Abstract
The T‐cell receptor (TCR) is the critical structure involved in antigen recognition of T lymphocytes. Although the pig has a large proportion of circulating T lymphocytes bearing the γδ TCR, their study has been impeded due to the lack of specific antibodies. Here a monoclonal antibody (mAb) PPT27 directed to γδ TCR is described. Flow cytometry analyses showed that the mAb recognized a subset of T lymphocytes of which the majority expressed no CD2, CD4 and CD8 whilst the minority bore CD2 and CD8. The mAb precipitated a protein of 86 000 MW under non‐reducing conditions and a doublet of 43 000 MW under reducing conditions from peripheral blood T lymphocytes lysed in nonidet P‐40 buffer, whilst it precipitated the CD3–TCR complex from the cells lysed in digitonin. Further analysis revealed that the antibody recognized the majority, but not all, of the γδ T cells, suggesting that there may be more isotypes of γδ TCR than currently believed. The antibody was unable to stimulate γδ T cells to proliferate in vitro, suggesting that these cells are activated by a different activation mechanism from that of αβ T cells.
Introduction
The T‐cell receptor (TCR) is a heterodimeric surface protein associated with the CD3 subcomplex to form the CD3–TCR complex, which mediates antigen recognition and subsequent signal transduction and activation of immunocompetent T lymphocytes.1 Hitherto, two types of TCR have been identified, the αβ TCR, formed by α‐ and β‐chains and the γδ TCR, formed by γ‐ and δ‐chains. In rodents and humans, most T cells express the αβ TCR (termed αβ T cells) whilst a small number of T cells bear γδ TCR (γδ T cells). In contrast, in ruminants and pigs, there are a large proportion of circulating γδ T cells.
Recently, it has become clear that γδ T cells are strikingly different from αβ T cells in a number of important respects, such as their early appearance in ontogeny,2 limited V gene usage but extensive junctional diversity,2,3 direct recognition of antigens without antigen processing,3 unique trafficking and tissue distribution,2,4 as well as performing some functions not directly related to antigen recognition.5–10 All these differences indicate that γδ T cells contribute to immune competence in a unique way which remains poorly understood.
The pig, with its abundant circulating γδ T‐cell population, is a useful model for studies of the nature and functions of this T‐cell subset. Although three types of porcine γδ TCR have been identified by biochemical and molecular biological methods,11–13 specific monoclonal antibodies (mAb) are still lacking to identify directly, and therefore study, these γδ T cells and their receptors. Although several mAb putatively recognizing porcine γδ TCR have been reported in the second international CD workshop,14 these mAb have yet to be fully characterized.
In order to address this issue, we have developed a mAb directed to porcine TCR and here report its tissue distribution, biochemical property and lack of mitogenic effect.
Materials and methods
Animals and antibodies
The animals used in this study were outbred Large White pigs of either sex.
The following anti‐porcine lymphocyte mAbs have been documented: anti‐CD2, MSA4 [immunoglobulin G2a (IgG2a)];15 anti‐CD3, PPT3 (IgG1);16 anti‐CD4, 74‐12‐4 (IgG2b);17 anti‐CD8, PPT21 and PPT22 (IgG1);18 and anti‐sheep γδ TCR, 86D (IgG1).19 B cells were identified with fluorescein isothiocyanate (FITC)‐conjugated goat anti‐porcine immunoglobulin (Southern Biotechnology Association, Inc, Birmingham, AL). FITC‐ or phycoerythrin‐ (PE) labelled goat anti‐murine subclass immunoglobulin antibodies and streptavidin‐PE/CY.5 were purchased from Southern Biotechnology Association, Inc.
Preparation of mAb
The immunization has been described elsewhere.16 Briefly, 2 × 106 porcine peripheral blood lymphocytes (PBL) were injected into a footpad of a BALB/c mouse at 3‐day intervals. On day 22, the mice were killed and draining popliteal lymph nodes were removed for fusion. Fusion of cells was carried out as described before.16 Supernatants of hybrids were tested for their binding to porcine PBL by flow cytometric (FCM) analysis and one mAb PPT27 was selected for further characterization.
Flow cytometry
This was done as described elsewhere.20 For two‐colour staining, PBL were treated with a mixture of mAb PPT27 (IgG2b) and anti‐CD2 (IgG2a), CD3 (IgG1), anti‐pan‐CD8 mAb PPT21 (IgG1), anti‐CD8hi mAb PPT22 (IgG1), or FITC‐conjugated anti‐pig immunoglobulin, followed by incubation with a mixture of PE‐conjugated anti‐mouse IgG2b and either FITC‐anti‐mouse IgG2a, FITC‐anti‐mouse IgG1, or plain buffer. For co‐staining with anti‐CD4, the cells were first incubated with PPT27, followed by PE‐anti‐mouse IgG2b, blocked with 10% normal mouse serum and finally stained with biotinylated anti‐CD4 followed by FITC‐streptavidin. Three‐colour staining was conducted as previously described.20
Immunoprecipitation and glycosidase treatments
Iodination and immunoprecipitation were carried out following procedures as previously described.16 Glycosidase digestion of iodinated antigen precipitated by the protein G beads (Sigma Chemical Co, St Louis, MO) coated with mAb PPT27 was done with Endo‐F and Endo‐H (Boehringer Mannheim, Mannheim, Germany) following the manufacturer’s instructions.
Cell separation and lymphocyte proliferation
Porcine peripheral blood mononuclear cells (PBMC) were prepared as reported elsewhere.16 Cell subsets were selectively depleted from PBMC using the mini MACS system (Miltenyi Biotec GmbH, 51429 Bergisch Gladbach, Germany) following the manufacturer’s instructions. Induction of lymphocyte proliferation was conducted as previously described.16
Results
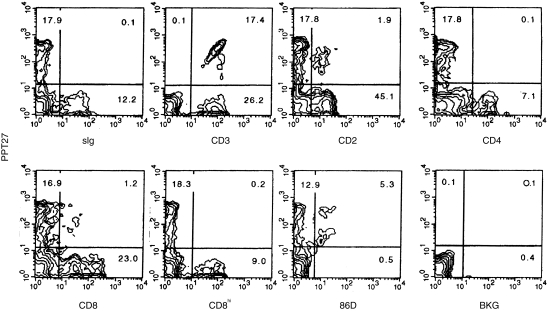
Screening hybridoma supernatants by FCM identified one mAb, code named PPT27, with reactivity to a proportion of PBL, but without reactivity to monocytes and granular cells (data not shown). In PBL, the mAb did not react with B cells but bound to a subset of T cells. The antigen was not expressed on CD4+ cells or CD8hi (CD8‐αβ)18 cells, and only a small proportion of cells bore CD2 or expressed CD8 at a lower level (CD8‐αα). Furthermore, cells recognized by 86D, a mAb directed to sheep γδ TCR19 and cross‐reactive with porcine T cells, were all reactive with the mAb (Fig. 1). Similarly, in all the lymphoid tissue tested, the mAb only reacted with a subpopulation of T cells, including all of the 86D+ cells. Interestingly, the phenotypes of the antigen‐positive cells in the tissues were different to that seen in PBL. For example, in the spleen, as in PBL, the mAb reacted with 30–40% of the T cells, but most of the positive cells expressed CD2 and a significant number expressed CD8‐αα. Similar phenotypes were found in the antigen‐bearing cells in mesenteric lymph nodes and tonsil, though here only a small percentage of T cells were stained (Table 1). In the thymus, the mAb reacted with a subpopulation of mature, large medullary thymocytes,16 and not with the smaller, immature cortical thymocytes.16 In contrast, CD3 was expressed on both smaller and larger thymocytes at different levels as previously reported16 (Fig. 2a). Unlike the peripheral tissues, the antigen‐bearing cells which were stained by pan‐CD8 mAb were also stained by anti‐CD8hi mAb, suggesting that these cells expressed CD8‐αβ dimer (Table 1).
Figure 1.
Phenotypes of the PPT27‐reactive cells as defined by two‐colour FCM. Porcine PBL were stained with the indicated mAb as described in the Materials and Methods. Figures in the quadrants are percentages. Background staining (BKG) was that of cells stained with medium instead of mAb.
Table 1.
Staining percentage of T lymphocytes by mAb PPT27 and subset composition of the mAb reactive cells from blood and lymphoid tissues. Figures are mean ± SD from five animals aged between 3 and 4 weeks
| Subset composition of PPT27+ cells (%) | ||||||
|---|---|---|---|---|---|---|
| Tissue | % stained T cells | CD2+ | CD4+ | CD8+ | CD8hi | 86D+ |
| PBL | 39·9 ± 17·1 | 9·6 ± 2·0 | – * | 6·6 ± 4·8 | – | 29·1 ± 15·3 |
| Spleen | 34·9 ± 14·8 | 95·6 ± 13·1 | – | 31·3 ± 4·1 | – | 40·9 ± 7·8 |
| MLN | 4·0 ± 4·1 | 77·8 ± 9·3 | – | 60·0 ± 7·3 | – | 51·9 ± 5·6 |
| Tonsil | 5·9 ± 3·9 | 46·2 ± 4·0 | – | 45·6 ± 5·5 | – | 42·9 ± 7·1 |
| Large thymocytes | 25·2 ± 7·7 | 96·7 ± 8·5 | 2·7 ± 0·9 | 15·9 ± 3·2 | 16·1 ± 5·0 | 17·4 ± 6·9 |
No significant number of cells with this marker found in the mAb reactive cells. PBL, peripheral blood lymphocytes; MLN, mesenteric lymph nodes.
Figure 2.
(a) Monoclonal antibody PPT27 reacts only with larger thymocytes. Porcine thymocytes were stained with either mAb PPT27 or anti‐CD3 followed by PE‐anti‐mouse immunoglobulin and analysed using FCM for fluorescence intensity versus forward scatter (FSC). (b) The antigen comodulates with CD3. Porcine PBL coated with anti‐CD3 mAb PPT3 (IgG1) were cultured at 37° on the monolayer of cell line 16.2 CG7 (a mouse L‐cell line stably transfected with CD32) for up to 48 hr. Aliquots of the cells were taken at the time‐points indicated and were stained with a mixture of PPT27 (IgG2b) and PPT3, followed by a mixture of PE‐anti‐mouse IgG2b and FITC‐anti‐mouse IgG1, then analysed by FCM.
A notable feature of the antigen expression was that it correlated linearly with that of CD3, suggesting that the antigen is coexpressed with CD3 (Fig. 1). This notion was confirmed by the antigenic modulation experiments. Unlike human CD3, the porcine CD3 molecule is difficult to modulate. Thus, the modulation was induced by culturing anti‐CD3‐coated PBL with the mouse L‐cell line expressing FcR. As shown in Fig. 2(b), a decreased expression of CD3 was accompanied by a decreased binding of mAb PPT27. The antigen recognized by PPT27 remained correlated linearly with CD3 and decreased accordingly until both of them disappeared from the cell surface (Fig. 2b). This indicates that the antigen is a surface structure which is coexpressed and associated with CD3. In other words, it is a component of the CD3–TCR complex.
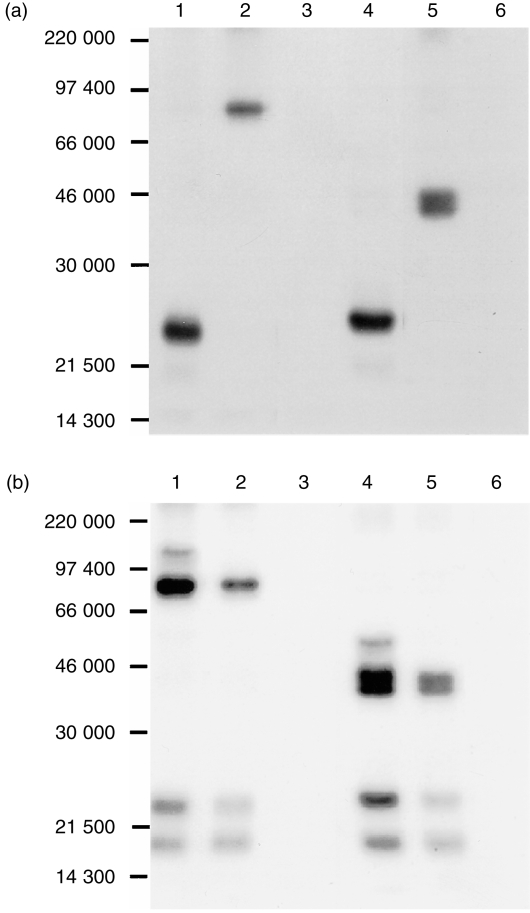
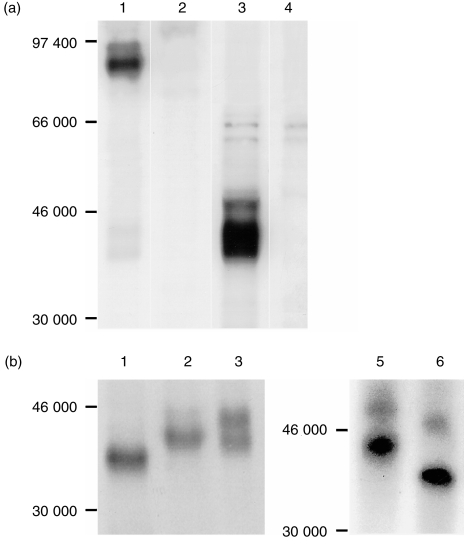
To verify this notion, immunoprecipitation experiments were conducted using the mAb in parallel with anti‐CD3 mAb. The anti‐CD3 mAb precipitated from PBL lysed, in nonidet P‐40 (NP‐40) lysis buffer, a major band of 23 000 MW under both reducing and non‐reducing conditions as previously reported,16 whereas the mAb precipitated a band of about 86 000 MW under non‐reducing conditions and a 43 000 MW doublet under reducing conditions, indicating that the antigen is a 43 000 MW dimer (Fig. 3a). When the cells were lysed in digitonin buffer, apart from the band of 23 000 MW, anti‐CD3 also precipitated a band of 18 500 MW which was not present in the precipitates of NP‐40 lysates. Although its size is similar to that of ζ‐ or η‐chain in other species, this polypeptide did not form a dimer. We therefore assume that this band is CD3 γ‐chain of which the size is quite different from that of CD3 ε‐ or δ‐chain in other species. In addition, two types of TCR of 43 000 and 55 000 MW dimers were also precipitated as previously reported.16 Meanwhile, the mAb precipitated CD3 in addition to the 43 000 MW dimer (Fig. 3b), suggesting that the mAb is directed to TCR. Combined with its tissue distribution, these results suggest that the antigen recognized by the mAb is γδ TCR.
Figure 3.
The mAb PPT27 precipitates TCR. 125I‐labelled porcine PBL were lysed either in NP‐40 (a) or digitonin (b) lysis buffer and precipitated with Protein G–Sepharose 4B beads coated with anti‐CD3 mAb PPT3 (lanes 1, 4), mAb PPT27 (lanes 2, 5), or normal mouse serum (lanes 3, 6). Gels (10%) run under non‐reducing (lanes 1–3) or reducing (lanes 4–6) conditions.
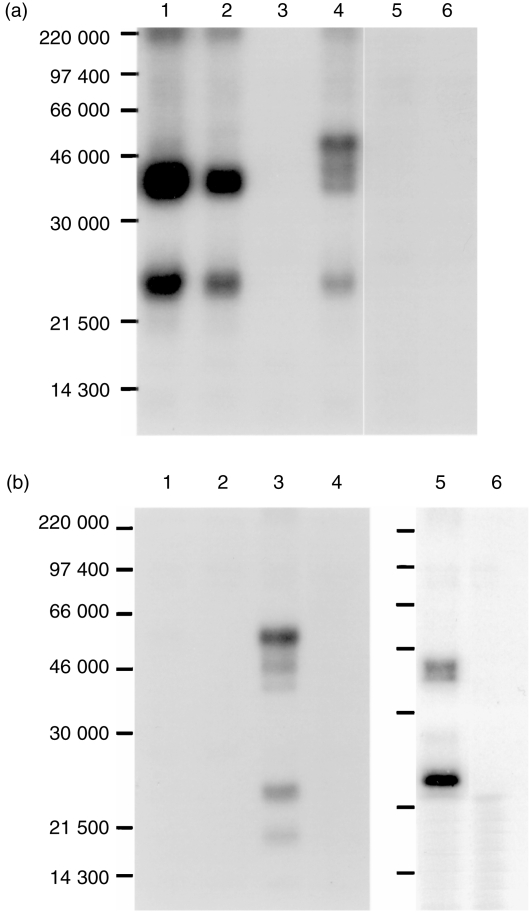
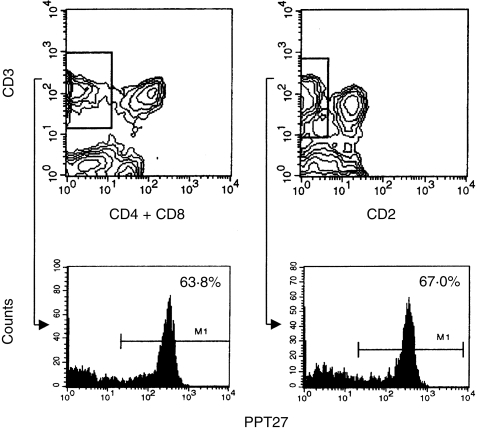
To address this issue further, different PBL subsets were prepared using negative selection and were used in immunoprecipitation. When PBL were depleted of CD4+ and CD8hi cells and lysed in digitonin, both PPT27 and anti‐CD3 mAb precipitated only the 43 000 MW dimer in addition to the CD3 molecules. As most αβ T cells had been removed by depletion, this suggests that the 55 000 MW dimer is αβ TCR and the 43 000 MW dimer is γδ TCR. When the cells were depleted of the antigen‐positive cells, on the other hand, the major type of TCR precipitated by anti‐CD3 was the 55 000 MW dimer, though a fainter 43 000 MW dimer also existed (Fig. 4a). In accordance, when PBL lysate in digitonin buffer was precleared with the mAb, anti‐CD3 still precipitated a faint doublet of 43 000 MW, whereas preclearing the lysate with anti‐CD3 completely removed the antigen. Furthermore, when CD4– CD8– cells were lysed in digitonin buffer and precleared with the mAb, anti‐CD3 still precipitated the doublet of 43 000 MW, indicating that this band is γδ TCR (Fig. 4b). Therefore, it seems that the mAb recognises an epitope present on the majority, but not all, of the γδ TCR. This interpretation was supported by the fact that more than 30% of the CD4– CD8– double negative or CD2– T cells, which are known to be γδ T cells, did not express the antigen, as revealed by FCM analysis (Fig. 5).
Figure 4.
The mAb PPT27 recognizes most, but not all, γδ TCR. (a) Immunoprecipitation of PBL subsets. Porcine PBL depleted with anti‐CD4+ anti‐CD8hi (lanes 1–3) or with PPT27 (lanes 4–6) were 125Iodinated and lysed in digitonin lysis buffer before being precipitated with anti‐CD3 (lanes 1, 4), PPT27 (lanes 2, 5) or normal mouse serum (lanes 3, 6). Gel (10%) run under reducing conditions. (b) Sequential immunuoprecipitation. 125Iodinated PBL (lanes 1–4) or CD4– CD8– cells (lanes 5, 6) lysed in digitonin buffer was precleared with anti‐CD3 (lanes 1, 2) or with PPT27 (lanes 3–6) before being precipitated with anti‐CD3 (lanes 1, 3, 5) or with PPT27 (lanes 2, 4, 6). Gel (10%) run under reducing conditions.
Figure 5.
Three‐colour FCM analysis of porcine PBL. T cells bearing no CD4/CD8 (upper left) or CD2 (upper right) were gated as indicated and analysed for reactivity with mAb PPT27.
As the mAb did not recognize all of the γδ TCR, we examined whether it reacted with a subset of γδ TCR which existed on CD2+ CD4– CD8– T cells.12 Immunoprecipitation was therefore conducted using spleen cells in which about 60% of the antigen‐positive cells were of this phenotype (Table 1). As shown in Fig. 6(a), in addition to the major 43 000 MW dimer seen in the PBL precipitates, the mAb precipitated a band of about 50 000 MW under reducing conditions and one of about 93 000 MW under non‐reducing conditions, indicating that mAb recognized another type of γδ TCR consisting of a 43 000/50 000 MW heterodimer (Fig. 6a).
Figure 6.
(a) Splenocytes express another type of γδ TCR. 125Iodinated splenocyte lysate in NP‐40 buffer was precipitated with PPT27 (lanes 1, 3) or normal mouse serum (lanes 2, 4). Gel (10%) run under non‐reducing (lanes 1, 2) or reducing (lanes 3, 4) conditions. (b) Deglycosylation of γδ TCR. 125Iodinated PBL (lanes 1–3) or splenocyte (lanes 4, 5) lysate in NP‐40 buffer precipitated with the mAb was treated with Endo‐F (lanes 1, 5), Endo‐H (lane 2) or buffer (lanes 3, 4). Gel (10%) run under reducing conditions.
A further experiment was conducted to explore the post‐translational modification of the antigen expressed on PBL. Treatment of the antigen with glycosidase Endo H diminished the amount of the larger band of the doublet, suggesting that it contained high‐mannose oligosaccharide, though the complex form might also exist. In contrast, the smaller band was resistant to the digestion, suggesting that its saccharide motif might be of the complex form. In support of this interpretation, treatment with Endo F reduced both the bands into one of about 40 000 MW. Treatment of splenocyte lysates with Endo F reduced the band of 50 000 MW to about 46 000 MW, suggesting that this larger band is not a more heavily glycosylated form of the 43 000 MW band (Fig. 6b).
Next, we examined the effect of mAb PPT27 on lymphocyte activation. We found that the mAb, whether used in the soluble form or immobilized onto the plate directly or indirectly through a second mAb, did not stimulate PBMC to proliferate. Removal of the αβ T cells from the cultures had no effect. Moreover, the mAb did not synergize the effect of anti‐CD3, which only induces αβ T cells to proliferate.21 Neither did the mAb co‐stimulate with interleukin‐2 or phorbol dibutyrate (PDB; data not shown).
Discussion
In this study, we report a mAb directed against porcine γδ TCR. The specificity of this mAb has been demonstrated by the distribution of the antigen among T‐cell subsets and the comodulation and coprecipitation of the antigen with CD3. The γδ TCR identified by this mAb is present on most, but not all, γδ T cells. The development of this mAb provides a tool for structural and functional studies of porcine γδ T cells.
Previously, porcine γδ TCR has been identified using a cross‐reactive antiserum of human δ‐chain. This antiserum precipitated a δ‐chain of 40 000 MW from T cells regardless of the phenotype of the cells. In contrast, three different γ‐chains with MW of 37 000, 38 000 and 46 000, respectively, were coprecipitated with the δ‐chain. Of these, however, only one type of γ‐chain was precipitated from a defined subset of γδ T cells. Thus, the 37 000, 38 000 and 46 000 MW γ‐chains were precipitated from CD2– 86D–, CD2– 86D+ and CD2+ CD4– CD8– T cells, respectively.11,12 In agreement with the biochemical work, molecular biological studies revealed the existence of at least three γ and one δ TCR‐constant region isotypes.13 However, because of the lack of mAb directed against different γ‐chains, it is still not clear whether their expression on different γδ T‐cell subsets is strictly related to the cell phenotypes mentioned above. Much less is known about the physiological implications of the existence of the γ‐chain isotypes. In this respect, the availability of an anti‐porcine γδ TCR mAb may help to address these issues.
Investigation into the reactivity of mAb PPT27 points to the possibility that there are probably more isotypes of γδ TCR present than currently believed. As the mAb reacts with a variety of γδ T cells, including all the three previously studied γδ T‐cell subsets, i.e. CD2– 86D–, CD2– 86D+ and CD2+ CD4– CD8– T cells, it is unlikely that the mAb is directed against an individual γ‐chain expressed only on one of these three subsets. As the δ‐chain expression is not subset‐restricted, it is tempting to conclude that the mAb is directed against the δ‐chain. However, as FCM and immunoprecipitation analyses have revealed, the mAb fails to react with a minor subset of γδ T cells. If mAb PPT27 is indeed directed to the δ‐chain, it follows that the δ‐chain is not a pan‐γδ T cell marker, and that there may be more than one type of δ‐chain present on different porcine γδ T cells. Alternatively, the mAb may be directed to a common epitope present on all three different γ‐chains, but absent on another unknown type of γ‐chain. Whichever the case, our study shows that the structure of porcine γδ TCR is more complicated than previously thought.
With respect to the chemical features of γδ TCR, our results are in general in support of previous reports. Minor differences in apparent MW upon sodium dodecyl sulphate–polyacrylamide gel electrophoresis analysis may be attributed to the different electrophoresis systems employed. Comparing our data with the previous report, we conclude that the larger band of the doublet in the PBL precipitate is the δ‐chain and the smaller is the γ‐chain. As the mAb reacts with both 86D+ and 86D– γδ T subsets, the γ‐chains precipitated from PBL should contain both the 86D+ and 86D– isotypes, though they could not be separated by size, or distinguished by differential N‐glycosylation. The third known type of γ‐chain, which is expressed on CD2+ CD4– CD8– T cells, is precipitated from splenocytes as a 50 000 MW protein. As this protein was only a minor component on the gel, in spite of the fact that 60% of antigen‐positive splenocytes are CD2+ CD4– CD8– (Table 1), it seems possible that not all of the CD2+ CD4– CD8– γδ T cells express this type of γ‐chain. To explore this and the functional and cellular correlates of the different porcine γ‐chain isotypes will clearly require specific monoclonal antibodies.
It is noteworthy that the mAb does not induce γδ T cells to proliferate. Although it is possible that the mAb is directed to a non‐mitogenic epitope, it is more likely that the lack of mitogenic effect of the mAb reflects the unique activation mechanism of porcine γδ T cells. In our previous studies, we have found that porcine γδ T cells, unlike αβ T cells, could not be induced to proliferate by anti‐CD3 mAb.21 This finding is reinforced by the current result. Hence, it seems that triggering of CD3–TCR alone is not sufficient to induce porcine γδ T cells to proliferate and additional signal(s) are probably needed. Indeed, evidence has indicated that mechanism of γδ T‐cell activation may be different from that of αβ T cells. For example, activation of γδ T cells has been reported to involve CD7 which is not involved in αβ T‐cell activation.22 Also, a certain surface structure expressed only on γδ T cells has been found to regulate γδ T‐cell activation.23 In this regard, the mAb may provide a useful tool for exploration of the distinct mechanism by which γδ T cells are activated.
References
- 1.Ashwell JD, Klausner RD. Genetic and mutational analysis of the T‐cell antigen receptor. Annu Rev Immunol. 1990;8:139. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- 2.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 3.Chien Y‐H, Jores R, Crowley MP. Recognition by γ/δ T cells. Ann Rev Immunol. 1996;14:511. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 4.Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol. 1991;9:679. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 5.Boismenu R, Havran W. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 6.Komano H, Fuijura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γ‐δ T‐cells. Proc Natl Acad Sci USA. 1995;92:6147. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann SHE, Blum C, Yamamoto S. Crosstalk between α/β T‐cells and γ/δ T‐cells in vivo: activation of α/β T‐cell response after γ/δ T‐cell modulation with the monoclonal antibody GL3. Proc Natl Acad Sci USA. 1993;90:9620. doi: 10.1073/pnas.90.20.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMenamin C, Pimm C, McKersey M, Holt P. Regulation of IgE responses to inhaled antigen in mice by antigen‐specific γδ T cells. Science. 1994;265:1869. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 9.Wen L, Roberts SJ, Viney JL, et al. Immunoglobulin synthesis and generalized autoimmunity in mice congenitally deficient in γ‐δ (+) T cells. Nature. 1994;369:654. doi: 10.1038/369654a0. [DOI] [PubMed] [Google Scholar]
- 10.Horner A, Jabara H, Ramesh N, Geha R. γ/δ T lymphocytes express CD40 ligands and induce isotype‐switching in B lymphocytes. J Exp Med. 1995;181:1239. doi: 10.1084/jem.181.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirt W, Saalmuller A, Reddehase MJ. Distinct γ/δ T cell receptors define two subsets of circulating porcine CD2–CD4–CD8– T lymphocytes. Eur J Immunol. 1990;20:265. doi: 10.1002/eji.1830200206. [DOI] [PubMed] [Google Scholar]
- 12.Saalmuller A, Hirt W, Reddehase MJ. Porcine γ/δ T lymphocyte subsets differing in their propensity to home to lymphoid tissue. Eur J Immunol. 1990;20:2343. doi: 10.1002/eji.1830201026. [DOI] [PubMed] [Google Scholar]
- 13.Thome M, Saalmuller A, Pfaff E. Molecular cloning of porcine T cell receptor α, β, γ and δ chains using polymerase chain reaction fragments of the constant regions. Eur J Immunol. 1993;23:1005. doi: 10.1002/eji.1830230503. [DOI] [PubMed] [Google Scholar]
- 14.Davis WC, Zuckermann FA, Hamilton MJ, et al. Analysis of monoclonal antibodies that recognize γδ T/null cells. Vet Immunol Immunopathol. 1998;60:305. doi: 10.1016/s0165-2427(97)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Hammerberg C, Schuig GG. Characterization of monoclonal antibodies directed against swine leucocytes. Vet Immuol Immunopathol. 1986;11:107. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Oura CAL, Kirkham PA, Parkhouse RME. Preparation of monoclonal anti‐porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology. 1996;88:577. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368. [PubMed] [Google Scholar]
- 18.Yang H, Parkhouse RME. Differential expression of CD8 epitopes amongst porcine CD8‐positive functional lymphocyte subsets. Immunology. 1997;92:45. doi: 10.1046/j.1365-2567.1997.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay CR, Hein WR. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;5:540. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Parkhouse RME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Parkhouse RME. Differential activation requirements associated with stimulation of T cells via different epitopes of CD3. Immunology. 1998;93:26. doi: 10.1046/j.1365-2567.1998.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrel S, Salvi S, Rafti F, Favrot M, Rapin C, Sekaly RP. Direct involvement of CD7 (gp40) in activation of TcR γ/δ+ cells. Eur J Immunol. 1991;21:1195. doi: 10.1002/eji.1830210515. [DOI] [PubMed] [Google Scholar]
- 23.Hanby‐flarida MD, Trask OJ, Yang TJ, Baldwin CL. Modulation of WC1, a lineage‐specific cell surface molecule of γ/δ T cells augments cellular proliferation. Immunology. 1996;88:116. doi: 10.1046/j.1365-2567.1996.d01-649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]