Abstract
To identify histamine-producing cells at the late phase of allergic inflammation, the expression of l-histidine decarboxylase (HDC) was examined in the infiltrating leucocytes in the inflammatory locus. HDC activity and HDC mRNA levels in the infiltrating leucocytes in the pouch fluid of the immunized rats (that were injected with the antigen solution into the air pouch) were increased compared with those in the infiltrating leucocytes of the non-immunized rats. When infiltrating leucocytes collected 8 hr after antigen injection were cultured, histamine production by the cells from the immunized rats was higher than that from the non-immunized rats. In situ hybridization of HDC mRNA revealed that almost all the infiltrating leucocytes of the immunized rats, 4 hr after injection of the antigen, expressed HDC mRNA with high intensity, while those of the non-immunized rats showed only a weak intensity of HDC mRNA. In the immunized rats, ≈ 90% of leucocytes infiltrating in the pouch fluid at 4 hr were neutrophils and 8% were monocytes/macrophages. Neither mast cells nor basophils were detected in the infiltrating leucocytes. When rat peritoneal neutrophils were incubated in the presence of 12-O-tetradecanoylphorbol 13-acetate, histamine production was significantly increased. These findings suggest that the leucocytes, mainly neutrophils, infiltrating at the inflammatory locus are responsible for histamine production at the late phase of allergic inflammation.
Introduction
Histamine plays crucial roles in immune and inflammatory reactions via H1 and H2 receptors. In an air pouch-type allergic inflammation model in rats,1 the histamine content of the pouch fluid was found to increase biphasically.2 Histamine at the anaphylaxis phase (0–30 min after the antigen challenge) induced a transient increase in vascular permeability via H1 receptors.3 An increase in histamine content in the pouch fluid during this phase was caused by the degranulation of subcutaneous mast cells by an immunoglobulin E (IgE)-dependent mechanism.4 In contrast, at the late phase (4–24 hr after the antigen challenge), histamine production, which is not caused by mast cell degranulation but by the increase in de novo synthesis of histamine at the inflammatory site,5 does not contribute to the increase in vascular permeability but plays a role in down-regulation of leucocyte infiltration into the inflammatory locus via H2 receptors.2
It has been previously reported that the increase in l-histidine decarboxylase (HDC) activity of the inflammatory tissue during the late phase of allergic inflammation is regulated by histamine-production-increasing factor (HPIF), which increases histamine production by bone marrow cells.5 The histamine-production-increasing activity in the pouch fluid has been shown to increase during 4 to 24 hr after antigen challenge and this was followed by an increase in HDC activity in the pouch wall tissue.5 Recently, it was demonstrated that one candidate for HPIF in the late phase of allergic inflammation is granulocyte–macrophage colony-stimulating factor (GM-CSF).6 However, the cells responsible for histamine production at the late phase still remain to be clarified. Topical application of 12-O-tetradecanoylphorbol 13-acetate (TPA), a protein kinase C activator, on mouse skin induces an increase in HDC activity.7 The TPA-induced increase in HDC activity was also observed in mast cell-deficient W/Wv mice.8 Therefore, it is possible that cells other than mast cells are responsible for histamine production at the late phase of allergic inflammation. In an in vitro system, it has been reported that macrophages9–11 and T lymphocytes12 produce histamine as a result of various types of stimulation. However, the analysis of histamine-producing cells in vivo has not been carried out. The present study was aimed at clarifying the type of cells at the inflammatory site that are responsible for histamine production at the late phase of allergic inflammation.
Materials and methods
Induction of allergic inflammation in rats
Immunization and induction of air pouch-type allergic inflammation in rats were carried out as described previously.1 Male rats of the Sprague-Dawley strain, specific pathogen-free and weighing 150–180 g (Charles River Japan Inc., Kanagawa, Japan), were used. An antigen, azobenzenearsonate-conjugated acetyl bovine serum albumin (ABA-AcBSA), was synthesized according to the procedure described by Tabachnick & Sobotka.13 The lyophilized ABA-AcBSA was dissolved in saline at a concentration of 20 mg/ml and emulsified with an equal volume of Freund’s complete adjuvant (FCA; Difco Laboratories, Detroit, MI). Rats were immunized by intradermal (i.d.) injection of 0·5 ml of the ABA-AcBSA/FCA emulsion into two nuchal and three lumbar sites of each rat (0·1 ml/site). Nine days later, 8 ml of air was injected subcutaneously (s.c.) on the dorsum to make an ellipsoid-shaped air pouch.
Twenty-four hours after the injection of air, 2 mg of the antigen dissolved in 4 ml of a sterilized solution of 2% (w/v) sodium carboxymethylcellulose (Cellogen F3H; Daiichi Kogyo Seiyaku, Niigata, Japan) in saline supplemented with 0·1 mg/ml of penicillin G potassium and 0·1 mg/ml of dihydrostreptomycin sulphate (Meiji Seika Co., Tokyo, Japan) was injected into the air pouch to induce allergic inflammation. A group of rats that had been injected i.d. with FCA emulsion without the antigen received the antigen solution into the air pouch in the same manner and were used as the non-immunized controls. The rats were treated in accordance with procedure approved by the Animal Ethics Committee of the Graduate School of Pharmaceutical Sciences, Tohoku University, Japan.
Collection of leucocytes infiltrating the pouch fluid
At appropriate times after antigen challenge, rats were killed by cutting the carotid artery under diethylether anaesthesia and the entire pouch fluid was collected. The pouch fluid was centrifuged at 450 g and 4° for 10 min. The leucocytes precipitated by this procedure were washed three times with phosphate-buffered saline (PBS) and finally resuspended at an appropriate concentration in the indicated buffer or in Eagle’s minimal essential medium (EMEM), as described below.
Measurement of HDC activity in the leucocytes infiltrating the pouch fluid
HDC activity in the infiltrating leucocytes was determined according to the method described by Watanabe et al.7 Briefly, the leucocytes collected from the pouch fluid from the immunized or the non-immunized rats, 8 or 24 hr after injection of the antigen solution into the air pouch, were resuspended at 1·0 × 108 cells/ml in an HDC reaction buffer (0·1 m potassium-phosphate buffer, pH 6·8, 0·2 mm dithiothreitol, 0·01 mm pyridoxal 5′-phosphate, 2 µg/ml of leupeptin, 2 µg/ml of pepstatin and 1% (v/v) polyethylene glycol, MW 300). The cell suspension was sonicated for 1 min and centrifuged at 10 000 g and 4° for 15 min. Three hundred microlitres of the supernatant fraction was preincubated for 10 min at 37°, then 200 µl of the HDC reaction buffer containing 2·5 mm l-histidine was added and further incubated for 3 hr. The reaction was stopped by adding 500 µl of 0·8 N perchloric acid. After centrifugation at 220 g and 4° for 3 min, the supernatant fraction was collected. The histamine content in the fraction was measured fluorometrically, as described by Shore et al.14 Protein content in the supernatant fraction was measured according to the method described by Lowry et al.15 HDC activity was expressed as the amount of histamine formed per min per mg of protein.
Detection of HDC mRNA levels by using the reverse transcription–polymerase chain reaction
Leucocytes in the pouch fluid were collected 0, 2, 4 and 8 hr after injection of the antigen solution into the air pouch of the immunized and the non-immunized rats. Total RNA in the cells was extracted by acid guanidinium thiocyanate–phenol–chloroform extraction,16 and the yield of RNA extracted was determined by spectrophotometry. One microgram of RNA from each sample was reverse transcribed at 37° for 1 hr in 40 µl of the buffer (50 mm Tris-HCl, pH 8·3, 75 mm KCl and 3 mm MgCl2) containing 5 µm of random hexamer oligonucleotides (Gibco BRL, Gaithersburg, MD), 200 U of the reverse transcriptase from Moloney murine leukaemia virus (Gibco BRL), 0·5 µm deoxynucleotide 5′-triphosphate and 10 mm dithiothreitol. Polymerase chain reaction (PCR) primers for rat HDC were designed according to Joseph et al.17 The primer sequences used were: (forward) 5′-GGATCCATGCTGGGGCTCCCGGACTTC-3′ and (reverse) 5′-GTCGACAGCCATGTCTGTACCGTGTCT-3′, which amplify a 792 base pair (bp) HDC fragment. The PCR was performed for 30 cycles in 50 µl of the PCR buffer (10 mm Tris-HCl, pH 8·3, 50 mm KCl, 1·5 mm MgCl2) containing 5 µl of the synthesized cDNA solution, 0·25 µm each primer, 125 µm dNTP and 0·5 U of Taq polymerase (Takara Shuzo Co., Ohtsu, Shiga, Japan) using a thermal cycler (GeneAmpTM PCR System 2400; Perkin Elmer Cetus, Norwalk, CT). Each cycle consisted of a 30-second denaturation at 94°, 1 min of annealing at 56° and 2 min of extension at 72°. The rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (a housekeeping gene) was used as an internal standard gene. The PCR primers for rat GAPDH were as described by Robbins & McKinney.18 The primer sequences used were: (forward) 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and (reverse) 5′-TCCTTGGAGGCCATGTAGGCCAT-3′, which amplify a 249-bp GAPDH fragment. The PCR was performed for 27 cycles as follows: 30 seconds of denaturation at 94°, 1 min of annealing at 57°, and 2 min of extension at 72°. The other conditions were the same as those for rat HDC. After the PCR was performed, 9 µl of the PCR reaction mixture was loaded onto a 1·5% (w/v) agarose gel, and the PCR products were visualized by ethidium bromide staining after electrophoresis.
Measurement of histamine production by the infiltrating leucocytes of the immunized rats and the non-immunized rats
Four-hundred microlitres of the cell suspension (1·0 × 107 cells/ml) of the infiltrating leucocytes (collected 8 hr after injection of the antigen solution) were seeded into each well of 12-well plastic tissue culture plates (Costar Co., Cambridge, MA) and incubated for 24 hr at 37° in EMEM containing 10% (v/v) heat-inactivated fetal bovine serum (FBS; Flow Laboratories, Mclean, VA), 5·0 mm l-histidine and 0·02 mm pyridoxal 5′-phosphate, in an atmosphere of 5% CO2/95% air.
After incubation, the conditioned medium was collected and centrifuged at 220 g and 4° for 3 min. Histamine contents in the supernatant fraction were measured fluorometrically, as described above. As FBS contains a small amount of histamine, histamine content in EMEM containing 10% (v/v) heat-inactivated FBS was also determined and subtracted from each value obtained above.
May–Grünwald–Giemsa staining of the infiltrating leucocytes
Leucocytes collected 4 hr after injection of the antigen solution into the air pouch of the immunized and the non-immunized rats were resuspended at 1·0 × 108 cells/ml in PBS, then an aliquot of the cell suspension was smeared onto glass slides. The slides were immersed in May–Grünwald solution for 3 min, followed by immersion in Giemsa solution for 5 min.
Preparation of hybridization probes
A biotin-16-UTP-labelled single-strand RNA probe for rat HDC was synthesized using either T7 or SP6 RNA polymerase in a reaction medium containing biotin RNA labelling mix (Boehringer Mannheim GmbH Biochemica, Mannheim, Germany), according to the manufacturer’s instructions. Briefly, a 792-bp HDC PCR product, which amplified one part of the region encoding HDC mRNA, was subcloned into plasmid vector pGEM-T (pGEM-T/HDC; Promega, Madison, WI).
A biotin-16-UTP-labelled RNA probe was generated by transcribing linearized pGEM-T/HDC with T7 or SP6 RNA polymerase followed by destruction of the original template plasmid with RNase free DNase I (Boehringer Mannheim GmbH Biochemica). RNA probes were precipitated by LiCl and washed with 70% ethanol. The dried pellets were dissolved to yield a concentration of 10 µg/ml in the hybridization buffer consisting of 50% (v/v) deionized formamide, 4 × SSC (1 × SSC = 150 mm NaCl, 15 mm Na-citrate, pH 7·0), 200 µg/ml of yeast tRNA, 1 × Denhardt’s solution and 10% (w/v) dextran sulphate (final concentration).
In situ hybridization
In situ hybridization was carried out according to the procedure described by Lawrence et al.19 and Cox et al.20 In brief, leucocytes (collected 4 hr after injection of the antigen solution into the air pouch of the immunized and the nonimmunized rats) were resuspended at 1·0 × 108 cells/ml in PBS, and an aliquot of the cell suspension was smeared onto 2% 3-aminopropyltriethoxysilane (Shin-etsu Chemical Industries Co., Tokyo, Japan)-coated glass slides. Cells were fixed in 4% (w/v) paraformaldehyde in PBS for 4 hr followed by treatment with proteinase K (Boehringer Mannheim GmbH Biochemica; 20 µg/ml in TE [10 mm Tris-HCl, pH 8·0, 1 mm EDTA]) at 37° for 30 min. The slides were saturated in 4% (w/v) paraformaldehyde in PBS for 10 min and then washed with PBS. To inactivate endogenous alkaline phosphatase in the cells, the slides were treated with 0·2 m HCl for 10 min. After washing with PBS, the slides were saturated in 0·25% (v/v) acetic anhydride in TAE (0·1 m triethanolamine-HCl, pH 8·0 and 0·15 m NaCl) for 10 min, washed in PBS, dehydrated in ethanol and air-dried. The hybridization mixture was denatured, applied to the slides and covered with parafilm. Hybridization was performed at 50° for 16 hr in a humidified chamber. Then, the slides were washed successively for 30 min in: 5 × SSC at 50°; 50% formamide, 2 × SSC at 50°; and TNE (10 mm Tris-HCl, pH 7·6, 500 mm NaCl and 1 mm EDTA) at 37°; followed by RNase A digestion (10 µg/ml in TNE) at 37°. The slides were then washed successively for 20 min in: TNE at 37°; 2 × SSC at 50°; and twice in 0·2 × SSC at 50°. Subsequently, the slides were washed with TNT (0·1 m Tris-HCl, pH 7·6, 0·15 m NaCl and 0·05% [v/v] Tween-20) three times, then treated with TNB (0·1 m Tris-HCl, pH 7·5, 0·15 m NaCl, 0·5% [w/v] DuPont Blocking Reagent) for 3 hr. To detect HDC mRNA, the slides were incubated with avidin–biotin–alkaline phosphatase complex (1 : 500 dilution; Vector Laboratories, Burlinghame, CA) overnight at room temperature, washed with TNT three times and then reacted with alkaline phosphatase using nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) solution (Promega) as a substrate.
Preparation of rat peritoneal neutrophils
Male Sprague-Dawley rats, specific pathogen free and weighing 350–450 g (Charles River Japan), were used. Peritoneal neutrophils were obtained according to the method described previously21 with minor modifications. Briefly, peritoneal cells collected 16 hr after intraperitoneal injection of 30 ml of a sterilized Ca2+-free Krebs-Ringer solution containing 1% (w/v) casein (casein from milk, vitamin-free, Wako Pure Chemical Ind., Osaka, Japan) were washed twice with PBS and resuspended in 1 ml of buffer 1 (137 mm NaCl, 12 mm NaHCO3, 5·6 mm dextrose, 2·7 mm KCl, 0·4 mm NaH2PO4, 5 mm HEPES, 5 mm MES, 0·1% BSA, pH 6·8). To remove peritoneal mast cells, the cell suspension was layered over 6 ml of 80% (v/v) Percoll solution, followed by centrifugation at 450 g and 4° for 10 min. The cells at the interface were harvested, washed with EMEM and finally resuspended in EMEM, containing 10% FBS, at 1·0 × 107 cells/ml. Determination of the cell population by May–Grünwald–Giemsa staining indicated that greater than 98% were neutrophils and the rest were mononuclear cells.
Measurement of histamine production by peritoneal neutrophils
Four hundred microlitres of the cell suspension (1·0 × 107 cells/ml) of rat peritoneal neutrophils and 400 µl of EMEM containing the indicated concentrations of TPA (Sigma Chemical Co., St. Louis, MO), 5·0 mm l-histidine and 0·02 mm dithiothreitol were added to each well of 12-well plastic tissue culture plates and incubated for 24 hr at 37° in an atmosphere of 5% CO2/95% air.
After incubation, the conditioned medium was collected and centrifuged at 220 g and 4° for 3 min. Histamine content in the supernatant fraction was measured fluorometrically, as described above.
Statistical analysis
Statistical significance of the results was analysed by using Dunnett’s test for multiple comparison and by using the Student’s t-test for unpaired observations.
Results
HDC activity and histamine production by infiltrating leucocytes collected from the immunized and the non-immunized rats
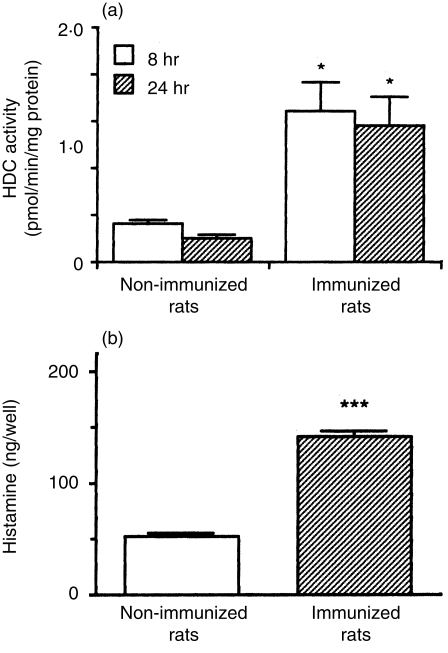
To determine whether the leucocytes infiltrating into the inflammatory site produce histamine, HDC activity in the leucocytes was determined. HDC activity in the leucocytes infiltrating the pouch fluid, at 8 and 24 hr after injection of the antigen solution into the air pouch of the immunized rats, was significantly higher than that in the leucocytes of the non-immunized rats (Fig. 1a). In addition, to determine whether the leucocytes produced histamine (consistent with HDC activity), the leucocytes collected 8 hr after injection of the antigen solution into the air pouch were cultured for 24 hr in EMEM containing 10% FBS, 5·0 mm l-histidine and 0·02 mm pyridoxal 5′-phosphate, and histamine levels in the conditioned medium were determined. As shown in Fig. 1(b), the leucocytes from the immunized rats produced a greater amount of histamine than leucocytes from the non-immunized rats.
Figure 1.
l-histidine decarboxylase (HDC) activity and histamine production by the leucocytes infiltrating the pouch fluid. (a) Leucocytes were collected from the pouch fluid of the immunized and the non-immunized rats 8 hr (open columns) and 24 hr (hatched columns) after injection of the antigen solution into the air pouch. Leucocytes (1·0 × 108 cells/ml) from each rat were sonicated, centrifuged and HDC activity in the supernatant fraction was determined as described in the Materials and methods. HDC activity is expressed as pmol/min/mg of protein. Values represent the mean ± SEM of three rats. Statistical significance: *P < 0·05 versus the corresponding non-immunized group. (b) Infiltrating leucocytes (4·0 × 106 cells/well) obtained from the immunized and the non-immunized rats 8 hr after injection of the antigen solution into the air pouch were cultured for 24 hr at 37° in Eagle’s minimal essential medium (EMEM) containing 10% fetal bovine serum (FBS), 5·0 mm l-histidine and 0·02 mm pyridoxal 5′-phosphate. Histamine contents in the conditioned medium were measured as described in the Materials and methods. Values represent the mean ± SEM of four rats. Statistical significance: ***P < 0·001 versus the non-immunized group.
HDC mRNA levels in leucocytes infiltrating into the pouch fluid of immunized and non-immunized rats
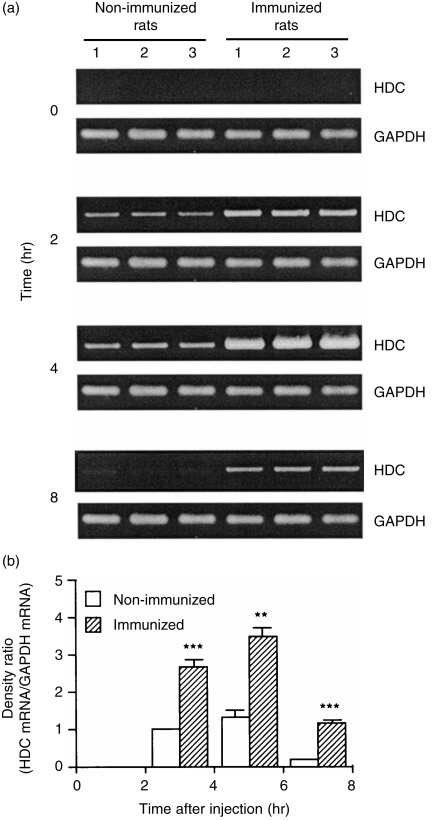
The levels of HDC mRNA in the infiltrating leucocytes of the immunized rats started to increase at 2 hr, peaked at 4 hr and then decreased at 8 hr (Fig. 2). In contrast, in the non-immunized rats, lower levels of HDC mRNA were detected at 2 and 4 hr (Fig. 2). The levels of GAPDH mRNA showed almost no difference between the two groups, remaining constant during 8 hr after injection of the antigen solution (Fig. 2).
Figure 2.
Time course of mRNA levels of l-histidine decarboxylase (HDC) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the leucocytes infiltrating the pouch fluid. (a) Leucocytes infiltrating the pouch fluid were collected, at the time-points indicated, after injection of the antigen solution into the air pouch of the immunized and the non-immunized rats. Total RNA was extracted and reverse transcription–polymerase chain reaction (RT–PCR) was performed as described in the Materials and methods. The mRNA levels were determined in the leucocytes from three individual rats in both groups. (b) The ratio of HDC mRNA density to GAPDH mRNA density is shown. Values represent the mean ± SEM from three individual rats. Statistical significance: ***P < 0·001, **P < 0·01 versus the corresponding non-immunized group. The ratio in the non-immunized rats at 2 hr was set to 1·0.
Analysis of the histamine-producing cells in the infiltrating leucocytes by in situ hybridization
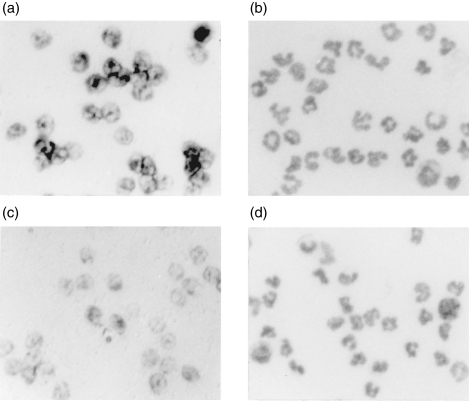
To identify the cells that express HDC mRNA, in situ hybridization was performed, using a biotin-labelled specific antisense probe for HDC mRNA, in leucocytes infiltrating the pouch fluid collected 4 hr after injection of the antigen solution into the air pouch. In the immunized rats, almost all the leucocytes expressed HDC mRNA, and the intensity of the HDC mRNA expression was marked to a greater extent in the immunized rats than in the nonimmunized rats (Fig. 3a, 3c). In contrast, no significant labelling was observed using the sense probe for HDC mRNA (data not shown).
Figure 3.
Detection of l-histidine decarboxylase (HDC) mRNA by in situ hybridization. The infiltrating leucocytes of the immunized rats (a and b) and the non-immunized rats (c and d) were collected 4 hr after injection of the antigen solution into the air pouch. In situ hybridization for HDC mRNA was performed using a biotin-labelled antisense HDC-specific RNA probe. HDC mRNA expression was visualized by nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) NBT/BCIP (a and c) using alkaline phosphatase as substrate. The population of leucocytes infiltrating the pouch fluid (collected 4 hr after injection of the antigen solution) from the immunized and the non-immunized rats, was examined by May–Grünwald–Giemsa staining (b and d). Magnification × 200.
The leucocytes infiltrating the pouch fluid 4 hr after injection of the antigen were examined by May–Grünwald–Giemsa staining (Fig. 3b, 3d). The proportions of neutrophils, monocytes/macrophages, eosinophils and mononuclear cells in the infiltrating leucocytes from the immunized rats were: 89·5 ± 0·3, 8·3 ± 0·4, 0·4 ± 0 and 1·8 ± 0·1%, respectively and those in the infiltrating leucocytes from the non-immunized rats were, respectively, 89·8 ± 0·2, 8·2 ± 0·2, 0·6 ± 0 and 1·4 ± 0·1% (mean ± SEM from four rats). There was no significant difference in cell population between the immunized rats and the non-immunized rats. Neither mast cells nor basophils were observed in the pouch fluid at 4 hr in either group.
Effects of TPA on histamine production by rat peritoneal neutrophils
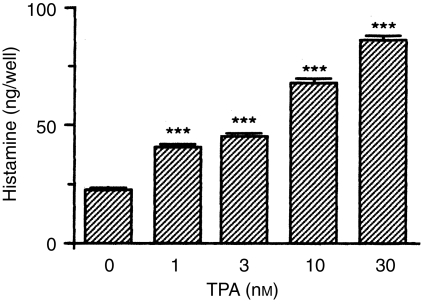
To show that neutrophils infiltrating the inflammatory site have histamine-producing ability, the effect of TPA on histamine production by rat peritoneal neutrophils was examined. When rat peritoneal neutrophils were incubated for 24 hr in EMEM containing 10% FBS, various concentrations of TPA, 5·0 mm l-histidine and 0·02 mm pyridoxal 5′-phosphate, histamine levels in the conditioned medium were increased in a concentration-dependent manner (Fig. 4).
Figure 4.
Effects of 12-O-tetradecanoylphorbol 13-acetate (TPA) on histamine production by rat peritoneal neutrophils. Rat peritoneal neutrophils (4·0 × 106 cells/well) were incubated for 24 hr at 37° in Eagle’s minimal essential medium (EMEM) containing 10% fetal bovine serum (FBS), various concentrations of TPA, 5·0 mm l-histidine and 0·02 mm pyridoxal 5′-phosphate. Histamine content in the conditioned medium was measured as described in the Materials and methods. Values represent the mean ± SEM of four wells. Statistical significance: ***P < 0·001 versus 0 nm TPA.
Discussion
In the air pouch-type allergic inflammation model in rats, histamine levels in the pouch fluid at the late phase are correlated with changes in HDC activity in the surrounding pouch wall tissue.5 The present work demonstrated that HDC activity and HDC mRNA levels in the infiltrating leucocytes were higher in immunized rats than in non-immunized rats (Figs 1, 2 and 3). Furthermore, when the infiltrating leucocytes from the immunized rats were cultured, histamine levels in the conditioned medium were increased and significantly higher than those from the non-immunized rats (Fig. 1). These results suggest that the leucocytes infiltrating the inflammatory locus are responsible for histamine production during the late phase of allergic inflammation.
Although it is reported that macrophages9–11 and T lymphocytes12 have the ability to produce histamine, it has not yet been clarified whether neutrophils produce histamine. Therefore, the present study is the first to suggest that neutrophils produce histamine. About 90% of the infiltrating leucocytes at 4 hr were neutrophils, and almost all leucocytes at 4 hr expressed HDC mRNA (Fig. 3). These findings indicate that neutrophils produce histamine. The possibility that neutrophils produce histamine was confirmed by the finding that rat peritoneal neutrophils produce histamine following TPA treatment (Fig. 4). Taken together, it was suggested that the neutrophils infiltrating the inflammatory locus are the major histamine-producing cells at the late phase. However, it is also possible that other types of infiltrating cells, such as macrophages, although their population is less than 10%, produce histamine at the inflammatory locus.
The population of each type of infiltrating leucocytes in the pouch fluid, 4 hr after injection of the antigen solution, was almost the same in immunized and non-immunized rats, although HDC activity and HDC mRNA level in the leucocytes were much higher in the immunized rats than in the non-immunized rats (Figs 1, 2 and 3). Therefore, it is probable that HDC protein in the leucocytes was induced by stimulation with factors produced at the inflammatory site of the immunized rats. The factor responsible might be HPIF5 because the level of HPIF activity in the pouch fluid of the immunized rats was much higher than that of the non-immunized rats and changed in parallel with histamine levels in the pouch fluid at the postanaphylaxis phase.5,22 Recently, it was reported that one candidate for HPIF is GM-CSF.6 GM-CSF is able to induce histamine production by immature cells in bone marrow.23 However, GM-CSF not only regulates haematopoiesis, such as differentiation and proliferation of monocytic and myelocytic progenitor cells via histamine synthesis,23,24 but also enhances various functions of mature inflammatory cells, such as degranulation, arachidonic acid release and cytokine secretion from neutrophils, superoxide anion release from macrophages and survival of eosinophils.25,26 Thus, GM-CSF might stimulate infiltrating neutrophils at the inflammatory site and induce histamine production.
It has been reported that histamine released in the late phase of allergic inflammation negatively regulates leucocyte infiltration via H2 receptors.2 It has also been reported that the infiltrating leucocytes in the pouch fluid of the immunized rats produce chemokines such as macrophage inflammatory protein-2 (MIP-2),27–29 and that an intrapouch injection of anti-MIP-2 antibody suppresses neutrophil infiltration.30 Thus, neutrophils produce both the positive regulator, MIP-2, and the negative regulator, histamine, for leucocyte infiltration. The balance of these regulators might be important for the control of neutrophil infiltration in allergic inflammation. Further investigations are necessary to clarify the mechanism of histamine production in neutrophils at the late phase of allergic inflammation.
Acknowledgments
This work was supported in part by a Grant-in-Aid for General Scientific Research (09672211), Exploratory Research (11877381) and Scientific Research (B) (11470481) from the Ministry of Education, Science, Sports and Culture of Japan.
Glossary
Abbreviations
- ABA-AcBSA
azobenzenearsonate-conjugated acetyl bovine serum albumin
- BCIP
5-bromo-4-chloro-3-indolyl-phosphate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HDC
l-histidine decarboxylase
- HPIF
histamine-production-increasing factor
- NBT
nitro blue tetrazolium
- TPA
12-O-tetradecanoylphorbol 13-acetate
References
- 1.Tsurufuji S, Yoshino S, Ohuchi K. Induction of an allergic air-pouch inflammation in rats. Int Arch Allergy Appl Immunol. 1982;69:189. doi: 10.1159/000233170. [DOI] [PubMed] [Google Scholar]
- 2.Hirasawa N, Ohuchi K, Watanabe M, Tsurufuji S. Role of endogenous histamine in postanaphylactic phase of allergic inflammation in rats. J Pharmacol Exp Ther. 1987;241:967. [PubMed] [Google Scholar]
- 3.Ohuchi K, Hirasawa N, Watanabe M, Tsurufuji S. Pharmacological analysis of the vascular permeability response in the anaphylactic phase of allergic inflammation. Eur J Pharmacol. 1985;117:337. doi: 10.1016/0014-2999(85)90007-x. [DOI] [PubMed] [Google Scholar]
- 4.Ohuchi K, Hirasawa N, Takeda H, Asano K, Watanabe M, Tsurufuji S. Mechanism of antianaphylactic action of β-agonists in allergic inflammation of air pouch type in rats. Int Arch Allergy Appl Immunol. 1987;82:26. doi: 10.1159/000234285. [DOI] [PubMed] [Google Scholar]
- 5.Hirasawa N, Ohuchi K, Kawarasaki K, Watanabe M, Tsurufuji S. Occurrence of histamine-production-increasing factor in the postanaphylactic phase of allergic inflammation. Int Arch Allergy Appl Immunol. 1989;88:386. doi: 10.1159/000234722. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi M, Hirasawa N, Mue S, Ohuchi K. Identification of histamine-production- increasing factor produced by stimulated RBL-2H3 rat basophilic leukemia cells as granulocyte–macrophage colony-stimulating factor. Biochim Biophys Acta. 1998;1403:273. doi: 10.1016/s0167-4889(98)00064-0. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, Taguchi Y, Sasaki K, Tsuyama K, Kitamura Y. Increase in histidine decarboxylase activity in mouse skin after application of the tumor promoter tetradecanoylphorbol acetate. Biochem Biophys Res Commun. 1981;100:427. doi: 10.1016/s0006-291x(81)80114-3. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi Y, Tsuyama K, Watanabe T, Wada H, Kitamura Y. Increase in histidine decarboxylase activity in skin of genetically mast-cell-deficient W/Wv mice after application of phorbol 12-myristate 13-acetate: evidence for the presence of histamine-producing cells without basophilic granules. Proc Natl Acad Sci USA. 1982;79:6837. doi: 10.1073/pnas.79.22.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi-nagata K, Watanabe T, Maeyama K, et al. Increase of histidine decarboxylase activity in murine myelomonocytic leukemia cells (WEHI-3B) in parallel to their differentiation into macrophage. Biochim Biophys Acta. 1988;972:249. doi: 10.1016/0167-4889(88)90199-1. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi-nagata K, Watanabe T, Yamatodani A, et al. In vitro increase of histidine decarboxylase activity and release of histamine by peritoneal resident cells of mast cell-deficient W/Wv mice; possible involvement of macrophages. J Biochem. 1988;103:24. doi: 10.1093/oxfordjournals.jbchem.a122233. [DOI] [PubMed] [Google Scholar]
- 11.Takamatsu S, Nakano K. Histamine synthesis by bone marrow-derived macrophages. Biosci Biotech Biochem. 1994;58:1918. doi: 10.1271/bbb.58.1918. [DOI] [PubMed] [Google Scholar]
- 12.Aoi R, Nakashima I, Kitamura Y, Asai H, Nakano K. Histamine synthesis by mouse T lymphocytes through induced histidine decarboxylase. Immunology. 1989;66:219. [PMC free article] [PubMed] [Google Scholar]
- 13.Tabachnick M, Sobotka H. Azoproteins. II. A spectrophotometric study of the coupling on diazotized arsanilic acid with proteins. J Biol Chem. 1960;235:1051. [PubMed] [Google Scholar]
- 14.Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182. [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Joseph DR, Sullivan PM, Wang Y-M, Kozak C, Fenstermacher DA, Behrendsen ME, Zahnow CA. Characterization and expression of the complementary DNA encoding rat histidine decarboxylase. Proc Natl Acad Sci USA. 1990;87:733. doi: 10.1073/pnas.87.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins M, McKinney M. Transcriptional regulation of neuromodulin (GAP-43) in mouse neuroblastoma clone N1E-115 as evaluated by the RT/PCR method. Mol Brain Res. 1992;13:83. doi: 10.1016/0169-328x(92)90047-f. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence JB, Singer RH. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucl Acids Res. 1985;13:1777. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox KH, Deleon DV, Angerer LM, Angerer RC. Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984;101:485. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- 21.Edamatsu T, Xiao Y-Q, Tanabe J, Mue S, Ohuchi K. Induction of neutrophil chemotactic factor production by staurosporine in rat peritoneal neutrophils. Br J Pharmacol. 1997;121:1651. doi: 10.1038/sj.bjp.0701322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirasawa N, Shiraishi M, Tokuhara N, et al. Pharmacological analysis of the inflammatory exudate-induced histamine production in bone marrow cells. Immunopharmacology. 1997;36:87. doi: 10.1016/s0162-3109(96)00164-6. [DOI] [PubMed] [Google Scholar]
- 23.Dy M, Schneider E, Gastinel L-N, Auffray C, Mermod JJ, Hamburger J. Histamine-producing cell-stimulating activity. A biological activity shared by interleukin 3 and granulocyte–macrophage colony-stimulating factor. Eur J Immunol. 1987;17:1243. doi: 10.1002/eji.1830170905. [DOI] [PubMed] [Google Scholar]
- 24.Piquet-pellorce C, Schneider E, Dy M. GM–CSF in association with IL-1 triggers day-8 CFU-S into cell cycle: role of histamine. J Cell Physiol. 1991;149:18. doi: 10.1002/jcp.1041490104. [DOI] [PubMed] [Google Scholar]
- 25.Gasson JC. Molecular physiology of granulocyte–macrophage colony-stimulating factor. Blood. 1991;77:1131. [PubMed] [Google Scholar]
- 26.Tachimoto H, Ebisawa M, Iikura Y, et al. Activated human mast cells release factors supporting eosinophil survival in vitro. Int Arch Allergy Immunol. 1997;113:293. doi: 10.1159/000237578. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe J, Watanabe M, Mue S, Ohuchi K. Leukocyte-derived neutrophil chemotactic factor-2 produced by infiltrated leukocytes in allergic inflammation model in rats is macrophage inflammatory protein-2. Immunol Invest. 1995;24:757. doi: 10.3109/08820139509060703. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe J, Watanabe M, Fujimoto N, Mue S, Ohuchi K. Characterization of leukocyte-derived neutrophil chemotactic factor-2 and its possible roles in neutrophil infiltration in allergic inflammation in rats. Int Arch Allergy Immunol. 1995;108:148. doi: 10.1159/000237132. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe J, Watanabe M, Mue S, Ohuchi K. The mechanism of neutrophil chemotactic factor production and the characteristic of the neutrophil chemotactic factor produced by the infiltrated leukocytes in allergic inflammation. Jpn J Inflammation. 1995;15:115. doi: 10.3109/08820139509060703. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Y-Q, Tanabe J, Edamatsu T, Hirasawa N, Mue S, Ohuchi K. Possible participation of macrophage inflammatory protein 2 in neutrophil infiltration in allergic inflammation in rats. Biochim Biophys Acta. 1997;1361:138. doi: 10.1016/s0925-4439(97)00034-3. [DOI] [PubMed] [Google Scholar]