Abstract
The repertoire of lymphocyte receptor genes encoded in a germline is further diversified by a number of processes, including the template-independent addition of nucleotides (N regions) by means of terminal deoxynucleotidyl transferase (TdT). Normally, mouse γδ T cells in the early fetal thymus, whose T-cell receptor (TCR) genes lack N regions and are encoded by Vγ3-Jγ1 and Vδ1-Dδ2-Jδ2 with canonical junctions (invariant Vγ3Vδ1), are thought to be the precursors of dendritic epidermal T cells (DETC). We generated mutant mice whose endogenous TdT promoter was replaced with the lck promoter through homologous recombination. These mutant mice expressed TdT in fetal thymus, had abundant N regions and infrequent canonical junctions in γ and δ rearrangements, and showed a decreased number of γδ T cells. Various Vγ3Vδ1 T cells, most of which had N regions in their TCR genes, were found to disseminate in the skin of newborn mutant mice, whereas normal numbers of DETCs with the invariant Vγ3Vδ1 rearrangement were observed in adult mutants. These data demonstrate that the regulation of TdT expression during fetal development is important for the generation of γδ T cells, and that Vγ3Vδ1 T cells, which have various junctional sequences in their TCR genes, randomly disseminate in skin, but invariant Vγ3Vδ1 T cells have a great advantage for proliferation in skin.
Introduction
Diversity of variable regions of T-cell receptor (TCR) and immunoglobulin genes is generated by the recombination of variable (V), diversity (D) and joining (J) segments, and is augmented by nibbling or addition of nucleotides at V-(D)-J junctions.1 The addition of nucleotides falls into two categories: template-dependent (P nucleotides)2 and template-independent (N regions).3 Gene targeting of terminal deoxynucleotidyl transferase (TdT) revealed that TdT, by which deoxynucleotides can be added to available 3′ ends, is the only major activity involved in physiological addition of N regions.4–6 TCR and immunoglobulin genes of TdT-deficient mice showed very restricted junctional diversity because of the lack of N regions and the frequent occurrence of particular junctions. TdT expression is regulated during ontogeny because TdT transcripts appear 3–5 days after birth but are not observed in the fetal stage,7 and TCR and immunoglobulin genes from fetal and neonatal repertoire lacked N regions and frequently had predominant junctional sequences.6
In the fetal thymus, γδ T cells appear as a series of overlapping waves.2,8 The Vγ3 and Vγ4 subsets appear first in two consecutive waves and form heterodimers with δ chains composed of Vδ1-Dδ2-Jδ2. Neither subset shows diversity in its V-J junctions. The Vγ3 subset comprises the vast majority of T cells in skin, whereas the Vγ4 subset is disseminated in tongue and the female reproductive tract in adult mice. Later in thymic development these subsets are replaced by the highly diversified Vγ2 and Vγ1 subsets, which form heterodimers with a variety of Vδ gene products and are exported to the blood and lymphoid organs.
Dendritic epidermal T cells (DETC), which are a unique population of γδ T cells in the epithelia of the skin of mice, have a highly restricted TCR repertoire.9 In the skin, most of the T cells express γ chains encoded by Vγ3-Jγ1 segments and δ chains encoded by Vδ1-Dδ2-Jδ2 segments. More than 80% of the productively rearranged γ and δ genes in skin have the same predominant (canonical) junctions that lack N regions.9,10 The use of Vγ3-Jγ1 and Vδ1-Dδ2-Jδ2 segments whose junctions are canonical in DETCs is also characteristic of the rearrangements found early in fetal development, indicating that the precursors of DETCs are produced in a series of overlapping waves during fetal thymic development.2,8
To investigate further the significance of the lack of TdT expression in fetal thymus and the reason why γδ T cells with the invariant Vγ3Vδ1 subset are disseminated in skin, we generated mutant mice in which TdT expression was regulated by the lck promoter through homologous recombination. In this work we showed that TdT expression in fetal thymus caused a decrease in the number of γδ T cells and that Vγ3Vδ1 T cells randomly disseminated in epidermis at the neonatal stage but invariant Vγ3Vδ1 DETCs selectively expanded in the epidermis.
Materials and methods
Mice
To construct the targeting vector, we cloned a 3·2-kb genomic KpnI–XhoI fragment, which contained exon 1, by partial digestion into pGEM-7Zf (Promega, Madison, WI). A DNA segment comprising the lck promoter was blunt-end ligated into a blunt-ended Csp451 site. To obtain the final targeting vector, we inserted a 6·3-kb ClaI–XhoI fragment containing the lck promoter and a genomic KpnI–XhoI fragment into a ClaI–XhoI site of pBluescript SK (Stratagene, La Jolla, CA); the latter contained an 8·7-kb genomic XbaI*–HindIII fragment 5′ of the TdT gene, a phosphoglycerol kinase-promoter–herpes simplex virus–thymidine kinase (PGK-HSV-tk) and a phosphoglycerol kinase–neomyan-resistant gene (PGK-neor), which was constructed previously4 (asterisk denotes a site in the cloning vector). Screening of embryonic stem (ES) cells and generation of homozygous mutants were carried out as described previously.11 Handling of mice and experimental procedures were conducted in accordance with institutional guidelines for animal care and use.
Polymerase chain reaction amplification and DNA sequencing
Total RNA and genomic DNA were prepared from fetal thymuses at embryonic day (E)14.5 to E17.5 and adult thymuses at 10 weeks of age, and from DETCs at birth and at 4 and 10 weeks of age, using Isogen (Nippongene, Toyama, Japan), and 1 µg of RNA was reverse transcribed by using Moloney murine leukaemia virus reverse transcriptase (Gibco-BRL, Gaithersburg, MD), in a 20-µl reaction volume, with random hexamers. Polymerase chain reaction (PCR) amplifications were performed using GeneAmp 2400 (Perkin-Elmer, Norwalk, CT) and AmpliTaq DNA polymerase (Perkin-Elmer), in a 50-µl reaction volume, with 1 µl of cDNA solution or 0·1 µg of genomic DNA. Nested primers for the analysis of junctional sequences were as follows: Vγ3, 5′-CTGGTACCAACTGAAAGAAG-3′ and 5′-CTCAAGCTTGGAAATTGATGAG-3′; Jγ1, 5′-GGAATTCCTTCTGCAAATACC-TTG-3′ and 5′-AGAGGGAATTCCTATGAGCT-3′; Cγ, 5′-CATGTATGTGTCGTTAGTCTT-3′ and 5′-AAAGAATTCT-TCAAGGAGAC-3′; Vδ1, 5′-ATGGATCTAATGCTCTG-TTTTTAG-3′ and 5′-AATAGGAATTCTACTGATGGTGG-3′; Jδ2, 5′-GGGATCCACAAAGAGCTC-3′ and 5′-GCCG-GATCCAAAAACATCTG-3′; and Cδ, 5′-GTTCAAAGTCAGTCGAGTGCA-3′ and 5′-TCTGGATCCAGACAAGCAGCATTTGTT-3′. Twenty-five cycles of amplification were carried out (at 94° for 30 seconds, 55–64° for 30 seconds and 72° for 30 seconds). A second round of amplification was carried out starting with 5 µl of the mixture from the first round. PCR products were digested with appropriate restriction enzymes, cloned into pBluescript SK (Stratagene) and sequenced using a 373A or 373S DNA sequencer (Applied Biosystems, Foster City, CA). Primers for the Vγ/Vδ usage analysis were as follows: Vγ1, 5′-GGGCTTGGGCAGCTGGAGCA-3′; Vγ2, 5′-GCAACCTG-AAATATCAATTT-3′; Vγ3, 5′-GACTCCTGGATATCTCAGGAT-3′; Vγ4, 5′-GGAACGAGTCTCACGTCACC-3′; Vγ5, 5′-AAGCTAGAGGGGTCCTCT-3′; Cγ, 5′-GGGGAAATGTC-TGCATCAAG-3′; Vδ1, 5′-CAGTTGCCAAAACTTTTAC-TGT-3′; Vδ2, 5′-AAGAAGGAGATGAAGTCACCA-3′; Vδ3, 5′-CTCTTCAGGGTCCAGAATAC-3′; Vδ4, 5′-AAGTCTGT-GCAGGTGGCAG-3′; Vδ5, 5′-CCCAGATTTATTTTGGTATCGCA-3′; Vδ6, 5′-AGCAAGCAGGCAGGAGGG-3′; Vδ7, 5′-AGCCTCAGGGTACCCAACCCTG-3′; and Cδ, 5′-CTGGGGGAGATGACTATAGC-3′. Thirty cycles of amplifications were carried out (at 94° for 45 seconds, 59° for 1·5 min and 72° for 1·5 min) using cDNA from thymocytes at E16·5 and 10 weeks and from skin at birth and 4 weeks. One-tenth of each PCR product was analysed by electrophoresis in a 1·8% agarose gel. Subsequently, gels were blotted onto nylon filters and hybridized to a 32P-labelled Cγ or Cδ probe. The Cγ and Cδ probes were generated by PCR amplification of the first 161 bp of Cγ and the first 159 bp of Cδ using cDNA from control C57BL/6 adult thymocytes.
Flow cytometric analysis
Fluorescein isothiocyanate (FITC)-conjugated antibodies (TCR-αβ and TCR-Vγ3) and phycoerythrin (PE)-conjugated antibodies (TCR-γδ, heat-stable antigen [HSA] and Thy-1.2) were purchased from Pharmingen (San Diego, CA). Epidermal cells were enriched for DETCs as described previously.12 Single-cell suspensions from thymuses and DETCs were stained with the monoclonal antibodies (mAbs) described above and with propidium iodide, as described previously.4 Viable cells (1 × 104 for thymus and 1 × 105 for skin) were analysed with a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Franklin Lakes, NJ) using cellquest software.
Results
TdT expression in fetal thymus of mutant mice
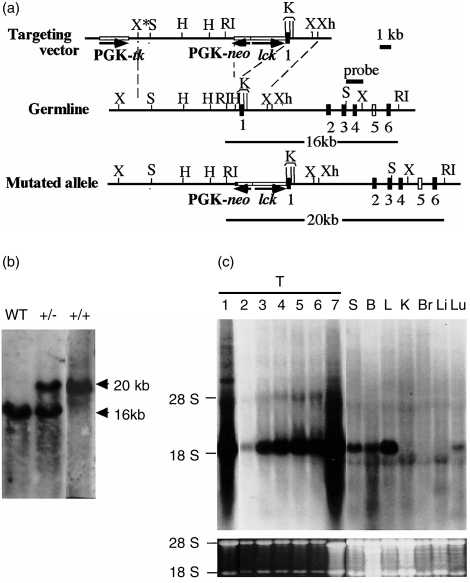
A targeting vector was constructed to replace the TdT promoter region13 with the lck promoter and PGK-neor. (Fig. 1a). The targeting vector was electroporated into the E14 line of ES cells and selected with G418 and gancyclovir. Targeted ES cells were injected into blastocysts of C57BL/6 mice. The chimeras were mated with C57BL/6 mice, and the mutation was transmitted through the germline. After interbreeding heterozygous (lckTdT+/–) mice, homozygous (lckTdT+/+) mice were generated (Fig. 1b).
Figure 1.
Generation of mutant mice in which the terminal deoxynucleotidyl transferase (TdT) promoter was replaced by the lck promoter. (a) Structure of the targeting vector and partial restriction map of the genomic TdT locus and mutated allele after homologous recombination. Exons 1–4 and 6 are depicted as black boxes. The location of exon 5 (white box) has not been determined. RI, EcoRI; H, HindIII; X, XbaI; S, SalI; Xh, XhoI; K, KpnI. (b) Southern blot analysis of representative mouse tail DNA. Genomic DNA was isolated from tails. DNA was digested with EcoRI and hybridized with the SalI–XbaI probe indicated. WT, wild-type littermate. (c) Expression of TdT in lckTdT+/+ and control C57BL/6 mice. RNA prepared from each tissue was examined by Northern blot analysis using a probe of full-length cDNA of TdT. Fetal thymus RNA was prepared from embryonic day (E)14.5–E18.5 embryos. Lane 1, C57BL/6 thymus (T) at 8 weeks; lanes 2–7, lckTdT+/+ thymuses (lane 2, E14.5; lane 3, E15.5; lane 4, E16.5; lane 5, E17.5; lane 6, E18.5; lane 7, 8 weeks). RNA from spleen (S), bone marrow (B), lymph node (L), kidney (K), brain (Br), liver (Li) and lung (Lu), was extracted from 8-week-old lckTdT+/+ mice. 28S and 18S ribosomal bands stained with ethidium bromide are shown in the lower panel.
TdT expression was studied in lymphoid (fetal and adult thymus, spleen, bone marrow and lymph node) and non-lymphoid (kidney, brain, liver and lung) (Fig. 1c) tissues. In normal C57BL/6 control mice, TdT expression was detected strongly in adult thymus and weakly in bone marrow but not in fetal thymus and the other tissues (Fig. 1c, data not shown). In lckTdT+/+ mice, TdT expression was detected in fetal and adult thymus, spleen, bone marrow, lymph node and lung, but not in kidney, brain or liver.
Reduction in the number of γδ T cells in lckTdT+/+ fetal thymus
At E15.5–E18.5, no significant difference was observed in the absolute number of thymocytes among lckTdT+/+ and control embryos, which included wild-type (WT) littermates, C57BL/6 and 129 mice (Table 1). Analyses of thymocytes of lckTdT+/+ and control embryos (WT littermates, C57BL/6 and 129) by flow cytometry at E15.5–E18.5 revealed that the populations of αβ– γδ+ cells and Vγ3+ Thy-1.2+ cells in lckTdT+/+ embryos were much smaller (by ≈ 30–50%) than those in control embryos (Table 2). The earliest Vγ3 cells to appear in the thymus expressed low levels of TCR and high levels of heat-stable antigen (HSAhigh), and the TCR expression increased and the HSA expression decreased (HSAlow) during maturation in the thymus.14 The Vγ3+ cells in lckTdT+/+ thymus, as well as those in control thymus, expressed low levels of Vγ3 and high levels of HSA at E15.5 and the levels of Vγ3 expression increased and those of HSA expression decreased during thymus development, indicating that Vγ3+ cells matured normally in lckTdT+/+ thymus (data not shown). However, both populations of Vγ3+ HSAhigh cells and Vγ3+ HSAlow cells were reduced in lckTdT+/+ embryos (Table 2, data not shown). The populations of CD4– CD8–, CD4+ CD8+, CD4– CD8+ and CD4+ CD8– cells in lckTdT+/+ embryos were similar to those in control embryos (data not shown).
Table 1.
Total number of thymocytes at embryonic day 15.5 (E15.5) to E18.5
| E15.5(×105) | E16.5(×105) | E17.5(×106) | E18.5(×106) | |
|---|---|---|---|---|
| WT | 2·5±0·6 (n=9) | 5·0±0·8 (n=9) | 3·1±0·7 (n=9) | 5·6±0·6 (n=9) |
| C57BL/6 | 2·1±0·3 (n=10) | 5·7±0·7 (n=10) | 3·2±0·4 (n=10) | 5·5±0·4 (n=10) |
| 129 | 2·2±0·1 (n=4) | 5·4±0·5 (n=9) | 2·8±0·2 (n=4) | 5·9±0·4 (n=9) |
| lckTdT+/+ | 2·4±0·9 (n=17) | 5·5±0·9 (n=14) | 3·4±0·6 (n=15) | 6·0±0·5 (n=17) |
All values represent mean ± SD.
n, number of embryos analysed. There was no significant difference in mice at any age.
WT, wild-type littermates.
Table 2.
Summary of flow cytometric analysis of fetal thymus
| E15.5(n) | E16.5(n) | E17.5(n) | E18.5(n) | |
|---|---|---|---|---|
| αβ–γδ+ | ||||
| WT | 2·1±0·9*(6) | 2·9±0·7 (7) | 2·9±0·8 (8) | 1·9±0·2 (5) |
| C57B/6 | 2·0±0·6 (6) | 2·6±0·6 (7) | 2·4±0·6 (7) | 2·7±0·3 (6) |
| 129 | 1·7 (1) | 2·9±0·3 (6) | 2·6*(1) | 1·6±0·4 (7) |
| lckTdT+/+ | 0·7±0·1 (5) | 1·2±0·2 (7) | 0·9±0·2 (9) | 1·0±0·2 (16) |
| Vγ3+Thy–1·2+ | ||||
| WT | 2·0±0·3 (7) | 2·2±0·5 (9) | 1·8±0·4 (6) | 1·0±0·5 (5) |
| C57BL/6 | 1·8±0·4 (8) | 3·0±0·6 (10) | 2·0±0·4 (7) | 0·9±0·2*(6) |
| 129 | 2·0*(1) | 3·7±0·1 (4) | 1·9*(1) | 1·1±0·3*(7) |
| lckTdT+/+ | 0·9±0·2 (5) | 1·0±0·3 (10) | 0·8±0·1 (10) | 0·6±0·1 (12) |
| Vγ3+HSA+ | ||||
| WT | 1·4±0·4*(9) | 2·7±0·5 (8) | 2·0±0·6 (8) | 1·1±0·4*(5) |
| C57BL/6 | 1·7±0·6*(7) | 2·5±0·3 (9) | 1·7±0·4 (7) | 1·0±0·4 (6) |
| 129 | 1·3*(1) | 2·6±0·5 (4) | 2·0*(1) | 1·2±0·3 (7) |
| lckTdT+/+ | 0·7±0·1 (5) | 1·2±0·2 (3) | 1·1±0·1 (12) | 0·5±0·2 (18) |
All values represent mean percentages ± SD. The population of Vγ3+ heat-stable antigen (HSA)+ cells includes Vγ3+HSAhigh cells and Vγ3+HSAlow cells. The percentages in lck-promoter terminal deoxynucleotidyl transferase (lckTdT)+/+ mice were significantly lower than those in the three control groups at P < 0·001 (no asterisk)
P < 0·01
P < 0·05
denotes no significant difference.
Statistical analysis was performed by using one-way analysis of variance (anova) with statview4·5.
WT, wild-type littermates; n, number of embryos analysed.
Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 junctional sequences in fetal thymus
Genomic DNA and total RNA were extracted from thymuses of lckTdT+/+ and control C57BL/6 embryos, and cDNA was synthesized from total RNA. Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 junctional sequences were amplified by the PCR using genomic DNA and cDNA, and sequenced (Table 3). The occurrence of N regions was observed at a rate of only ≈ 3% of Vγ3-Jγ1 junctions in analyses using both genomic DNA and cDNA of C57BL/6 thymuses at E16.5. More than one-third of the Vγ3-Jγ1 junctions of genomic DNA and four-fifths of the Vγ3-Jγ1 junctions of cDNA were canonical, and the majority of the junctions of in-frame sequences were canonical in the sequences of both genomic DNA and cDNA of C57BL/6 thymus. In contrast, more than two-thirds of Vγ3-Jγ1 junctions had N regions in the sequences of genomic DNA from lckTdT+/+ thymus at E14.5–E17.5 (Table 3). The frequency of canonical Vγ3-Jγ1 junctions in lckTdT+/+ thymus was markedly reduced: one-eighth and one-third of the junctions were canonical in the sequences of genomic DNA and cDNA, respectively. Even in in-frame sequences from lckTdT+/+ thymus, less than one-third and less than one-half of Vγ3-Jγ1 junctions were canonical in the sequences of genomic DNA and cDNA, respectively.
Table 3.
Summary of Vγ3-Jγ1/Vδ1-Dδ-Jδ2 junctional sequences in fetal thymus
| Number of seq. analysed | In-frame seq.(%) | N+ seq. (%) | Canonical seq. (%) | Canonical seq./in-frame (%) | ||
|---|---|---|---|---|---|---|
| Vγ3-Jγ1 junctional sequences | ||||||
| C57BL/6 | ||||||
| E16.5 | genome | 37 | 14 (38) | 1 (3) | 14 (38) | 14/14 (100) |
| E16.5 | cDNA | 35 | 32 (91) | 1 (3) | 28 (80) | 28/32 (88) |
| lckTdT+/+ | ||||||
| E14.5 | genome | 37 | 14 (38) | 25 (68) | 4 (11) | 4/14 (29) |
| E15.5 | genome | 39 | 17 (44) | 27 (69) | 5 (13) | 5/17 (29) |
| E16.5 | genome | 43 | 24 (56) | 32 (74) | 6 (14) | 6/24 (25) |
| E17.5 | genome | 37 | 19 (51) | 25 (68) | 5 (14) | 5/19 (26) |
| E16.5 | cDNA | 44 | 35 (80) | 20 (45) | 16 (36) | 16/35 (46) |
| E17.5 | cDNA | 45 | 34 (76) | 24 (53) | 17 (38) | 17/34 (50) |
| Vδ1-Dδ-Jδ2 junctional sequences | ||||||
| C57BL/6 | ||||||
| 16.5 | genome | 37 | 33 (89) | 1 (3) | 33 (89) | 33/33 (100) |
| E16.5 | cDNa | 36 | 35 (97) | 1 (3) | 29 (81) | 29/35 (83) |
| lckTdT+/+ | ||||||
| E16.5 | genome | 33 | 15 (45) | 26 (79) | 3 (9) | 3/15 (20) |
| E16.5 | cDNA | 25 | 23 (92) | 17 (68) | 1 (4) | 1/23 (4) |
Junctional sequences (seq.) of genomic DNA and cDNA from embryonic day (E)14.5–17.5 thymuses of control C57BL/6 mice and lck-promoter terminal deoxynucleotidyl transferase (lckTdT)+/+ mice. Two embryos were analysed at each age, and two independent polymerase chain reaction (PCR) amplification reactions were performed for each embryo. Identical sequences detected in products from the same PCR reaction were counted only once, except when they were found multiple times in independent amplifications.
In the Vδ1-Dδ-Jδ2 junctions of C57BL/6 thymus at E16.5, the rate of occurrence of N regions was only ≈ 3%, and the majority of the sequences were in-frame and canonical (Table 3). In lckTdT+/+ fetal thymus, a significant increase was observed in the appearance of N regions and a remarkably reduced frequency of Vδ1-Dδ2-Jδ2 canonical junctions (Table 3). More than two-thirds of the junctions had N regions and less than one-tenth of the junctions were canonical in the sequences of both genomic DNA and cDNA. It is noteworthy that less than one-half of the genomic sequences analysed were in-frame in lckTdT+/+ fetal thymus, a frequency that was ≈ 50% of that in C57BL/6 fetal thymus.
Flow cytometric analysis of skin
In flow cytometric analyses of skin, the percentages of each population of γδ+, Vγ3+ HSA+ (Vγ3+ HSAhigh and Vγ3+ HSAlow), and Vγ3+ γδ+ cells from newborn mice were less than one-tenth of those from 4- and 24-week-old mice, both in WT and lckTdT+/+ littermates (Fig. 2). Furthermore, these percentages in lckTdT+/+ newborn mice were ≈ 50% of those in WT newborn mice. However, after 4 weeks of age, no significant differences in these percentages were noted between WT and lckTdT+/+ littermates, and Vγ3+ γδ T cells predominated. Similar results for γδ+, Vγ3+ HSA+, and Vγ3+ γδ+ populations in 4- and 24-week-old mice were also obtained in WT and lckTdT+/+ mice at 10, 15 and 20 weeks of age (data not shown).
Figure 2.
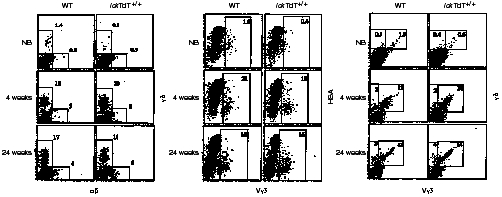
Flow cytometric analysis of skin. Epidermal cells from wild-type (WT) and lck-promoter terminal deoxynucleotidyl transferase (lckTdT)+/+ mice at birth and at 4 and 24 weeks of age were stained for αβ/γδ, Vγ3/heat stable antigen (HSA) and Vγ3/γδ, and analysed by fluorescence-activated cell sorter (FACScan). All nucleated cells gated by forward scatter and side scatter were analysed. Numbers indicate the percentages of cells stained for a particular phenotype in the respective boxed regions. Representative data are shown. NB, newborn.
Reverse transcription–polymerase chain reaction analysis of Vγ and Vδ usage in skin
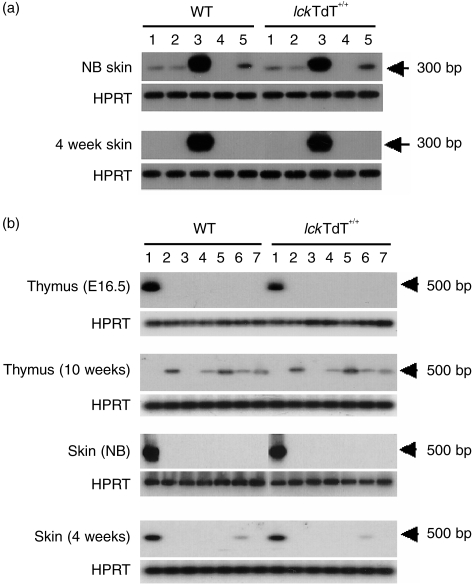
To determine Vγ gene usage, total RNA from WT and lckTdT+/+ skin at birth and at 4 weeks of age was reverse transcribed into cDNA, and TCR Vγ chain transcripts were selectively amplified by PCR using a Cγ primer and one of five Vγ (Vγ1-Vγ5) primers. Southern blot analysis with a 5′ Cγ probe showed that four amplified DNA fragments (Vγ1, 2, 3 and 5) were detected in both WT and lckTdT+/+ skin of newborn mice, with the strongest intensities noted in Vγ3 fragments, whereas Vγ3 fragments were predominantly detected in both WT and lckTdT+/+ skin at 4 weeks of age (Fig. 3a).
Figure 3.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis of T-cell receptor (TCR) γ and δ mRNA. RNA was extracted from thymocytes (at embryonic day [E]16.5 and 10 weeks old) and skin (at birth and 4 weeks) of wild-type (WT) and lck-promoter terminal deoxynucleotidyl transferase (lckTdT)+/+ mice. cDNA was amplified by PCR using a Cγ primer and one of five Vγ (Vγ1-Vγ5) primers (a) or with a Cδ primer and one of seven Vδ (Vδ1-Vδ7) primers (b), and an aliquot was separated by electrophoresis and transferred onto nylon membranes. The blotted membranes were hybridized with a Cγ probe (a) or a Cδ probe (b). Hypoxanthine-guanine phosphoribosyl transferase (HPRT) was used as an internal control for loading equal amounts of cDNA for analysis. NB; newborn. (a) 1, Vγ1; 2, Vγ2; 3, Vγ3; 4, Vγ4; 5, Vγ5. (b) 1, Vδ1; 2, Vδ2; 3, Vδ3; 4, Vδ4; 5, Vδ5; 6, Vδ6; 7, Vδ7.
We also examined Vδ gene usage in thymus at E16.5 and 10 weeks of age, and in skin at birth and at 4 and 24 weeks of age, by reverse transcription–polymerase chain reaction (RT–PCR) using a Cδ primer and one of seven Vδ (Vδ1-Vδ7) primers (Fig. 3b). The Vδ1 segment was predominant in both WT and lckTdT+/+ thymus at E16.5, whereas Vδ2, Vδ4, Vδ5, Vδ6 and Vδ7 fragments were observed in both WT and lckTdT+/+ adult thymus. In newborns, Vδ1 fragments were predominant in both WT and lckTdT+/+ skin. Vδ1 fragments were preferentially detected and weak bands of Vδ6 were also observed in skin of both WT and lckTdT+/+ mice at 4 and 24 weeks of age (Fig. 3b and data not shown). The weak expression of Vδ6 in skin has been described previously.15
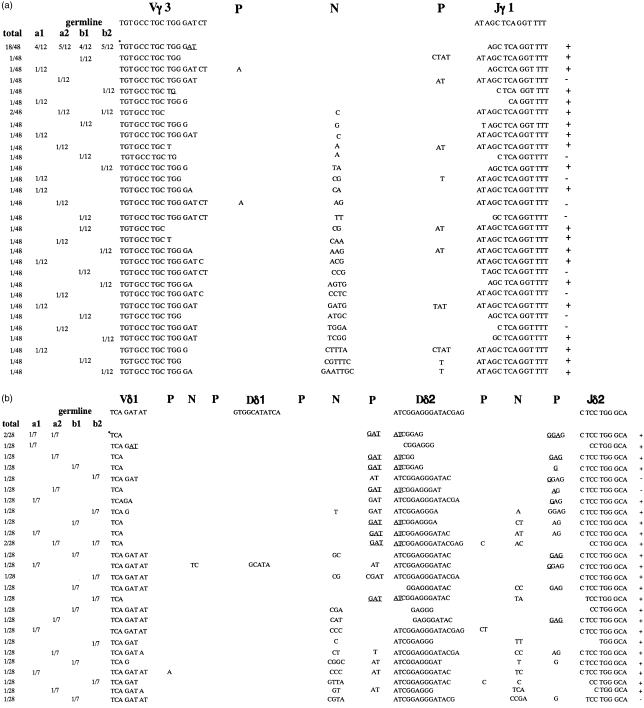
Vγ3-Jγ1/Vδ1-Dδ-Jδ2 junctional sequences in skin
Total RNA was extracted from the skin of newborn WT mice and the skin of newborn and 10-week-old lckTdT+/+ mice, and Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 junctional sequences were analysed. In newborn WT littermates, most of the junctions of both Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 were canonical (Table 4). In newborn lckTdT+/+ mice, more than one-half of the Vγ3-Jγ1 junctions had N regions, and about two-fifths of the junctions were canonical (Fig. 4a, Table 4). However, a greatly decreased occurrence of N regions (one-sixth of the Vγ3-Jγ1 junctions) and greatly increased frequency of canonical junctions (four-fifths of the junctions) were observed in 10-week-old lckTdT+/+ mice (Table 4). Analysis of Vδ1-Dδ-Jδ2 junctions revealed that more than four-fifths of the junctions had N regions and less than one-tenth of the junctions were canonical in newborn lckTdT+/+ mice (Fig. 4b), whereas a greatly decreased occurrence of N regions (one-seventh of the junctions) and greatly increased frequency of canonical junctions (four-fifths of the junctions) were observed in 10-week-old lckTdT+/+ mice (Table 4). It is noteworthy that both frequencies of N addition and the canonical junction observed in Vγ3-Jγ1 junctional sequences of newborn lckTdT+/+ skin were similar to those observed in the junctional sequences of lckTdT+/+ fetal thymus cDNA; this was also the case in Vδ1-Dδ-Jδ2 junctional sequences (Tables 3, 4).
Table 4.
Summary of junctional sequences of Vγ3-Jγ1/Vδ1-Dδ-Jδ2 cDNA in skin
| Number of seq.analysed | In-frame seq. (%) | N+ seq. (%) | Canonical seq. (%) | Canonical seq./in-frame (%) | |
|---|---|---|---|---|---|
| Vγ3-Jγ1 junctional sequences | |||||
| WT | |||||
| NB | 20 | 20 (100) | 1 (5) | 19 (95) | 19/20 (95) |
| lckTdT+/+ | |||||
| NB | 48 | 39 (81) | 25 (52) | 18 (38) | 18/39 (46) |
| 10 weeks | 46 | 45 (99) | 8 (17) | 38 (83) | 38/45 (84) |
| Vδ1-Dδ-Jδ2 junctional sequences | |||||
| WT | |||||
| NB | 14 | 14 (100) | 0 (0) | 13 (93) | 13/14 (93) |
| lckTdT+/+ | |||||
| NB | 28 | 25 (89) | 23 (82) | 2 (7) | 2/25 (10) |
| 10 weeks | 29 | 29 (100) | 4 (14) | 23 (79) | 23/29 (79) |
Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 junctional sequences (seq.) of cDNA from skin of newborn (NB) and 10-week-old lck-promoter terminal deoxynucleotidyl transferase (lckTdT)+/+ mice and newborn wild-type littermates (WT) were analysed. Two embryos were analysed at each age, and two independent polymerase chain reaction (PCR) amplification reactions were analysed in each embryo. Identical sequences detected in products from the same PCR reaction were counted only once, except when they were found multiple times in independent amplifications.
Figure 4.
Junctional sequences of Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 cDNA from skin of newborn lck-promoter terminal deoxynucleotidyl transferase (TdT)+/+ mice. Vγ3-Jγ1 junctional sequences (a) and Vδ1-Dδ-Jδ2 junctional sequences (b) were obtained using cDNA from skin of lckTdT+/+ mice. Two mice (a and b) were analysed, and two separate polymerase chain reaction (PCR) amplification reactions were performed for each mouse. The sequences are aligned with the germline sequences of the T-cell receptor (TCR) Vγ3 segment16and Vδ1 segment.17The frequency of each junction is listed to the left of the sequence. Overlapping nucleotides that could be encoded by either germline segment (including P nucleotides) are underlined. In-frame and out-of-frame sequences are indicated as + and –, respectively, on the right of the sequence. Canonical sequence is indicated by an asterisk.
Discussion
Mutant mice were generated whose TdT promoter was replaced with the lck promoter through homologous recombination. The protein Lck (p56lck) is expressed as soon as haematopoietic progenitors first colonize the thymic anlage and in all thymocyte subpopulations.18 Therefore, the lck promoter is normally active when γδ T cells first appear in the fetal thymus. We also detected TdT expression in lckTdT+/+ thymus at E14·5 (Fig. 1c), when Vγ3+ cells are first recognized in normal fetal thymus.19 The expression pattern of TdT observed in the mutant mice was identical to that of p56lck under the lck promoter in normal mice.20 TdT expression during thymus development in lckTdT+/+ embryos led to more abundant N additions in γδ TCR genes than that in TdT transgenic mice,21 and severely inhibited the generation of both Vγ3-Jγ1 and Vδ1-Dδ-Jδ2 canonical junctions.
Increased TdT activity in fetal thymus was associated with a decrease in the number of γδ T cells. The in-frame sequences in Vδ1-Dδ-Jδ2 rearrangements of lckTdT+/+ mice were ≈ 50% of those of control mice (Table 3). This indicates that the increase in out-of-frame sequences of Vδ1-Dδ-Jδ2 rearrangements caused the reduction in the number of γδ T cells in lckTdT+/+ fetal thymus, although it is possible that TdT expression in fetal thymocytes may affect their survival as a result of the addition of nucleotides at sites of double-stranded breaks and cause a decrease in the number of γδ T cells. However, the total number of thymocytes was similar among lckTdT+/+ and control embryos (Table 1), and flow cytometric analyses using anti-CD4 and anti-CD8 antibodies showed that αβ T cells developed normally and the number of αβ T-cell lineages during development was similar among lckTdT+/+ and control mice (data not shown). Thus, our data show that abundant N addition in Vδ1-Dδ-Jδ2 junctions is responsible for the decrease in the number of γδ T cells in fetal thymus and that the short stretch of sequence homologies near the 3′ end of the coding segments greatly favours not only the generation of canonical junctions but also the generation of γδ T cells in the absence of TdT expression, demonstrating the importance of the regulation of TdT expression in fetal thymus.
In the skin of normal newborn mice, γδ T cells with invariant Vγ3Vδ1 were predominantly disseminated, although γδ T cells with Vγ1, 2, or 5 were also detected (Fig. 3, Table 4). Vγ3Vδ1 T cells were also predominantly detected in lckTdT+/+ newborn skin, but most of the junctional sequences of their TCR genes had N regions, and less than 5% of Vγ3Vδ1 T cells were invariant, a similar frequency to that of the generation of invariant Vγ3Vδ1 T cells in fetal thymus of mutant mice (Tables 3, 4). These data indicate that Vγ3Vδ1 T cells, which have various junctional sequences in their TCR genes, randomly disseminate in skin. Two previous studies using transgenic mice demonstrated that DETCs were able to express transgene-encoded TCRs that were different from the TCR encoded by invariant Vγ3Vδ1.22,23 Furthermore, Vγ3-deficient mice showed normal numbers of DETCs, one-third of which were positively stained by mAb 17D1, which specifically recognizes invariant Vγ3Vδ1 T cells.24 These reports and our data demonstrate that invariant Vγ3Vδ1 TCR is not essential for the migration of γδ T cells into skin. Moreover, our results show that migration of Vγ3Vδ1 T cells into skin occurs randomly.
After 4 weeks of age, the number of DETCs were similar in WT and mutant mice, and the majority of cells had invariant Vγ3Vδ1 in their TCR genes. This indicates that invariant Vγ3Vδ1 T cells expanded rapidly in epidermis during the first 4 weeks of life. A marked increase in the number of CD3+ cells in epidermis during the first few weeks of life has been shown in normal mice.25 A rapid increase in 17D1-positive cells during the first three weeks of life was also reported in normal mice.26 As invariant Vγ3Vδ1 T cells were already predominant in newborn skin of normal mice (Fig. 3, Table 4), the increase in 17D1-positive cells was not an indication that they selectively expanded in epidermis. However, our data clearly demonstrate that invariant Vγ3Vδ1 T cells have a great advantage for proliferation in epidermis.
Much evidence exists for the interaction of DETCs and keratinocytes in epidermis. DETCs exist in close contact with keratinocytes and support their growth by keratinocyte growth factor.27 Keratinocytes stimulate DETCs to secrete interleukin (IL)-2 through a TCR-dependent pathway,28 and IL-7 produced by keratinocytes may play an important role in the survival and growth of DETCs in epidermis.29 Furthermore, localized proliferation of DETCs was induced by keratinocytes treated with contact sensitizers.30 This evidence suggests that keratinocytes play an important role in the maintenance and growth of DETCs in epidermis. As invariant Vγ3Vδ1 T cells selectively expanded in epidermis, the existence of a ligand, which recognizes invariant Vγ3Vδ1 TCR or equivalent epitopes, could be proposed on keratinocytes.
Our mutant mice expressed TdT abundantly in fetal thymus. TdT caused a decrease in the number of γδ T cells in fetal thymus, and clearly showed random migration of Vγ3Vδ1 T cells into skin and selective expansion of invariant Vγ3Vδ1 T cells in the skin. These results also demonstrate the usefulness of this novel approach to deregulating gene expression by ‘replacing the promoter by gene targeting’, as it is often difficult to produce adequate gene expression in a specific tissue by generating transgenic mice.
Acknowledgments
We thank R. Perlmutter for the lck promoter, K. Deguchi and T. Matsunashi for technical assistance, R. Hiraiwa for maintaining mouse colonies and A. Kimura for secretarial assistance. This work was supported by grants from the Ministry of Education, Science and Culture, Japan, and Sankyo Foundation of Life Science.
Glossary
Abbreviations
- DETC
dendritic epidermal T cell
- ES
embryonic stem
- HPRT
hypoxanthine-guanine phosphoribosyl transferase
- TdT
terminal deoxynucleotidyl transferase
- WT
wild type
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;14:575. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Lafaille J, Decloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor γδ gene: implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1987;59:859. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 3.Alt FW, Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci USA. 1982;79:4118. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komori T, Okada A, Stewart V, Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 5.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 6.Komori T, Pricop L, Hatakeyama A, Bona CA, Alt FW. Repertoires of antigen receptors in TdT congenitally deficient mice. Int Rev Immunol. 1996;13:317. doi: 10.3109/08830189609061755. [DOI] [PubMed] [Google Scholar]
- 7.Bogue M, Gilfillan S, Benoist C, Mathis D. Regulation of N-region diversity in antigen receptors through thymocyte differentiation and thymus ontogeny. Proc Natl Acad Sci USA. 1992;89:11011. doi: 10.1073/pnas.89.22.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havran WL, Carbone A, Allison JP. Murine T cells with invariant γδ antigen receptors: origin, repertoire, and specificity. Semin Immunol. 1991;3:89. [PubMed] [Google Scholar]
- 9.Asarnow DM, Kuziel WA, Bonyhadi M, Tiggelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 10.Asarnow DM, Goodman T, Lefrancois L, Allison JP. Distinct antigen receptor repertoires of two classes of murine epithelium-associated T cells. Nature. 1989;341:60. doi: 10.1038/341060a0. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki K, Yagi H, Bronson RT, et al. Absence of fetal liver hematopoiesis in transcriptional co-activator, Core Binding Factor β (Cbfb) deficient mice. Proc Natl Acad Sci USA. 1996;93:12359. doi: 10.1073/pnas.93.22.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nixon-fulton JL, Bergstresser PR, Tigellaar RE. Thy-1+ epidermal cells proliferate in response to concanavalin A and interleukin 2. J Immunol. 1986;136:2776. [PubMed] [Google Scholar]
- 13.Smale ST, Baltimore D. The ‘initiator’ as a transcription control element. Cell. 1989;57:103. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq G, Plum J, Nandi D, Smedt MD, Allison JP. Intrathymic differentiation of Vγ3 T cells. J Exp Med. 1993;178:309. doi: 10.1084/jem.178.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota Y, Kobata T, Seki M, et al. Extrathymic origin of Vγ1/Vδ6 T cells in the skin. Eur J Immunol. 1992;22:595. doi: 10.1002/eji.1830220245. [DOI] [PubMed] [Google Scholar]
- 16.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 17.Chien YH, Iwashima M, Wettstein DA, et al. T-cell receptor δ gene rearrangements in early thymocytes. Nature. 1987;330:722. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds PJ, Lesley J, Trotter J, Schulte R, Hyman R, Sefton BM. Changes in the relative abundance of type I and type II lck mRNA transcripts suggest differential promoter usage during T-cell development. Mol Cell Biol. 1990;10:4266. doi: 10.1128/mcb.10.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 20.Marth JD, Peet R, Krebs EG, Perlmutter RM. A lymphocyte-specific protein tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985;43:393. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Cado D, Asarnow DM, et al. The role of short homology repeats and TdT in generation of the invariant γδ antigen receptor repertoire in fetal thymus. Immunity. 1995;3:439. doi: 10.1016/1074-7613(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 22.Ferrick DA, Sambhara SR, Ballhausen W, et al. T cell function and expression are dramatically altered in T cell receptor Vγ1.1Jγ4Cγ4 transgenic mice. Cell. 1989;57:483. doi: 10.1016/0092-8674(89)90923-9. [DOI] [PubMed] [Google Scholar]
- 23.Bonneville M, Itohara S, Krecko EG, et al. Transgenic mice demonstrate that epithelial homing of γ/δ T cells is determined by cell lineages independent of T cell receptor. J Exp Med. 1990;171:1015. doi: 10.1084/jem.171.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallick-wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science. 1998;279:1729. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 25.Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells. J Immunol. 1989;143:2431. [PubMed] [Google Scholar]
- 26.Robert ET, Lewis JM. Factors involved in the localization and activation of murine γδ positive dendritic epidermal T cells. In: Lambert WC, Giannotti B, Vloten WV, editors. Basic Mechanisms of Physiologic and Aberrant Lymphoproliferation in the Skin. New York: Plenum Press; 1994. p. 39. [Google Scholar]
- 27.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 28.Havran WL, Chien Y-H, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 29.Matsue H, Bergstresser PR, Takashima A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J Immunol. 1993;151:6012. [PubMed] [Google Scholar]
- 30.Huber H, Descossy P, van Brandwijk R, Knop J. Activation of murine epidermal TCR-γδ+ T cells by keratinocytes treated with contact sensitizers. J Immunol. 1995;155:2888. [PubMed] [Google Scholar]