mice) (lane 4), or Sephadex G-10 pass-through normal thymocytes (lane 5). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LN, lymph node. (d) Western blotting pattern of laminin α2-chain expression in normal BALB/c mouse spleen. Human merosin (lane 1 as a positive control), spleens (lanes 2–6 from 54-, 40-, 29-, 21- and 12-day-old mice, respectively), thymus (35-day-old mice) (lane 7), or muscle (54-day-old mice) (lane 8).
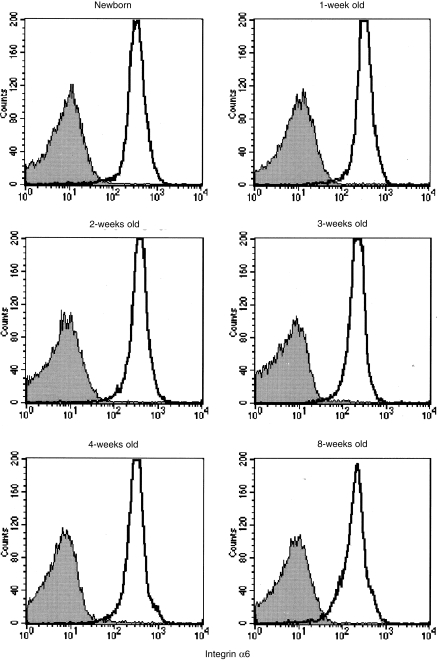
VLA-6 is known to be a ligand for merosin in the thymus.13,14 Therefore, the expression of integrin α6 on thymocytes was examined by using flow cytometry. As shown in Fig. 5, integrin α6 expression on thymocytes was observed for mice at various stages of development (from neonates to adults). Thus, both laminin α2 and integrin α6 are already expressed in neonatal mice.
Figure 5.
Expression of integrin α6 on thymocytes from normal BALB/c mice. Thymocytes were isolated from mice at the ages indicated, stained with anti-integrin α6 plus fluorescein isothiocyanate (FITC)-anti-hamster immunoglobulin G (IgG) and analysed by using flow cytometry. Shaded areas indicate controls without the first antibody.
Merosin and anti-VLA-6 antibodies rescue DP thymocytes from cell death
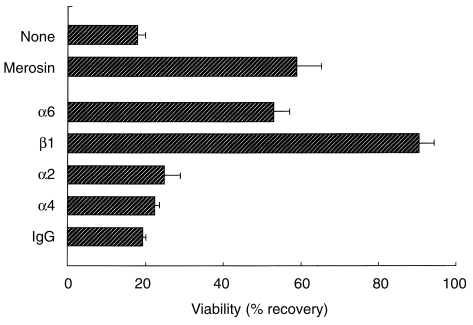
To study the mechanisms for the selective death of DP cells in the absence of merosin, the role of merosin or VLA-6 in the viability of DP cells was examined in vitro. Normal adult thymocytes were cultured in the presence or absence of merosin or various integrin mAbs, and cell survival was examined. As shown in Fig. 6, a reduced cell recovery was observed in the group receiving no proteins, or in the presence of control rat IgG. In contrast, the viability of adult BALB/c thymocytes was significantly enhanced in the presence of merosin, or mAb to integrins α6 or β1. The mAb to integrin β1 produced better viability than the mAb to integrin α6. Integrin α4, but not integrin α2, is expressed on thymocytes; however, mAbs to these integrins had no effect on the viability of DP cells.
Figure 6.
Increased viability of normal thymocytes cultured in the presence of merosin or anti-very late activation antigen-6 (VLA-6) monoclonal antibodies (mAbs). Normal BALB/c thymocytes (from 4-week-old mice) were prepared by passing the thymus cells through a Sephadex G-10 column to remove adherent cells. The cells were cultured for 8 hr in serum-free medium with various anti-integrin mAbs or human merosin (protein concentration 10 µg/ml/5 × 105 cells). Immunoglobulin G (IgG) indicates normal rat IgG. 7-amino actinomycin D (7-AAD)-negative cells were counted. The data shown are mean ± SD (of three independent cultures) and are representative of three separate experiments.
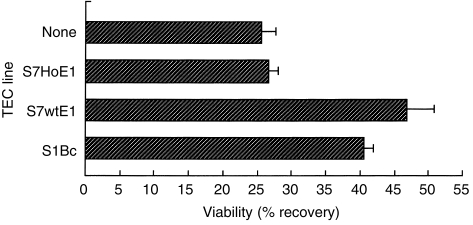
To elucidate the role of merosin on the interaction between thymocytes and TECs, normal thymocytes were cultured on various TEC lines. S7HoE1, the TEC line from mer–/– mice, had almost no enhancing effect on thymocyte viability when compared with the absence of TECs (Fig. 7). On the other hand, S1Bc and S7wtE1, which are TEC lines from WT and normal BALB/c mice, respectively, produced better recovery of viable DP cells from culture. We attempted co-culture of thymocytes and S7wtE1 cells in the presence of an antilaminin α2 antibody, but the thymocyte viability was inhibited only partially (data not shown). This result may be explained by the fact that the antibody used in the experiment did not sufficiently block the binding of merosin with thymocytes.
Figure 7.
Increased viability of double-positive (DP) thymocytes cultured on laminin α2-chain-expressing thymic epithelial cell (TEC) lines. Sephadex G-10 pass-through normal BALB/c thymocytes (from 6-week-old mice) were cultured for 42 hr in the 2·5% FCS′ (see the Materials and methods for a description of FCS′)-containing medium, and stained with anti-CD4, anti-CD8 and 7-amino actinomycin D (7-AAD). 7-AAD-negative DP cells were counted by using flow cytometry. The frequencies of double-negative (DN), DP, CD4 single-positive (SP), or CD8 SP thymocytes at 0 hr were 1·8%, 84·5%, 9·3%, or 4·4%, respectively. The data shown are mean ± SD (of three independent cultures) and are representative of three separate experiments.
Taken collectively, these results suggested that the interaction of merosin from TEC with VLA-6 (or with some undefined molecule(s) in the β1-integrin family) in DP thymocytes may play a crucial role in the survival of DP thymocytes.
Discussion
Severe thymic atrophy has previously been observed in laminin α2-chain null mutant (dy3k/dy3k) mice in association with growth retardation and muscular dystrophy. Histological and cellular analyses revealed that the thymic atrophy was a result of selective apoptotic cell death of DP thymocytes. Thymic and splenic abnormalities associated with bodyweight loss in naturally occuring dystrophic mice (dy/dy) was first described in 1976.30,31 The histological features of the dy/dy mice were similar to the results obtained in this study, although TCR-β-positive mature-type T cells were detected in the dystrophic thymus. Merosin is expressed in the spleen and the splenocyte number is also reduced in mer–/– mice, but the proportions of mature T and B lymphocytes are similar to those in normal mice (data not shown). This suggests that merosin has some functions in the maintenance of splenocytes, but the mechanisms underlying this issue are not yet known.
It will be of interest to discover why thymocyte death occurs at a certain age after birth in association with the onset of muscular dystrophy. We examined the developmental changes in merosin and VLA-6 expression in normal thymus. Merosin expression in the thymus was observed from the neonatal to adult (day 54) stages. VLA-6 expression was also found from the neonatal period. These results suggest that the apoptotic death of DP thymocytes is not associated with the timing of the expression of either merosin or VLA-6 in the thymus. It is speculated that neonatal mutant muscles contain some unidentified trophic factors which prevent apoptotic cell death. Such a similar mechanism may be applicable to the thymus.
In a previous study we demonstrated that the degenerating muscle in the mer–/– mice contains a markedly higher number of TUNEL-positive nuclei compared to the muscles of their WT littermates.23 As described in this work, the number of DP thymocytes was also decreased by apoptotic cell death in association with muscle cell death. It is well known that the injection of glucocorticoids or severe physical stress induces thymic atrophy associated with a selective loss of DP cells. Very recently, Vacchio et al. reported that a significant amount of glucocorticoids can be synthesized by TECs, and that locally produced glucocorticoids may be a key element in antigen-specific thymocyte selection.32,33 We do not intend to exclude the role of glucocorticoids produced by either the adrenal gland or the thymus in the deletion of DP cells; however, questions arise as to whether the apoptotic death of DP thymocytes is a result of a simple secondary effect of muscular dystrophy and which role merosin plays in the thymus.
Experiments were therefore designed to determine the effect of merosin or VLA-6, a ligand of merosin in the thymus, on the survival of DP thymocytes in vitro. Because merosin is produced by TECs, normal thymocytes were depleted of TECs and then cultured in the presence or absence of exogenous proteins. Moreover, the addition of fibronectin supported the viability of the DP thymocytes (data not shown), and FCS was depleted of fibronectin by passing it through gelatin-conjugated gel. These experiments enabled us to determine the in vitro effects of merosin and integrins on the survival of DP thymocytes. As clearly shown in Figs 6 and 7, merosin, as well as the mAb to integrins α6 or β1, supports the viability of DP thymocytes. That anti-integrin β1 produced better cell viability than anti-α6 suggests that β1 integrin(s) other than VLA-6, such as VLA-3,14 may also participate in the interaction with merosin in the thymus. The mAbs to integrins α2 or α4 had no supportive effect. These effects were confirmed using TEC lines from WT or mer–/– mice. TECs from mer–/– had little effect on thymocyte viability, while TECs from WT or normal BALB/c mice enhanced viability. This result is in agreement with the earlier observation by Vachon et al.34 that merosin promotes myotube stability by preventing apoptosis in vitro. Such a preventive mechanism may be operating through merosin and VLA-6 in the thymus. Recently, it has also been shown that the co-ligation of VLA-4 and TCR rescues human thymocytes from dexamethasone-induced apoptosis.35 Agea et al. reported that costimulation of CD3/TCR with integrin protects against the apoptotic cell death of antigen-specific T lymphocytes.36
Here we hypothesize that a certain apoptotic death signal(s), including glucocorticoids, is delivered continuously to DP thymocytes and muscular cells, but is blocked by the interaction of merosin-integrin α6β1 in the thymus, and merosin-dystroglycan or merosin-integrin α7β137 in muscle. It is suggested that the interaction of ECM proteins and thymocytes influences the intrathymic migration and differentiation of T lymphocytes in the thymus.1 There is increasing evidence that integrins transmit signals into cells and suppress anchorage-related apoptosis (anoikis).38,39 Investigation of signalling pathways via ECM proteins will reveal the molecular mechanisms that prevent apoptotic cell death of lymphocytes and muscular cells.
Acknowledgments
This work was supported by grants-in-aid from the Ministries of Health and Welfare, and Education, Science, Culture and Sports, of Japan. The authors are grateful to Dr H. Hori and Dr T. Kina for providing various mAbs, and to Dr Margaret Dooley Ohto for reading this manuscript.
References
- 1.Savino W, Villa-verde D-MS, Lannes-vieira J. Extracellular matrix proteins in intrathymic T-cell migration and differentiation. Immunol Today. 1993;14:158. doi: 10.1016/0167-5699(93)90278-S. [DOI] [PubMed] [Google Scholar]
- 2.van Ewijk W. T-cell differentiation is influenced by thymic microenvironments. Annu Rev Immunol. 1991;9:591. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Burgeson RE, Chiquet M, Deutzmann R, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 6.Miner JH, Patton BL, Lentz SI, et al. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuolteenaho R, Nissinen M, Sainio K, et al. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124:381. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernier SM, Utani A, Sugiyama S, Doi T, Polistina C, Yamada Y. Cloning and expression of laminin alpha 2 chain (merosin) in the mouse. Matrix Biol. 1995;14:447. doi: 10.1016/0945-053x(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamada H, Hori H, Tanaka T, et al. Secretion of laminin alpha 2 chain in cerebrospinal fluid. FEBS Lett. 1995;376:37. doi: 10.1016/0014-5793(95)01240-3. [DOI] [PubMed] [Google Scholar]
- 12.Simon-assmann P, Duclos B, Orian-rousseau V, et al. Differential expression of laminin isoforms and alpha 6-beta 4 integrin subunits in the developing human and mouse intestine. Dev Dyn. 1994;201:71. doi: 10.1002/aja.1002010108. [DOI] [PubMed] [Google Scholar]
- 13.Chang AC, Wadsworth S, Colligan JE. Expression of merosin in the thymus and its interaction with thymocytes. J Immunol. 1993;151:1789. [PubMed] [Google Scholar]
- 14.Chang AC, Salomon DR, Wadsworth S, et al. α3β1 and α6β1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol. 1995;154:500. [PubMed] [Google Scholar]
- 15.Ibraghimov-beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 16.Tome FM, Evangelista T, Leclerc A, et al. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994;317:351. [PubMed] [Google Scholar]
- 17.Michelson AM, Russel E, Harman PJ. Dystrophia muscularis: a hereditary primary myopathy in the house mouse. Proc Natl Acad Sci USA. 1955;41:1079. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arahata K, Hayashi Y, Koga R, et al. Laminin in animal models for muscular dystrophy: Defect of laminin M in skeletal and cardiac muscles and peripheral nerve of the homozygous dystrophic dy/dy mice. Proc Japan Acad. 1993;69:259. Series B. [Google Scholar]
- 19.Xu H, Christmas P, Wu X-R, Wewer UM, Evgvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA. 1994;91:5572. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269:13729. [PubMed] [Google Scholar]
- 21.Xu H, Wu X-R, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994;8:297. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- 22.Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2j mice. Hum Mol Genet. 1995;4:1055. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- 23.Miyagoe Y, Hanaoka K, Nonaka I, et al. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 24.Hiramine C, Hojo K, Koseto M, Nakagawa T, Mukasa A. Establishment of a murine thymic epithelial cell line capable of inducing both thymic nurse cell formation and thymocyte apoptosis. Lab Invest. 1990;62:41. [PubMed] [Google Scholar]
- 25.Miyake S, Sakurai T, Okumura K, Yagita H. Identification of collagen and laminin receptor integrins on murine T lymphocytes. Eur J Immunol. 1994;24:2000. doi: 10.1002/eji.1830240910. [DOI] [PubMed] [Google Scholar]
- 26.Noto K, Kato K, Okumura K, Yagita H. Identification and functional characterization of mouse CD29 with a mAb. Int Immunol. 1995;7:835. doi: 10.1093/intimm/7.5.835. [DOI] [PubMed] [Google Scholar]
- 27.Wada K, Kina T, Kawamoto H, Kondo M, Katsura Y. Requirement of cell interactions through adhesion molecules in the early phase of T cell development. Cell Immunol. 1996;170:11. doi: 10.1006/cimm.1996.0128. [DOI] [PubMed] [Google Scholar]
- 28.Hori H, Kanamori T, Mizuta T, Yamaguchi N, Liu Y, Nagai Y. Human laminin M chain: epitope analysis of its monoclonal antibodies by immunoscreening of cDNA clones and tissue expression. J Biochem (Tokyo) 1994;116:1212. doi: 10.1093/oxfordjournals.jbchem.a124666. [DOI] [PubMed] [Google Scholar]
- 29.Hiramine C, Nakagawa T, Miyauchi A, Hojo K. Thymic nurse cells as the site of thymocyte apoptotic cell clearance in the thymus of cyclophosphamide-treated mice. Lab Invest. 1996;75:185. [PubMed] [Google Scholar]
- 30.De Kretser TA, Livett BG. Evidence of a thymic abnormality in murine muscular dystrophy. Nature. 1976;263:682. doi: 10.1038/263682a0. [DOI] [PubMed] [Google Scholar]
- 31.Karmali RA, Horrobin DF. Abnormality of thymus growth in dystrophic mice. Nature. 1976;263:684. doi: 10.1038/263684a0. [DOI] [PubMed] [Google Scholar]
- 32.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vacchio MS, Ashwell JD. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med. 1997;185:2033. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaitseva MB, Mojcik CF, Salomon DR, Shevach EM, Golding H. Co-ligation of α4β1 integrin and TCR rescues human thymocytes from steroid-induced apoptosis. Int Immunol. 1998;10:1551. doi: 10.1093/intimm/10.10.1551. [DOI] [PubMed] [Google Scholar]
- 36.Agea E, Bistoni O, Bini P, et al. Costimulation of CD3TCR complex with either integrin or nonintegrin ligands protects CD4+ allergen-specific T-cell clones from programmed cell death. Allergy. 1995;50:677. doi: 10.1111/j.1398-9995.1995.tb02585.x. [DOI] [PubMed] [Google Scholar]
- 37.Vachon PH, Xu H, Liu L, et al. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frisch SM, Vuori K, Ruoslahti E, Chan P-Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]