Abstract
Accumulating evidence suggests that macrophages function as major effector cells in the pathological process of various human diseases. We examined here the role of nuclear factor‐κB (NF‐κB) and caspases in the regulation of activation and apoptosis of macrophages. Activation of the human monoblastic leukaemia cell line, U937, by phorbol 12‐myristate 13‐acetate (PMA) increased the expression of CD14/CD86, and cytokine production. PMA stimulation also increased the expression of both pro‐caspase‐8 and pro‐caspase‐3 in U937, but not apoptosis or intracellular caspase‐3 activity. PMA also increased the expression of X‐chromosome‐linked inhibitor of apoptosis protein (XIAP) in U937, suggesting an inhibitory action for XIAP on the caspase cascade in PMA‐stimulated U937. Electrophoretic mobility shift assay (EMSA) showed a significant increase of nuclear NF‐κB activity in PMA‐stimulated U937. When a potent NF‐κB inhibitor, pyrrolidine dithiocarbamate (PDTC), was added to U937 cell culture in the presence of PMA, apoptosis was triggered by activation of caspase‐3, which was induced by caspase‐8 activation. XIAP expression was markedly suppressed in PMA‐treated U937 in the presence of PDTC. The inhibitors of caspase‐8 and caspase‐3 mostly inhibited apoptosis of U937 treated with PMA in the presence of PDTC. Furthermore, a phenotype of U937 treated with PMA and PDTC in the presence of caspase inhibitor was almost identical to that of unstimulated U937. Our results suggest that the signalling pathways involved in the activation and apoptosis of human macrophages could be co‐operatively regulated by the use of NF‐κB and caspase inhibitors, thus enabling the control of macrophage function and number.
Introduction
The transcription of most cytokine and costimulatory molecule genes in macrophages is dependent on the activation of nuclear transcriptional factors, such as nuclear factor κB (NF‐κB) or activator protein‐1 (AP‐1).1 In fact, NF‐κB is highly expressed in the nucleus of synovial infiltrating macrophages.2 Furthermore, NF‐κB is also a dominant regulator of apoptosis.3–6 Histological studies have detected apoptotic cell death of macrophages in both inflammatory and non‐inflammatory human diseases, such as synovial tissue of patients with rheumatoid arthritis and atheromatous plaque,7–12 suggesting that the signalling pathways leading to activation and/or apoptosis of macrophages in situ are regulated by NF‐κB activity.
Major effector gene products that induce apoptosis are members of the caspase family, which are conserved from nematodes to mammals.6,13 Of these, caspase‐3, which seems to be the most downstream caspase, activates a caspase‐activated deoxyribonuclease (CAD) through the cleavage of its inhibitor, ICAD, leading to fragmentation of DNA.14 Activation of inactive pro‐caspase‐3 by proteolysis is mediated by caspase‐8 or caspase‐9, located upstream of caspase‐3.6,13 On the other hand, activation of caspases is regulated by other gene products, including Bcl‐2‐related proteins and inhibitor of apoptosis protein (IAP) family proteins.6,13,15 Thus, antiapoptotic effects of NF‐κB seem to be mediated through regulation of apoptosis‐related gene expression.
To understand the molecular mechanisms involved in signalling pathways leading to activation and/or apoptosis of human macrophages, we examined in the present study the relationship among NF‐κB activity, activation of the caspase cascade and the expression of cell surface molecules on the human monoblastic leukaemia cell line, U937.
Materials and methods
Cell culture
U937 cells were maintained in RPMI‐1640 (Gibco, Paisley, Strathclyde, UK) supplemented with 10% fetal bovine serum (FBS; Medical & Biological Laboratories Co. [MBL], Nagoya, Japan), 100 units/ml of penicillin and 100 µg/ml of streptomycin, and grown in an atmosphere of 5% CO2 at 37°.
Activation of U937 by PMA
Cells were activated by the addition of phorbol 12‐myristate 13‐acetate (PMA; Sigma Chemical Co., St. Louis, MO). U937 cells were cultured with PMA (10 ng/ml) for 24 hr in RPMI‐1640 supplemented with 10% FBS. After incubation, the morphological features of U937 were examined using phase‐contrast microscopy. Cells were harvested and cell surface molecules were analysed by flow cytometry. In brief, 1 × 106 cells were washed with phosphate‐buffered saline (PBS) and incubated with phycoerythrin (PE)‐conjugated anti‐human CD14 monoclonal antibody (mAb) (PharMingen, San Diego, CA) or CD86 mAb (Immunotech, Marseille, France) for 30 min on ice. Stained cells were rewashed three times and analysed by flow cytometry using an Epics XL flow cytometer (Beckman Coulter, Hialeah, FL). In addition, the concentrations of various cytokines, such as interleukin (IL)‐1β, IL‐6, IL‐8 and tumour necrosis factor‐α (TNF‐α), produced in the culture supernatant of U937 cells were measured by using enzyme‐linked immunosorbent assay (ELISA) (Ohtsuka, Osaka, Japan), as described previously.16
Induction of apoptosis through NF‐κB inactivation
Apoptosis of PMA‐treated U937 cells was induced by the addition of pyrrolidine dithiocarbamate (PDTC), a potent NF‐κB inhibitor, as previously described.17,18 U937 cells were cultured with PMA in the presence or absence of PDTC (5 µm, Sigma) for 24 hr. After incubation, apoptotic cell death was quantified by determining the percentage of cells with hypodiploid DNA, as previously described.19,20 Briefly, cells (1 × 106) were fixed with 70% ethanol and treated with RNase (100 µg/ml, Sigma) then stained with propidium iodide (100 µg/ml, Sigma) for 30 min on ice. Stained cells were analysed by flow cytometry.
NF‐κB nuclear translocation in PMA‐stimulated U937 was detected by electrophoretic mobility shift assay (EMSA) using the Gel Shift Assay System (Promega Co., Madison, WI). Cells (1 × 107) were washed twice with PBS and then centrifuged (1400 g, 1 minute, 4°). The pellet was resuspended in low‐salt lysis buffer (10 mm HEPES, pH 7·9, 10 mm KCl, 0·1 mm EDTA, 0·1 mm EGTA, 1 µm dithiothreitol [DTT], and 1 mm phenylmethylsulphonyl fluoride [PMSF]) at 4° for 15 min, and Nonidet P‐40 (NP‐40) was added to a final concentration of 1·25%. Samples were then centrifuged (12 500 g, 5 min, 4°) and the cytosolic fraction was removed. The nuclear pellet was resuspended in extraction buffer (20 mm HEPES, pH 7·9, 400 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 µm DTT, 1 mm PMSF) at 4° for 30 min. The samples were then centrifuged (12 500 g, 5 min, 4°) and the resultant supernatant was stored at −80° prior to use.
Nuclear extract (7·5 µg) was mixed with 5 × binding buffer (50 mm Tris, pH 7·5, 250 mm NaCl, 5 mm MgCl2, 20% glycerol, 2·5 mm EDTA, 2·5 mm DTT), 2·5 µg poly (di‐dc)/poly (di‐dc) (0·25 mg/ml) and 3 × 104 counts per minute (c.p.m.) of double stranded oligonucleotide probe containing the NF‐κB binding site (5′‐AGTTGAGGGGACTTTCCCAGGC‐3′) labelled with [c‐32P]ATP, and incubated at room temperature for 20 min. Samples were run on a pre‐electrophoresed 89 mm Tris, 89 mm borate, 2 mm EDTA (TBE) 5% polyacrylamide gel for 120 min at 120 V. The gel was dried and autoradiographed by exposure to Hyperfilm. Nuclear extract from the human T‐cell lymphocytotropic virus‐I (HTLV‐I) ‐infected T‐cell line MT‐2 was used as a positive control for nuclear NF‐κB activity in EMSA.
Western blot analysis
Cells were washed with PBS and lysed using lysis solution containing 50 mm Tris, pH 8·0, 150 mm NaCl, 0·1% sodium dodecyl sulphate (SDS), 1% NP‐40, and 100 µg/ml PMSF. The protein concentration in cell extracts was determined by using the Bio‐Rad (Melville, NY) protein assay kit. An identical amount of protein in each lysate (20 µg/well) was subjected to 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Proteins were transferred to a nitrocellulose filter, and the filter was blocked for 1 hr using 5% non‐fat dried milk in PBS containing 0·1% Tween‐20 (PBS‐T), washed with PBS‐T, and incubated at room temperature for 1 hr in the presence of mouse anti‐human caspase‐3 mAb (Transduction Laboratories, Lexington, KY), mouse anti‐human caspase‐8 mAb (MBL), mouse anti‐human Bcl‐2 mAb (Dako Japan, Kyoto, Japan), rabbit anti‐human Bax polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti‐human Bcl‐xL mAb (Trevigen, Gaithersburg, MA), or mouse anti‐human X‐linked inhibitor of apoptosis protein (XIAP) mAb (MBL). mAb against β‐actin (Sigma) was used as an internal control. The filter was washed with PBS‐T and incubated with a 1 : 1000 dilution of secondary antibody, coupled with horseradish peroxidase. The enhanced chemiluminescence (ECL plus) system (Amersham, Bucks., UK) was used for detection. Anti‐caspase‐8 and anticaspase‐3 mAbs used in the experiments preferentially recognized a 55/54 000 MW form of pro‐caspase‐8 and a 32 000 MW form of pro‐caspase‐3, respectively. These forms are rapidly converted into the active subunits during the course of activation.21,22 Thus, a decrease in pro‐caspase‐8/pro‐caspase‐3 on Western blotting represented activation of caspase‐8/caspase‐3, respectively.21,22
In some experiments, we added Ac‐Asp‐Glu‐Val‐Asp‐aldehyde (DEVD‐CHO, 200 µm; Peptide Institute, Inc., Osaka, Japan), a caspase‐3 inhibitor, or Z‐Ile‐Glu (OMe)‐Thr‐Asp (OMe)‐FMK (IETD‐FMK, 200 µm; Enzyme Systems Products, Livermore, CA), a caspase‐8 inhibitor, to the cell culture 3 hr before adding PDTC, followed by examination of expression of the above molecules.
Measurement of intracellular caspase‐3 activity
Intracellular caspase‐3 activity was measured by using the PhiPhiLux G1D2 Kit (MBL), as previously described.23 Briefly, treated cells (1 × 106) were centrifuged to remove all of the culture medium. Then, 50 µl of rhodamine containing DEVD substrate at a concentration of 10 µm was added to the cell pellet and incubated in a 5% CO2 incubator at 37° for 60 min. Cells were washed once by ice‐cold flow cytometry dilution buffer and then resuspended in 500 µl of fresh dilution buffer. Samples were analysed by flow cytometry to determine the percentage of intracellular active caspase‐3+ cells.
Results
Activation of U937 by PMA
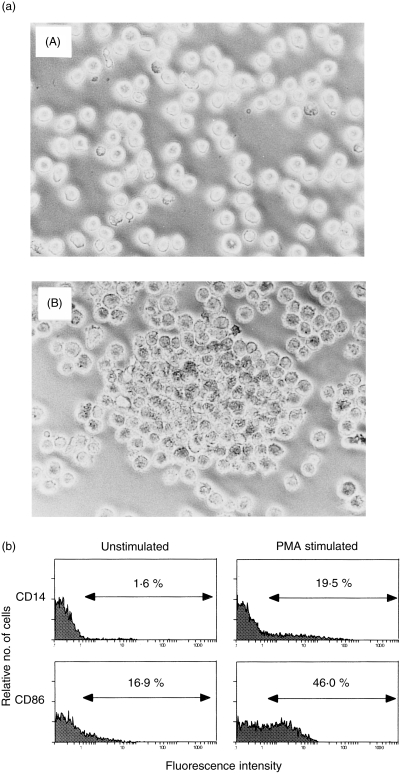
We initially examined the effect of PMA on activation of U937 cells. Figure 1(a) shows phase‐contrast microscopic features of U937 incubated with or without PMA. U937 cells tightly adhered to Petri dishes following stimulation with PMA. Flow cytometric analysis showed that CD86 expression on U937 was markedly augmented by PMA stimulation (Fig. 1b). CD14 expression could not be detected on unstimulated U937; however, its expression was clearly induced on PMA‐stimulated U937 (Fig. 1b). Furthermore, the production of various types of cytokines from U937 was also significantly increased by treatment with PMA (Table 1). These data indicate that PMA efficiently activates U937, thus confirming the results of previous studies.24
Figure 1.
(a) Phase‐contrast microscopic features of U937 cells stimulated or unstimulated with phorbol 12‐myristate 13‐acetate (PMA). U937 were cultured with or without 10 ng/ml of PMA for 24 hr, and morphological changes were observed by phase‐contrast microscopy. (A) unstimulated U937; (B) PMA‐stimulated U937. Note that U937 were found to be tightly adhered to Petri dishes after stimulation with PMA. Data are representative examples of four similar experiments. (b) Expression of CD14 and CD86 on U937 stimulated or unstimulated with PMA. U937 were cultured with or without 10 ng/ml of PMA for 24 hr, and the expression of CD14 and CD86 on U937 was examined as described in the text. Note that CD14 expression on U937 was induced by PMA, and CD86 expression on U937 was augmented by PMA. Numbers represent the percentage of positive cells. Data are representative examples of four similar experiments.
Table 1.
Cytokine production in U937 cells
| Cytokine production (pg/ml) | ||
|---|---|---|
| PMA (−) | PMA (+) | |
| IL‐1β | 47 | 435 |
| IL‐6 | 47 | 1011 |
| IL‐8 | 17130 | 286675 |
| TNF‐α | LT | 1584 |
Data are representative examples of four similar experiments.
IL, interleukin; PMA, phorbol 12‐myristate 13‐acetate; TNF‐α, tumour necrosis factor‐α.
LT: production < 20 pg/ml.
Induction of apoptosis in PMA‐treated U937 by NF‐κB inactivation
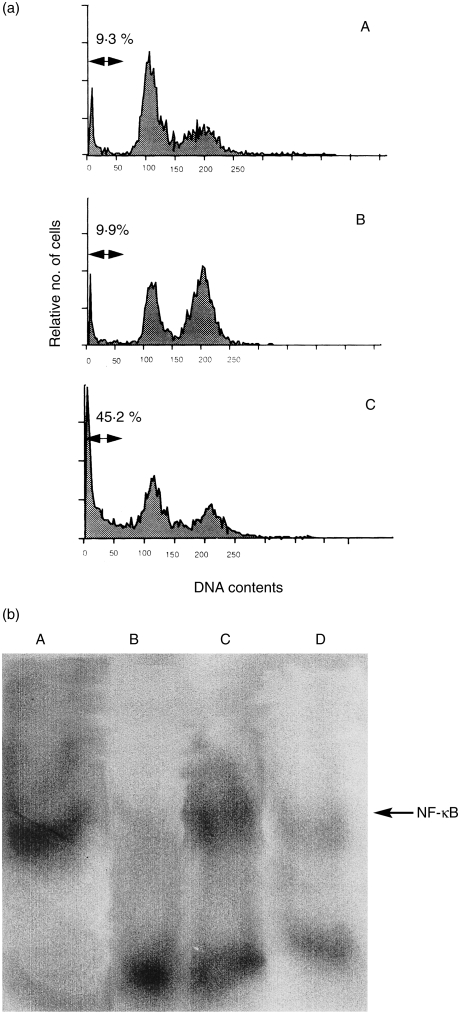
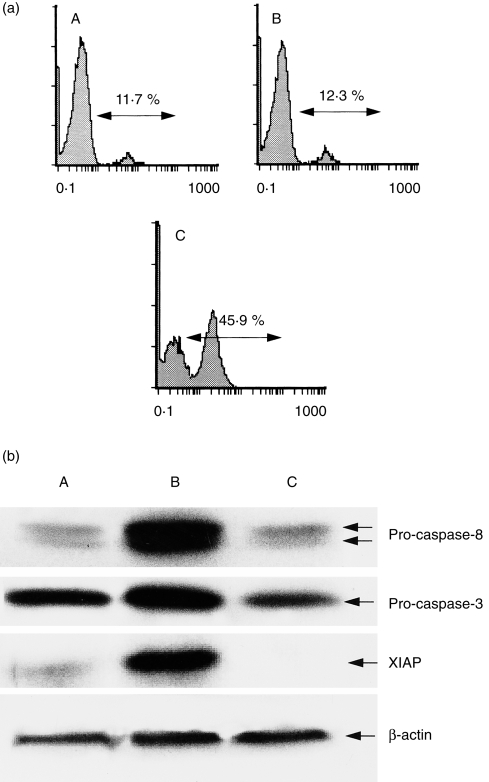
Previous studies have shown that certain T‐cell lines or T‐cell hybridomas undergo apoptosis following stimulation with PMA.25,26 However, in this study, apoptosis of cultured U937 was not augmented by PMA (Fig. 2a). NF‐κB nuclear translocation is accelerated by PMA,17 and recent experimental studies have revealed that NF‐κB nuclear translocation is a significant process that inhibits apoptosis.3–6 Therefore, we next examined the role of NF‐κB in PMA‐stimulated U937. NF‐κB nuclear translocation was significantly increased in PMA‐stimulated U937 (Fig. 2b). Suppression of NF‐κB nuclear translocation in PMA‐treated U937 by PDTC (lane D in Fig. 2b) was associated with apoptotic cell death (Fig. 2a). We also examined activation of the caspase cascade during this process. Caspase‐3 activation in U937 cells was not augmented by PMA treatment alone (Fig. 3a). However, the expressions of pro‐caspase‐8 and pro‐caspase‐3 were significantly increased in PMA‐stimulated U937 (Fig. 3b). These data suggest that the expression of certain antiapoptotic molecules is also induced by PMA stimulation, thus inhibiting the activation of pro‐caspases to active forms. Figure 3(b) shows that XIAP is weakly expressed in unstimulated U937, and its expression is significantly increased in PMA‐stimulated U937. Furthermore, increased caspase‐3 activity and reduced XIAP expression were evident in PMA‐treated U937 in the presence of PDTC (Fig. 3). The expression of pro‐caspase‐8 in PMA‐treated U937 was decreased by the addition of PDTC (Fig. 3b), suggesting the involvement of caspase‐8 activation in the apoptotic process. No Bcl‐xL expression was detected in U937, even in PMA‐stimulated cells, and Bcl‐2 expression on U937 was decreased by PMA stimulation (data not shown). Furthermore, Bax expression in U937 remained unchanged in either the presence or absence of PMA/PDTC (data not shown). These data suggest that activation of the caspase cascade (caspase‐8 to caspase‐3) during the apoptotic process is induced by NF‐κB inactivation in PMA‐treated U937, and that XIAP is a key molecule that regulates activation of the caspase cascade.
Figure 2.
(a) Apoptosis of U937 as determined by flow cytometry. U937 were cultured with or without phorbol 12‐myristate 13‐acetate (PMA) in the presence or absence of pyrrolidinedithiocarbamete (PDTC) for 24 hr, and apoptosis was examined as described in the text. Numbers represent the percentage of hypodiploid DNA‐positive cells. (A) unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 in the presence of PDTC. Note that apoptosis of U937 was not increased by PMA treatment alone, whereas it was significantly augmented in PMA‐treated U937 in the presence of PDTC. (b) Nuclear NF‐κB activity in U937 as determined by the electrophoretic mobility shift assay (EMSA). U937 were cultured with or without PMA in the presence or absence of PDTC for 6 hr, and nuclear NF‐κB activity in the cells was examined by EMSA, as described in the text. (A) positive control (MT‐2); (B) unstimulated U937; (C) PMA‐treated U937; (D) PMA‐treated U937 in the presence of PDTC. Note that nuclear NF‐κB activity was clearly increased in PMA‐treated U937, whereas it was significantly suppressed by PDTC. Data are representative examples of four similar experiments.
Figure 3.
(a) Percentage of intracellular active caspase‐3+ cells as determined by flow cytometry. U937 cells were cultured with or without phorbol 12‐myristate 13‐acetate (PMA) in the presence or absence of pyrrolidinedithiocarbamete (PDTC) for 24 hr, and the percentage of intracellular active caspase‐3+ cells was examined by flow cytometry. (A) Unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 in the presence of PDTC. Numbers represent the percentage of positive cells. Note that the percentage of intracellular active caspase‐3+ U937 was significantly increased in PMA‐treated U937 in the presence of PDTC. (b) Expression of pro‐caspase‐8, pro‐caspase‐3 and XIAP in U937 as determined by Western blot analysis. U937 were cultured with or without PMA in the presence or absence of PDTC for 24 hr, and the expression of the above molecules was examined by Western blot analysis. (A) Unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 in the presence of PDTC. Note that the expression of pro‐caspase‐8, pro‐caspase‐3 and XIAP was increased in PMA‐treated U937 whereas it was significantly decreased in PMA‐treated U937 in the presence of PDTC. Data are representative examples of four similar experiments.
NF‐κB and caspases co‐operatively regulate activation and apoptosis of U937
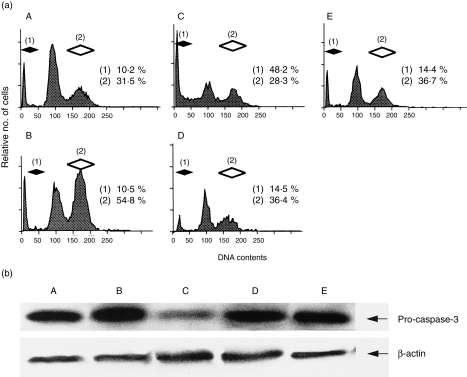
Experiments were performed to confirm the sequential activation of caspase‐8 to caspase‐3 in the apoptotic process induced by inactivation of NF‐κB in PMA‐treated U937. Flow cytometric analysis showed that PMA significantly augmented the percentage of U937 cells in G2/M although the percentage of cells with hypodiploid DNA did not change upon PMA stimulation (Fig. 4a, panel B). These results confirmed that PMA stimulation alone activates U937 but does not induce apoptosis. In contrast, the percentage of PMA‐treated U937 cells with hypodiploid DNA clearly increased in the presence of PDTC (Fig. 4a, panel C) However, the addition of IETD‐FMK or DEVD‐CHO inhibited most of the PDTC‐induced apoptosis (Fig. 4a, panels D and E). Furthermore, the decrease in pro‐caspase‐3 determined by Western blotting in PDTC‐treated U937 was blocked by adding IETD‐FMK or DEVD‐CHO (Fig. 4b). The increase in intracellular active caspase‐3+ cells detected by flow cytometry was also suppressed by IETD‐FMK or DEVD‐CHO (Fig. 4c). These data suggest that the sequential activation of caspase‐8 to caspase‐3 is necessary during PDTC‐induced apoptosis in U937 treated with PMA. Interestingly, the cell cycle and cell surface molecule analysis showed that a phenotype of U937 treated with PMA and PDTC in the presence of caspase inhibitor (IETD‐FMK or DEVD‐CHO) was almost identical to unstimulated U937 (Fig. 4a, 4d).
Figure 4.
(a) Cell cycle analysis of U937 as determined by flow cytometry. U937 were cultured with or without phorbol 12‐myristate 13‐acetate (PMA) in the presence or absence of pyrrolidinedithiocarbamete (PDTC)/caspase inhibitor for 24 hr. After incubation, both the percentage of cells with hypodiploid DNA and those at G2/M were examined as described in the text. (A) Unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 with PDTC; (D) PMA‐treated U937 with PDTC in the presence of Z‐Ile‐Glu (OMe)‐Thr‐Asp (OMe)‐FMK (IETD‐FMK); (E) PMA‐treated U937 with PDTC in the presence of Ac‐Asp‐Glu‐Val‐Asp‐aldehyde (DEVD‐CHO). The percentage of U937 with G2/M was significantly increased by PMA stimulation whereas that with hypodiploid DNA was unchanged. The percentage of cells with hypodiploid DNA was markedly increased in PMA‐treated U937 in the presence of PDTC; however, it was mostly inhibited by the addition of caspase‐8 (IETD‐FMK) or caspase‐3 (DEVD‐CHO) inhibitor. In addition, the percentage of G2/M cells of PMA‐treated U937 in the presence of PDTC and caspase inhibitor was almost similar to that of unstimulated U937. Numbers represent the percentage of positive cells (filled diamond, cells with hypodiploid DNA; open diamond, cells with G2/M). Data are representative examples of five similar experiments. (b) and (c) Inhibition of caspase‐3 activity by caspase inhibitor n PMA‐treated U937 in the presence of PDTC. (b) Detection of pro‐caspase‐3 by Western blotting. (c) Intracellular active caspase‐3+ cells as determined by flow cytometry. Numbers represent the percentage of positive cells. Note that both the decrease of pro‐caspase‐3 and the increase of intracellular active caspase‐3+ cells were blocked by the addition of caspase inhibitor. (A) Unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 with PDTC; (D) PMA‐treated U937 with PDTC in the presence of IETD‐FMK; (E) PMA‐treated U937 with PDTC in the presence of DEVD‐CHO. Data are representative examples of four similar experiments. (d) Regulation of cell surface molecule expression on U937 treated with or without PMA in the presence of PDTC/caspase inhibitor. U937 were cultured with or without PMA in the presence or absence of PDTC/caspase inhibitor for 24 hr. After incubation, the expression of CD14 and CD86 was examined by flow cytometry as described in the text. (A) Unstimulated U937; (B) PMA‐treated U937; (C) PMA‐treated U937 with PDTC in the presence of IETD‐FMK; (D) PMA‐treated U937 with PDTC in the presence of DEVD‐CHO. Note that the expression of CD14 and CD86 on PMA‐treated U937 with PDTC in the presence of caspase inhibitor was almost similar to that on unstimulated U937. Numbers represent the percentage of positive cells (dotted areas, negative control staining; open areas, CD14 or CD86 staining). Data are representative examples of three similar experiments.
Discussion
Activation of macrophages is a pivotal pathological feature of various diseases, including inflammatory and non‐inflammatory conditions.1,27–29 Activated macrophages produce various types of cytokines and increase the expression of costimulatory molecules in situ, thus acting in autocrine and paracrine fashions to perpetuate the pathological process.1,27–29 Recent studies have demonstrated the importance of regulation of cell growth and apoptotic cell death in the treatment of various human diseases.30,31 However, the mechanisms involved in the molecular interaction of the process in macrophages remain to be elucidated. In the present study, we examined the molecular mechanisms that regulate the activation and apoptotic cell death of the human monoblastic leukaemia cell line, U937.
Our results showed that PMA‐stimulated U937 tightly adhered to Petri dishes. These activated U937 expressed CD14, but particularly showed overexpression of CD86. Furthermore, PMA also activated cytokine production and the cell cycle. Previous studies have found that PMA‐stimulated T‐cell hybridomas are committed to activation‐induced cell death;25,26 however, PMA alone did not enhance apoptosis of U937 cells despite their activation. Thus, we next examined the expression of apoptosis‐related molecules in U937 cultured with or without PMA. Although the expressions of pro‐caspase‐8 and pro‐caspase‐3 on U937 were significantly increased by PMA, both hypodiploid DNA+ and intracellular active caspase‐3+ U937 cells were not increased by PMA, suggesting that the expression of antiapoptotic molecules in U937 was also augmented by PMA. Western blot analysis showed that the expression of XIAP, an inhibitor of caspase‐3 activation,15 was clearly increased in PMA‐stimulated U937 while Bcl‐2 expression on U937 was suppressed by PMA. On the other hand, Bcl‐xL expression could not be detected in U937 cultured in the presence or absence of PMA. These data suggest that XIAP is an important antiapoptotic molecule that suppresses the activation of caspase‐3 in PMA‐stimulated U937, thus inhibiting proteolytic cleavage of pro‐caspase to an active form.
NF‐κB is a nuclear transcriptional factor known to regulate the gene expression of cytokines and costimulatory molecules.17 In addition, recent studies have found that NF‐κB inhibits the apoptotic process, probably through the induction of expression of antiapoptotic molecules.3–6 Other studies have shown that NF‐κB is activated by PMA.17 As suspected, the activity of nuclear NF‐κB was augmented in PMA‐stimulated U937. When the potent NF‐κB inhibitor PDTC was added together with PMA, PMA‐induced NF‐κB nuclear translocation in U937 was markedly suppressed, resulting in apoptotic cell death. Activation of both caspase‐8 and caspase‐3 was observed during this process. Furthermore, the addition of caspase‐8 or caspase‐3 inhibitor almost abrogated PDTC‐induced apoptosis of U937, and the addition of caspase‐8 inhibitor markedly suppressed the activation of caspase‐3, confirming the sequential activation of caspase‐8 to caspase‐3 during the apoptotic process. Overexpression of XIAP in PMA‐stimulated U937 was also suppressed in the presence of PDTC, indicating again the importance of XIAP in the suppression of activation of the caspase cascade. PMA is also known to activate other nuclear transcriptional factors, such as AP‐1, in addition to NF‐κB.32 Thus, we speculate that the expression of each apoptosis‐related molecule is regulated by a different nuclear transcriptional factor, for example, XIAP by NF‐κB and caspases by AP‐1. Thus, NF‐κB inactivation may efficiently promote apoptotic cell death. A recent report has shown that XIAP expression in endothelial cells is positively regulated by NF‐κB, and its expression protects the cells from TNF‐α‐induced apoptosis.33
Although induction of apoptosis has been recently used as a novel strategy for the treatment of various human diseases, including inactivation of NF‐κB,30,31 potential adverse effects of this strategy are of concern as it may also result in elimination of excess cells in a non‐specific manner. For example, administration of anti‐Fas mAb to mice elicits severe hepatic injury.34,35 In this study, inhibitors of caspase‐8 and caspase‐3 almost abrogated PDTC‐induced apoptotic cell death of PMA‐treated U937. Furthermore, both cell cycle and cell surface molecule analyses in the present study showed that a phenotype of U937 treated with caspase inhibitor in the presence of PMA and PDTC was almost similar to that of unstimulated U937. These findings indicate that the use of inhibitors for both NF‐κB and caspases results in deactivation of macrophages without induction of apoptosis. These results suggest that the use of NF‐κB and caspase inhibitors could co‐operatively regulate the signalling pathways involved in the activation and apoptotic cell death of human macrophages. This could avoid the excess elimination of effector cells by induction of apoptotic cell death.
There is ample evidence to suggest that the key molecules involved in the regulation of apoptotic and antiapoptotic pathways are caspases and NF‐κB, which may interact with each other.36 The present data showed the importance of NF‐κB and caspases in the signalling pathways of human macrophages. These findings may allow the development of gene therapy for various human diseases, targeting the regulation of activation and apoptosis of macrophages.
Acknowledgments
This study was supported in part by Grants from the Ministry of Education, Science, Sports and Culture of Japan (no. 10922103). We thank Miss Y. Matsuo, Miss N. Fukuda and Miss Y. Imai for their excellent technical assistance.
Glossary
Abbreviations
- AP‐1
activator protein‐1
- CAD
caspase‐activated deoxyribonuclease
- DEVD‐CHO
Ac‐Asp‐Glu‐Val‐Asp‐aldehyde
- DTT
dithiothreitol
- EMSA
electrophoretic mobility shift assay
- IAP
inhibitor of apoptosis protein
- ICAD
inhibitor of CAD
- IETD‐FMK
Z‐Ile‐Glu (OMe)‐Thr‐Asp (OMe)‐FMK
- mAb
monoclonal antibody
- NF‐κB
nuclear factor‐κB
- PDTC
pyrrolidinedithiocarbamete
- PE
phycoerythrin
- PMA
phorbol 12‐myristate 13‐acetate
- PMSF
phenylmethylsulphonyl fluoride
- XIAP
X‐chromosome‐linked inhibitor of apoptosis protein
References
- 1.Burmester GR, Stuhlmüller B, Keyszer G, Kinne RW. Mononuclear phagocytes and rheumatoid synovitis. Arthritis Rheum. 1997;40:5. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- 2.Handel ML, McMorrow LB, Gravallese EM. Nuclear factor‐κB in rheumatoid synovium. Arthritis Rheum. 1995;38:1762. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- 3.Beg AA, Baltimore D. An essential role for NF‐κB in preventing TNF‐α‐induced cell death. Science. 1996;274:782. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 4.Wang C‐Y, Mayo MW, Balwin AS., Jr TNF‐ and cancer therapy‐induced apoptosis: potentiation by inhibition of NF‐κB. Science. 1996;274:784. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 5.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF‐α‐induced apoptosis by NF‐κB. Science. 1996;274:787. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S. Apoptosis by death factor. Cell. 1997;88:355. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima T, Aono H, Hasunuma T, et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- 8.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin‐1β‐converting enzyme. Am J Pathol. 1995;147:251. [PMC free article] [PubMed] [Google Scholar]
- 10.Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerosis lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149:367. [PMC free article] [PubMed] [Google Scholar]
- 11.Mallat Z, Ohan J, Leseche G, Tedgui A. Colocalization of CPP‐32 with apoptotic cells in human atherosclerotic plaques. Circulation. 1997;96:424. doi: 10.1161/01.cir.96.2.424. [DOI] [PubMed] [Google Scholar]
- 12.Isner JM, Kearney M, Bortman S, Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995;91:2703. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- 13.Reed JC. Cytochrome C: can’t live with it‐can’t live without it. Cell. 1997;91:559. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 14.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase‐activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 15.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X‐linked IAP is a direct inhibitor of cell‐death proteases. Nature. 1997;388:300. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami A, Eguchi K, Matsuoka N, et al. Inhibitory effects of interleukin‐10 on synovial cells of rheumatoid arthritis. Immunology. 1997;91:252. doi: 10.1046/j.1365-2567.1997.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeuerle PA, Henkel T. Function and activation of NF‐κB in the immune system. Annu Rev Immunol. 1994;12:141. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki K, Takeda H, Iwahashi H, Kitano S, Hanazawa S. NF‐κB inhibitors stimulate apoptosis of rabbit mature osteoclasts and inhibit bone resorption by these cells. FEBS Lett. 1997;410:297. doi: 10.1016/s0014-5793(97)00653-4. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami A, Eguchi K, Matsuoka N, et al. Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Mineral Res. 1997;12:1637. doi: 10.1359/jbmr.1997.12.10.1637. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami A, Eguchi K, Matsuoka N, et al. Inhibition of Fas antigen‐mediated apoptosis of rheumatoid synovial cells in vitro by transforming growth factor β1. Arthritis Rheum. 1996;39:1267. doi: 10.1002/art.1780390802. [DOI] [PubMed] [Google Scholar]
- 21.Cui H, Matsui K, Omura S, et al. Proteasome regulation of activation‐induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase‐8/a and caspase‐8/b. J Biol Chem. 1997;272:26953. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 23.Zapata JM, Takahashi R, Salvesen GS, Reed JC. Granzyme release and caspase activation in activated human T‐lymphocytes. J Biol Chem. 1998;273:6916. doi: 10.1074/jbc.273.12.6916. [DOI] [PubMed] [Google Scholar]
- 24.Barry OP, Pratico D, Savani RC, Fitzgerald GA. Modulation of monocyte–endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunner T, Mogil RJ, Laface D, et al. Cell‐autonomous Fas (CD95) /Fas–ligand interaction mediates activation‐induced apoptosis in T‐cell hybridomas. Nature. 1995;373:441. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 26.Ju ST, Panka DJ, Cui H, et al. Fas (CD95) / FasL interactions required for programmed cell death after T‐cell activation. Nature. 1995;373:444. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 27.Arend WP, Dayer J‐M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Hansson GK. Biology of disease. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991;64:5. [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS. Bisindolymaleimide VIII facilitates Fas‐mediated apoptosis and inhibits T cell‐mediated autoimmune disease. Nature Med. 1999;5:42. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 31.Miagkov AV, Kovalenko DV, Brown CE, et al. NF‐κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Cell signaling. In: Robertson M, Adams R, editors. Molecular Biology of the Cell. 3. New York, NY: Garland Publishing; 1994. p. 721. [Google Scholar]
- 33.Stehlik C, Martin R, Kumabashiri I, Schimid JA, Binder BR, Lipp J. Nuclear factor (NF)‐κB‐regulated X‐chromosome‐linked iap gene expression protects endothelial cells from tumor necrosis factor α‐induced apoptosis. J Exp Med. 1998;188:211. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogasawara J, Watanabe‐fukunaga R, Adachi M, et al. Lethal effect of the anti‐Fas antibody in mice. Nature. 1993;364:806. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 35.Fujisawa K, Asahara H, Okamoto K, et al. Therapeutic effect of the anti‐Fas antibody on arthritis in HTLV‐I tax transgenic mice. J Clin Invest. 1996;98:271. doi: 10.1172/JCI118789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giri DK, Aggarwal BB. Constitutive activation of NF‐κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT‐78 cells: autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]