Abstract
An interleukin-12 (IL-12) expression plasmid was transferred, using a gene gun, to mice infected with Leishmania major or Trypanosoma cruzi. Transfer of the IL-12 gene to susceptible BALB/c mice resulted in regression of lesion size and reduced the number of parasites in draining lymph nodes (LN) at the site of L. major infection. Coincident with these protective effects, the T-helper type (Th) response shifted towards Th1, as evaluated by cytokine production in vitro and L. major-specific antibody responses. Protective effects of the IL-12 gene were also observed in T. cruzi infection. Treatment of BALB/c mice infected with T. cruzi enhanced the production of interferon-γ (IFN-γ) by spleen cells, while suppressed production of interleukin-10 (IL-10) compared with control mice. Administration of anti-CD4 or anti-CD8 monoclonal antibody (mAb) abolished the protective immunity against T. cruzi infection, and treatment with the IL-12 gene could not restore the resistance in these mice. Mice depleted of natural killer (NK) cells with anti-asialo GM1 also became susceptible to infection, while the resistance was restored when these mice were treated with the IL-12 gene. Thus, target cells for the treatment appear to be CD4+ and CD8+ T cells, which are ordinarily activated by NK cells. These results suggest that the transfer of cytokine genes using a gene gun is an effective method for investigating the roles of cytokines and gene therapy in infectious diseases.
Introduction
The particle-mediated method for gene delivery using a gene gun utilizes a shock wave to accelerate DNA-coated gold particles into target cells or tissues. This gene delivery method is effective in various somatic tissues in vitro and in vivo. High levels of transgene activity have been readily detected, both in tissue extracts and at the cellular level, from the tissues of animals bombarded with various reporter genes.1,2 This in vivo gene delivery method has been used for DNA vaccination because it is simple, and a small amount of DNA is sufficient for stimulating both cellular and humoral immune responses, which can lead to protection from various infectious pathogens.3,4 Recently, the gene gun system has been employed in gene therapy. In fact, treatment with a particle-mediated, in vivo cytokine gene after the implantation of tumour cells inhibits tumour growth and prolongs the survival of tumour-bearing mice.5,6
Interleukin-12 (IL-12), a heterodimeric cytokine comprising p35 and p40 chains, was originally described as a natural killer cell stimulating factor (NKSF) and cytotoxic lymphocyte maturation factor (CLMF).7,8 It displays a potent array of biological activities, including the ability to enhance proliferation on natural killer (NK) and T cells,9,10 to induce production of interferon-γ (IFN-γ),11,12 to enhance NK and T-cell cytolytic activity,7,8,13 and differentiates CD4+ T-helper type 1 (Th1) cells from Th0 cells.14 Because of its multiple and unique properties, IL-12 has been shown to play important roles in many types of infectious diseases, Th1-mediated autoimmune diseases and, moreover, shows potent antitumour effects.15
In the present study, we examined the effects of in vivo gene treatment with IL-12, using a gene gun, on the immune response and course of infection with the obligate protozoa, Leishmania major and Trypanosoma cruzi. We demonstrate here that transfer of an IL-12 expression plasmid in vivo regulates systemic immune responses. Furthermore, this treatment controls the progression of both experimental leishmaniasis and trypanosomiasis.
Materials and methods
Animals and parasites
Female BALB/c and C57BL/6 mice used in these experiments were purchased from the Shizuoka Laboratory Animal Center (Hamamatsu, Japan).
A clone of L. major (MHOM/SU/73/5ASKH) was used in these experiments. L. major was maintained by serial passages in the footpads of BALB/c mice. Mice were injected subcutaneously (s.c.) in the hind footpad with 5 × 106 L. major promastigotes. The swelling of the footpad was measured weekly by using a metric calliper.
The Tulahuèn strain of T. cruzi was maintained by weekly passage in BALB/c mice. Mice were infected with 100 blood-derived T. cruzi trypomastigotes by s.c. injection at the base of the tail. The levels of parasitaemia were evaluated in 5-µl aliquots of blood drawn from the tail vein. In some experiments, in order to deplete CD4+ T cells, CD8+ T cells or NK cells, 0·5 mg of anti-CD4 monoclonal antibody (mAb) (GK1.5), anti-CD8 mAb (57-6-7), or 100 µg of anti-asialo GM-1 polyclonal serum was injected intraperitoneally (i.p.) into the mice on day 0 and 5, 10 and 15 days after infection with T. cruzi. To assess the depletion of CD4+ T cells, CD8+ T cells and NK cells, we analysed the proportion of T-cell receptor (TCR)-α/β+ CD8– cells, TCR-α/β+ CD4– cells and CD3– IL-2 receptor β-chain+ cells, respectively. Ninety-five percent of the cells of the appropriate subsets were invariably found to be depleted.
IL-12 expression plasmid
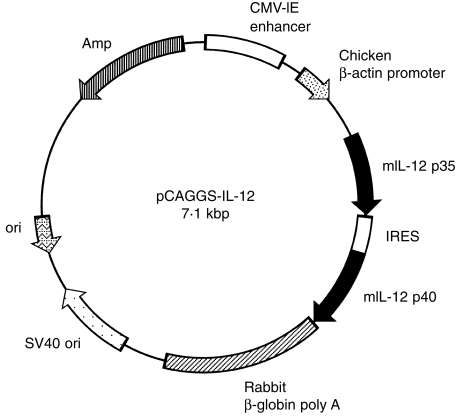
Both IL-12 p35 and IL-12 p40 cDNA were kindly provided by Dr S. Wolf (Genetic Institute, Cambridge, MA). IL-12 expression plasmid, designated pCAGGS-IL-12, was constructed by inserting mouse IL-12 p35 and p40 cDNA into the unique EcoRI site between the CAG promoter and a 3′-flanking sequence of the rabbit β-globin gene of the pCAGGS expression vector.16 To express the IL-12 p35/p40 heterodimer under control of the CAG promoter, an internal ribosomal entry site (IRES) was cloned between IL-12 p35 and p40 (Fig. 1). Plasmid was replicated in Escherichia coli DH5α and prepared using the QIAGEN Plasmid Kit Maxi or Mega (QIAGEN, Chatsworth, CA). Expression of IL-12 p70 in COS7 cells transfected with pCAGGS-IL-12 was 81 ± 3 ng/ml.
Figure 1.
Map for plasmid pCAGGS-IL-12. Plasmid pCAGGS-IL-12 was constructed by inserting interleukin-12 (IL-12) p35 and p40 cDNA into the unique EcoRI site between the CAG promoter and a 3′-flanking sequence of the rabbit β-globin gene of the pCAGGS expression vector. Transcription is initiated from the CAG promoter, continues through a mini-intron splice-donor splice-acceptor site, the IL-12 p35, IL-12 p40 gene, and is terminated by rabbit β-globin polyadenylation sequence. Amp, ampicillin resistance gene; IRES, internal ribosome entry site.
Gene gun-based in vivo gene transfer
IL-12 expression plasmid was precipitated onto 1·6-µm gold particles. Briefly, 12.5 mg of gold particles was suspended in 200 µl of 0·1 m spermidine, mixed with 200 µg of plasmid in a 200-µl volume and then mixed with 200 µl of 0·05 m CaCl2. These plasmid/gold particles were suspended in 6 ml of ethanol containing 0·05 mg/ml polyvinylpyrrolidone. The plasmid/gold particles were coated onto the inner surface of a tube using a tube loader, and the tube was cut into 1·2-cm segments, resulting in the delivery of 0·125 mg of gold particles and 2 µg of plasmid DNA per transfection. Plasmid DNA was delivered to shaved abdominal skin of mice via two injections using the Helios Gene Gun (Bio-Rad, Hercules, CA) at a helium pressure of 300 p.s.i. In vivo gene transfer was performed three or four times at 7-day intervals in L. major or T. cruzi infection, respectively. As controls, mice were transfected with the pCAGGS expression plasmid.
Determination of IL-12 protein and mRNA in the tissue
Following gene transfer, the marked sites of abdominal skin were biopsied on various days. For determination of IL-12 protein, the skin was homogenized in buffer solution comprising 0·25 m Tris-HCl (pH 7·4), 1 mm phenylmethylsulphonyl fluoride (PMSF) and 0·5% Triton-X-100, centrifuged at 7000 g to remove the debris, and the supernatants were collected. The level of IL-12 protein in the supernatant was determined using mouse IL-12 p70 enzyme-linked immunosorbent assay (ELISA) kits (Genzyme, Cambridge, MA). Detection of IL-12 mRNA was carried out by reverse transcription–polymerase chain reaction (RT–PCR) analysis. Total RNA was purified from the gene-transferred skin using ISOGEN (WAKO, Osaka, Japan), according to the manufacturer’s protocol. Two micrograms of total RNA was reverse transcribed with 200 U of M-MLV reverse transcriptase (using 1 µg of random hexanucleotide as the primer), in a 20-µl reaction mixture, and the cDNA product was used as a template for the PCR. For IL-12, the sense and antisense primers were: 5′-TCTGACTGACCGCGTTACTC-3′ and 5′-CTGGTTTGGTGGTCCCGTGAGAT-3′, respectively. For β-actin, the sense and anti-sense primers were: 5′-ATGTGCACAGAAAGCATGATC-3′ and 5′-AGATGATCTGAGTGTGAGGG-3′, respectively. The reaction mixture for PCR was as follows: 200 µm of each primer, 200 µm of each deoxynucleotide, 50 mm KCl, 10 mm Tris-HCl (pH 8·8), 1·5 mm MgCl2, 2·5 U of Taq polymerase and 1 µl of the reverse transcription product described above. The PCR was performed in a DNA thermal cycler for 30 or 40 cycles (denaturation for 1 min at 94°, annealing for 1·5 min at 58° and extension for 1·5 min at 72°). After amplification, PCR products were analysed by electrophoresis in 2% agarose gels. To avoid contamination of plasmid in RNA samples, we designed the IL-12 sense primer to hybridize with the non-coding exon in the chicken β-actin promoter of the pCAGGS expression plasmid. The expected size of the PCR product from IL-12 mRNA was 340 bp and that from β-actin was 576 bp.
Concanavalin A (Con A) stimulation
Single-cell suspensions of splenocytes from individual mice transfected 48 hr earlier with IL-12 expression or control plasmid, were prepared, washed and resuspended in 10% fetal calf serum (FCS)-RPMI medium containing 100 U/ml of penicillin and 100 μg/ml of streptomycin. Cells (5 × 106 cells/ml) were cultured in 24-well plates with 5 µg/ml of Con A for 48 hr. Supernatants were collected and assayed for IFN-γ and Interleukin-4 (IL-4) production by ELISA.
Determination of L. major load
For quantification of parasite number, a limiting-dilution in vitro culture was performed. Briefly, twofold dilutions of popliteal lymph node (LN) cells were plated (four replicates at each dilution) in 96-well plates containing Schnider medium supplemented with 20% FCS. The plates were incubated at 25° for 10 days and assessed, microscopically, for parasite growth. Parasitic load was expressed as the number of parasites per LN.
Antigen preparation
L. major was propagated in Schneider medium containing 20% FCS. T. cruzi trypomastigotes were obtained from supernatants of L-929 cell monolayers infected 3–5 days earlier and cultured in RPMI-1640 medium containing 5% FCS, 100 U/ml of penicillin, 100 µg/ml of streptomycin, 10 mm HEPES and 4 mm l-glutamine. The parasites were resuspended in phosphate-buffered saline (PBS) and three cycles of freeze–thawing were carried out. The parasites were then sonicated at 4° and viewed microscopically to ensure that all parasites had been disrupted. The parasite suspensions were centrifuged at 25 000 g and supernatants were collected. The protein concentrations were determined by using the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL)
Cell culture and cytokine ELISA
Lymphocytes were prepared from popliteal LN or spleen in L. major or T. cruzi infection, respectively. Lymphocytes were cultured at 5 × 106 cells/ml with parasite antigen (Ag) for 3 days, then the supernatants were collected. For determination of IFN-γ or IL-4, 96-well plates were coated overnight with primary anti-cytokine capture Ab. Plates were washed with PBS containing 0·05% Tween-20, blocked with PBS containing 10% FCS and dilutions of supernatants or standards were added. Dilutions of culture supernatant were incubated overnight at 4° and, after washing, the wells were incubated with biotin-conjugated anti-cytokine-detecting mAb. After a 2-hr incubation, the plates were washed and streptavidin-conjugated alkaline phosphatase was added. After developing, the absorbance was measured at 415 nm. The amount of cytokine in each supernatant was extrapolated from a standard curve. The Ab pairs used were as follows (capture antibody/biotinylated detection Ab): IFN-γ, R4-6A2/XMG1.2; and IL-4, 11B11/BVD6-24G2. IL-10 was measured by using the mouse IL-10 ELISA system (Amersham, Bucks, UK), according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean± SD. Differences between experimental groups within each experiment were analysed by using the unpaired Student’s t-test and were considered significant if P < 0·05.
Results
Expression of IL-12 in gene-transfected tissue
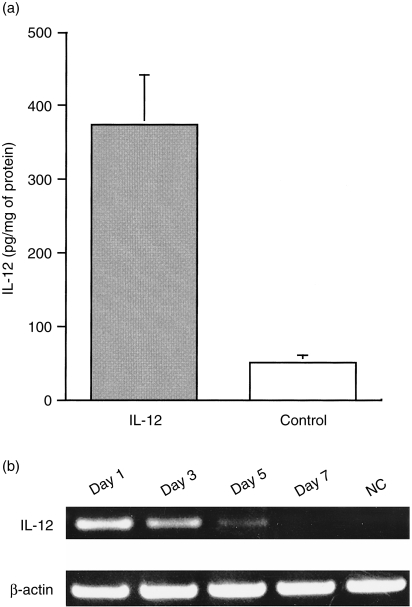
We directly transferred the IL-12 expression plasmid to the abdominal skin of mouse using the gene gun and examined the expression of IL-12 in the skin tissue. First, we measured the expression of IL-12 p70 protein using ELISA. As shown in Fig. 2(a), significant expression of IL-12 p70 was observed at the site of gene transfection 1 day after treatment. However, expression of IL-12 p70 rapidly decreased after 3 days to background levels (data not shown). We further determined the duration of IL-12 gene expression by using RT–PCR. IL-12 p35 mRNA derived from pCAGGS-IL-12 was detected until 5 days after gene transfer (Fig. 2b).
Figure 2.
Expression of the interleukin-12 (IL-12) in gene gun-treated skin tissues. Plasmid DNA (pCAGGS-IL-12 or pCAGGS) was precipitated onto 1·6-µm gold particles. Mice were shaved in the abdominal area, and abdominal skin was transfected with a 300 p.s.i. helium gas pulse by using the Helios Gene Gun. (a) Detection of IL-12 p70 protein in IL-12 gene-transfected skin tissues at 24 hr after transfection. Transfected skin tissues were removed, homogenized with extraction buffer and centrifuged at 7000 g to remove the debris. The content of IL-12 p70 in the supernatant was estimated by using enzyme-linked immunosorbent assay (ELISA), as described in the Materials and methods. (b) Detection of IL-12 mRNA in IL-12 gene-transfected skin tissues. RNA was extracted from IL-12 gene-transfected skin tissues at various time-points after treatment. cDNA products prepared from RNA were amplified by using the polymerase chain reaction (PCR). For IL-12 mRNA detection, specific primers were designed to encompass the intron sequence. The IL-12 forward primer was designed to hybridize with the sequence immediately downstream of the transcription start site of the CAG promoter and thus the IL-12 primer pair could amplify IL-12 cDNA derived from pCAGGA-IL-12, but not from endogenous IL-12. NC, sample from skin tissue transfected with the pCAGGS expression plasmid.
Transfer of the IL-12 gene systemically induces its effect
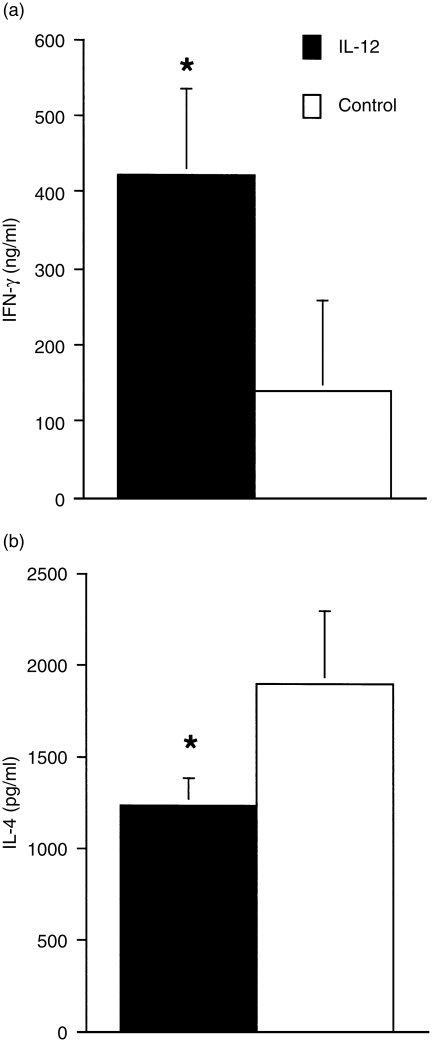
To determine whether or not transfer of the IL-12 gene influences cytokine production, we measured IFN-γ and IL-4 levels in Con A-stimulated splenocytes from mice transfected with IL-12 or a control gene. IFN-γ production by spleen cells from mice transfected with the IL-12 gene was twofold higher than that from mice treated with the control plasmid. IL-4 production was significantly reduced in the treated mice (Fig. 3), suggesting that transfer with the IL-12 gene can systemically induce its effects on mice and consequently develop a Th1-dominant immune response.
Figure 3.
Transfer with the interleukin-12 (IL-12) gene regulates the pattern of splenocyte cytokine production. Spleens were removed from mice at 48 hr after gene transfer and a single-cell suspension of splenocytes was cultured with 5 µg/ml of concanavalin A (Con A) for 48 hr. After culture, supernatants were collected and the levels of interferon-γ (IFN-γ) and interleukin-4 (IL-4) were assayed by using a sandwich enzyme-linked immunosorbent assay (ELISA) with specific antibody pairs. Data represents the mean ± SD of four mice. *Signifies a statistically significant difference (P < 0·05) between the group treated with pCAGGS (Control) and that treated with pCAGGS-IL-12 (IL-12). Results are representative of two independent experiments.
Effect of transfer with the IL-12 gene on the course of infection with L. major
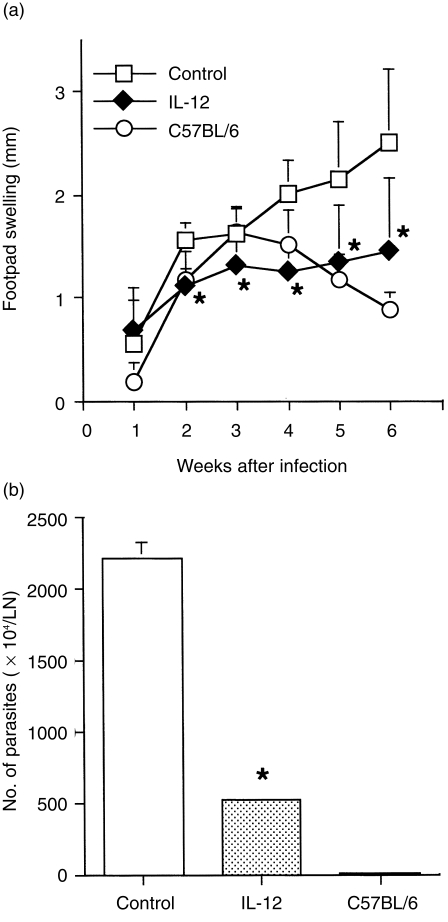
Although infection with an intracellular protozoan parasite, L. major, induces strong Th1 responses in most strains of inbred mice, BALB/c mice develop aberrant Th2 responses that underlie the susceptibility of this mouse strain.17–21 To assess whether transfer with the IL-12 gene could influence growth of the leishmanial parasite and the course of infection, footpad swelling was monitored in infected mice treated with IL-12 or the control gene four times at 7-day intervals. Footpad swelling rapidly increased in BALB/c mice that received the control plasmid, while resistant C57BL/6 mice showed a low grade and transient swelling of the footpad following infection. No statistical difference in footpad swelling was observed between treatment with the IL-12 gene and the control gene 1 week after infection, whereas the swelling was significantly less in mice that had been treated with the IL-12 gene compared with that in control BALB/c mice after the 2nd week (Fig. 4a). Seven weeks after infection, most of the mice treated with the control plasmid had developed necrotic lesions, whereas the IL-12 gene-treated mice did not (data not shown). Parasite burdens in infected mice were determined by limiting-dilution analysis. Consistent with the results of footpad swelling, the parasite load in LN of resistant C57BL/6 mice was markedly lower compared with that of BALB/c mice. Strikingly, the number of L. major in LN cells from IL-12 gene-treated mice was fourfold lower than that of control BALB/c mice (Fig. 4b). These results suggest that in vivo transfer with the IL-12 gene suppresses the growth of L. major.
Figure 4.
The effect of interleukin-12 (IL-12) gene transfer on progression of leishmaniasis in BALB/c mice. (a) Footpad swelling measurements represent the mean ± SD values of eight mice. BALB/c mice were treated with IL-12 or a control gene four times at 0, 1, 2 and 3 weeks after infection with 5 × 106 Leishmania major promastigotes. As a resistant strain, C57BL/6 mice were infected with the same doses of L. major. (b) Parasite burdens from IL-12 gene-treated BALB/c mice. After 5 weeks of infection, four animals (representative of each group) were killed and parasite density was quantified by enumerating the number of parasites in the lymph nodes (LN). *Signifies a statistically significant difference (P < 0·05) between the group treated with pCAGGS (Control) and that treated with pCAGGS-IL-12 (IL-12). Results are representative of two independent experiments.
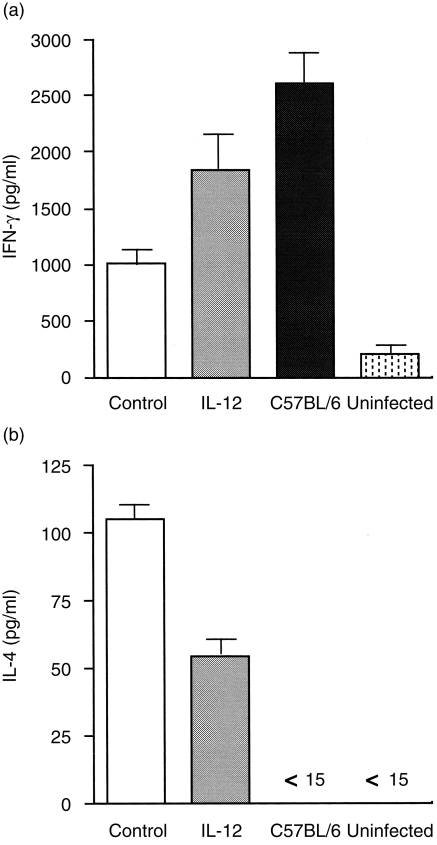
The IL-12 gene shifts the Th1/Th2 balance towards Th1 following L. major infection
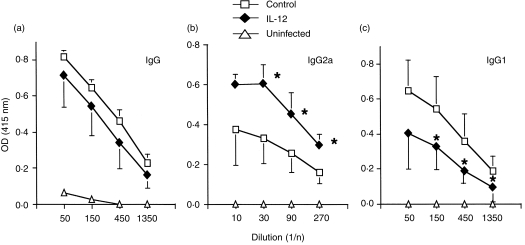
It is known that the susceptibility of infection with L. major is closely associated with the Th1/Th2 balance following infection. To determine whether resistance resulting from IL-12 gene transfer was caused by the generation of a Th1 response, the cytokine profile was measured using popliteal LN. Five weeks after infection, LN cells from BALB/c mice treated with IL-12 or the control gene were stimulated in vitro with L. major Ag, and cytokine production in the supernatant was assayed using ELISA. IFN-γ production by LN cells from mice treated with the IL-12 gene was significantly higher, whereas IL-4 production was twofold lower compared with that of control gene-treated mice (Fig. 5). As cytokines such as IFN-γ and IL-4 direct immunoglobulin class switching for IgG2a and IgG1, respectively, we measured L. major-specific isotypes to ascertain the pattern of cytokine production. As shown in Fig. 6, mice transfected with the IL-12 gene had substantially higher levels of L. major-specific IgG2a Ab responses compared with control mice. In contrast to the specific IgG2a level, the IgG1 level was lower in IL-12 gene-treated mice than in control mice. Thus, the isotype pattern of L. major-specific Ab is consistent with that of the cytokine production, indicating that transfer of the IL-12 gene shifts the immunoresponse towards Th1 from Th2 following L. major infection.
Figure 5.
Cytokine profile in interleukin-12 (IL-12) gene-treated mice after Leishmania major infection. Production of interferon-γ (IFN-γ) and interleukin-4 (IL-4) were measured in the culture supernatants of popliteal lymph node (LN) cells from mice infected for 5 weeks with L. major. Cells from four mice were pooled and cultured for 3 days in 12·5 µg/ml of soluble leishmanial Ag (SLA). Columns and bars represent mean ± SD of duplicate cytokine assays. Results are representative of two independent experiments.
Figure 6.
Leishmania major-specific antibody (Ab) production in interleukin-12 (IL-12) gene-treated BALB/c mice infected with L. major. Sera was collected 5 weeks after L. major infection in gene-treated mice. Sera was tested for L. major-specific antibody: (a) IgG; (b) IgG2a; and (c) IgG1. Data shown represent the mean± SD for four mice. Results are representative of two independent experiments. *Signifies a statistically significant difference (P < 0·05) between the group treated with pCAGGS (Control) and that treated with pCAGGS-IL-12 (IL-12).
Transfer with the IL-12 gene confers protection against T. cruzi infection
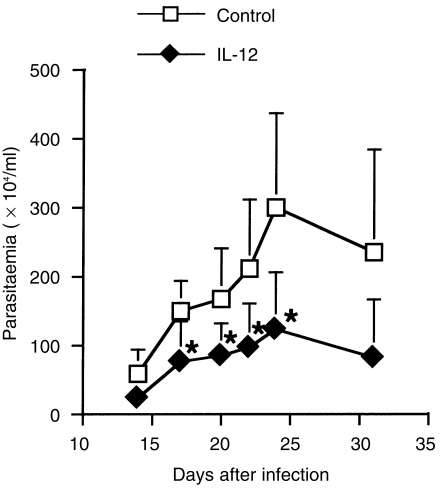
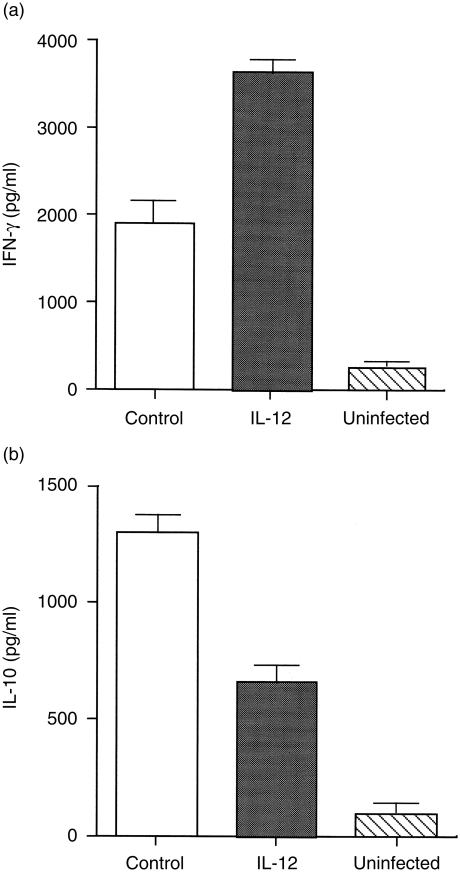
Next, we investigated the effects of IL-12 gene treatment on infection with another type of intracellular protozoan parasite, T. cruzi. In control gene-treated mice, the level of parasitaemia gradually increased and reached peak levels 25 days after infection. On the other hand, transfer with the IL-12 gene apparently reduced the levels of parasitaemia compared with those of control mice (Fig. 7). These results indicate that treatment with the IL-12 gene also enhances protective immunity to T. cruzi infection, as in the case of L. major infection. It is well established that IFN-γ plays a protective role in experimental T. cruzi infection, preventing acute disease and immunosuppression,22,23 whereas IL-10 promotes infection.24,25 Production of IFN-γ and IL-10 from spleen cells in response to T. cruzi Ag was compared between IL-12 gene- and control gene-treated mice. IFN-γ production in IL-12 gene-treated mice was twofold higher than in control mice. In contrast to IFN-γ production, IL-10 production was significantly decreased in IL-12 gene-treated mice (Fig. 8). Therefore, the different cytokine profile pattern, according to treatment with the IL-12 gene, appears to reflect the potential of the protective immunity in the host mice.
Figure 7.
The effect of interleukin-12 (IL-12) gene transfer on the course of parasitaemia in BALB/c mice infected with Trypanosoma cruzi. BALB/c mice were treated with IL-12 or a control gene three times, at 0, 1 and 2 weeks after infection with 100 T. cruzi trypomastigotes. The levels of parasitaemia were estimated by counting blood drain and represent the mean ± SD for five mice. *Signifies a statistically significant difference (P < 0·05) between the group treated with pCAGGS (Control) and that treated with pCAGGS-IL-12 (IL-12). Results are representative of three independent experiments.
Figure 8.
Cytokine profile in interleukin-12 (IL-12) gene-treated BALB/c mice after Trypanosoma cruzi infection. Production of interferon-γ (IFN-γ) and interleukin-10 (IL-10) was measured in the culture supernatants of spleen cells from mice 20 days after infection with T. cruzi. Spleen cells from three gene-treated mice were pooled and cultured for 3 days in 50 µg/ml of T. cruzi antigen (Ag). The levels of IFN-γ and IL-10 in supernatant were estimated by sandwich enzyme-linked immunosorbent assay (ELISA) using specific Ab pairs. Columns and bars represent mean ± SD of duplicate cytokine assays. Results are representative of two independent experiments.
CD4+ and CD8+ T cells, but not NK cells, contribute to IL-12 gene-mediated protection in T. cruzi infection
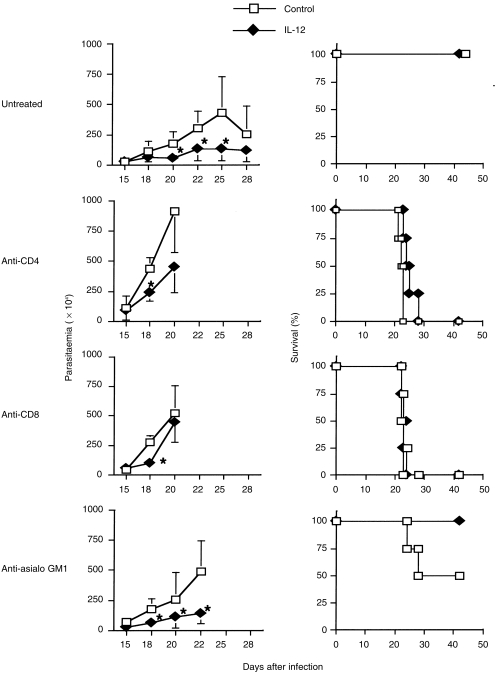
It has been reported that CD4+ T cells, CD8+ T cells and NK cells contribute to protective immunity in T. cruzi infection;26–31 however, accurate effector mechanisms still remain obscure and different from those of L. major infection. In order to identify the cell populations that are responsible for contributing to IL-12 gene-mediated protective immunity, we administered anti-CD4, anti-CD8 or anti-asialo GM-1 Ab into infected and gene-treated mice, then investigated the effects. In control gene treatment, mice treated with anti-CD4 or anti-CD8 mAb showed high levels of parasitaemia at an early stage of infection and all had died by day 23 after infection. Mice treated with anti-asialo GM1 Ab, which exclusively depletes NK cells, also became susceptible to infection and 50% died. In IL-12 gene-treated mice, IL-12 gene-mediated protection was abolished by the depletion of either CD4+ or CD8+ T cells. However, depletion of NK cells modulated the protective effect of IL-12 gene transfer (Fig. 9). These results suggest that IL-12 acts on NK cells by activating either CD4+ or CD8+ T cells.
Figure 9.
Interleukin-12 (IL-12) gene-mediated protection against Trypanosoma cruzi infection is dependent on both CD4+ and CD8+ T cells, but not on natural killer (NK) cells. BALB/c mice infected with 100 bloodstream trypomastigote T. cruzi were treated with IL-12 or a control gene on day 0, and 7 and 14 days after infection. To deplete CD4+ T cells, CD8+ T cells or NK cells, specific monoclonal antibody (mAb) (GK1.5 for CD4+ T cells, 57-6-7 for CD8+ T cells) or antiserum (asialo GM-1 for NK cells) was injected intraperitoneally into mice 0, 5, 10 and 15 days after infection. Data represents the parasitaemia (left panels) and mortality (right panels) of four mice. *Signifies a statistically significant difference (P < 0·05) between the group treated with pCAGGS (Control) and that treated with pCAGGS-IL-12 (IL-12).
Discussion
The present study demonstrates, for the first time, that direct transfer of the IL-12 expression plasmid in vivo can protect against an infection with intracellular protozoan parasites. Currently, various vectors and gene delivery systems are being developed and applied in the fields of gene therapy and DNA vaccination. The gene gun-mediated delivery system of expression plasmid has been shown to be a useful tool in DNA vaccination. In fact, transfer with expression plasmid, encoding pathogen-derived Ag, by a gene gun induces both humoral and cellular immunity to target Ag and results in the development of protective immunity against various infections.3,4 We have, herein, applied this gene delivery system to cytokine gene therapy for systemic infectious models in mice.
IL-12 has been described as a potent inducer of IFN-γ production by NK cells and an activator of different subsets of T cells. IL-12 is required in the development of T-cell-dependent and -independent protective immunity for a variety of micro-organisms.15 Administration of recombinant IL-12 (rIL-12) confers resistance to L. major and T. cruzi infections, while neutralization of IL-12 by mAb increases susceptibility to these infections,32–36 suggesting that IL-12 plays an important role in resistance to these protozoan infections. Although IL-12 is a powerful and effective cytokine, frequent systemic administration of high doses of rIL-12 causes dose-dependent toxicity in mice.37 Thus, relatively low, but long-term, in vivo secretion of IL-12, might be a suitable strategy for IL-12 gene therapy.
As shown in Figs 4 and 7, the transfer of IL-12 expression plasmid to abdominal skin efficiently enhanced protective immunity to two different types of intracellular protozoan parasites. Two mechanisms may exist to account for the protective immunity induced by the IL-12 gene. One possibility is that IL-12 protein produced at the site of gene transfection is released into the bloodstream where it systemically exerts its biological function. Peak expression of IL-12 gene levels were 373 ± 69 pg/mg of protein at 24 hr post-transfection. Furthermore, IL-12 mRNA was detected during a 5-day period at the gene transfection site (Fig. 2). However, serum IL-12 levels were not detected during these time-periods (data not shown). This might be the result of the limited sensitivity of the IL-12 assay (> 20 pg/ml). In contrast, other groups have reported that after transfer with IFN-γ, IL-6, or granulocyte–macrophage colony-stimulating factor (GM-CSF) genes, using gene gun delivery, low levels of these cytokines were apparent in the serum of mice.38 The other possibility is that IL-12 gene-transfected Langerhans’ cells in the epidermis migrate to local LNs and release the cytokine at the draining LN, which results in the development of IFN-γ-producing Th1 cells and activation of T cells. This idea is supported by a mechanism of antigen presentation in gene gun-based DNA immunization.39,40
Multiple cellular components are simultaneously required for establishing resistance to acute infection with T. cruzi, including CD4+ T cells, CD8+ T cells and NK cells.26–31 Macrophages rapidly produce IL-12 after T. cruzi infection in vivo and in turn induce IFN-γ production by NK cells.35 IFN-γ has been shown to be involved in limiting the replication of T. cruzi in host macrophages during the early acute phase of infection,30,31 leading to subsequent activation by both CD4+ and CD8+ T cells. In the present study, BALB/c mice depleted of CD4+ T cells, CD8+ T cells, or NK cells were susceptible to T. cruzi infection, indicating that CD4+ T cells, CD8+ T cells and NK cells all contribute to protective immunity against T. cruzi infection, as previously reported.26–31 In NK cell-depleted mice, transfer of the IL-12 gene restored resistance, whereas this treatment was not effective in CD4+ or CD8+ T-cell-depleted mice. Thus, IL-12 appears to directly activate T cells without requiring the assistance of IFN-γ-producing NK cells, resulting in the growth of Th1 cells and then activating cytotoxic CD8+ T cells.
Recently, a naked plasmid DNA-expressing cytokine has been applied as therapy in models of murine tumour and immunoinflammatory diseases. Gene gun-mediated transfer of the IL-12 gene via the skin was shown to result in regression of primary and metastatic murine tumours.5,6 Furthermore, direct intradermal injection of an IL-12 expression plasmid inhibits tumour growth at sites distant from gene transfection.41 In the case of immunoinflammatory diseases, intramuscular injection with an IL-10 expression plasmid, which possesses immunosuppressive properties, prevents the progression of autoimmune diabetes in non-obese diabetic (NOD) mice42 and suppresses immunopathological changes caused by ocular infection with herpes simplex virus43,44 The advantage of direct DNA transfection into living animals is the simplicity and safety of the technique. Direct transfection with plasmid DNA can be accomplished without the use of infectious virus and without the need for integration of the gene into the host genome. Therefore, this approach may be useful in gene therapy and of value in better understanding the mechanism of immunoregulation seen in a wide spectrum of diseases.
In conclusion, the present results show that the expression of biologically active IL-12 in vivo can be achieved following gene gun-based IL-12 gene delivery, resulting in the systemic modulation of an immunoresponse. This immunoregulation confers protection against infection with the intracellular protozoan parasites, L. major and T. cruzi.
Acknowledgments
We thank Dr R. A. Good for his critical review of the manuscript. This work was supported by research grants from the Ministry of Education, Science and Culture of Japan.
Glossary
Abbreviations
- Con A
concanavalin A
- IFN-γ
interferon-γ
- IL-12
interleukin-12
- LN
lymph node
- mAb
monoclonal antibody
- NK
natural killer
- Th1
T helper type 1
- RT–PCR
reverse transcription-polymerase chain reaction
References
- 1.Yang N-S, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci USA. 1990;87:9568. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng L, Ziegelhoffer RP, Yang N-S. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA. 1993;90:4455. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 5.Rakhmilevich AL, Turner J, Ford MJ, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci USA. 1996;93:6291. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakhmilevich AL, Jansse K, Turner J, Culp J, Yang N-S. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997;8:1303. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Fitz J, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biological effects on human lymphocytes. J Exp Med. 1989;170:827. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern AS, Padlasky FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valiante NM, Rengraju M, Trinchieri G. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of the B cell lines to stimulate T and NK proliferation. Cell Immunol. 1992;145:187. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 10.Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874. [PubMed] [Google Scholar]
- 11.Wu CY, Demeure CE, Kiniwa M, Gately M, Delespesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4+ T cells. J Immunol. 1993;151:1938. [PubMed] [Google Scholar]
- 12.Macatonia SE, Hsieh C, Murphy M, O'garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from αβ-TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-γ production is IFN-γ-dependent. Int Immunol. 1993;5:1119. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 13.Bloom ET, Horvath JA. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T cell activity: implications for the age-related decline in CTL. J Immunol. 1994;152:4242. [PubMed] [Google Scholar]
- 14.O'garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 16.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon γ or IL-4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott P. The role of Th1 and Th2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989;68:369. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- 19.Scott P, Natovitz P, Coffman R, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis: T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzel FP, Sadick MS, Mutha S, Locksley RM. Production of interferon-γ, interleukin 2, interleukin 4 and interlukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holaday BJ, Sadick MD, Wang Z, et al. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991;147:1653. [PubMed] [Google Scholar]
- 22.Torrico F, Heremans H, Rivera MT, van Marck E, Billiau A, Carlier Y. Endogenous IFN-γ is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626. [PubMed] [Google Scholar]
- 23.Minoprio P, Cheikh MCE, Murphy E, et al. Xid- associated resistance to experimental Chagas’ disease is IFN-γ dependent. J Immunol. 1993;151:4200. [PubMed] [Google Scholar]
- 24.Silva J, Morrissey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. Interleukin-10 and interferon-γ regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed SG, Brownell CE, Russo DM, Silva JS, Grabstein KH, Morrissey PJ. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135. [PubMed] [Google Scholar]
- 26.Rottenberg ME, Riarte A, Sporrong L, et al. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol Lett. 1995;45:53. doi: 10.1016/0165-2478(94)00221-c. [DOI] [PubMed] [Google Scholar]
- 27.Rottenberg ME, Bakhiet M, Olsson T, et al. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarleton RL, Grusby MJ, Postan M, Glimcher LH. Trypanosoma cruzi infection: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol. 1996;8:13. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 29.Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 30.Rottenberg M, Cardoni RL, Andersson R, Segura EL, Örn A. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanomoma cruzi infection. Scand J Immunol. 1988;28:573. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 31.Cardillo F, Voltarelli JC, Reed SG, Silva J. Regulation of Trypanosoma cruzi infection and interleukin 10: role of NK cells. Infect Immun. 1996;64:128. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sypek JP, Chung CL, Mayor SEH, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattner F, Padova KD, Alber G. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect Immun. 1997;65:4378. doi: 10.1128/iai.65.11.4378-4383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aliberti JCS, Cardoso MAG, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediated resistance to Trypanosoma cruzi in mice is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter CA, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect Immun. 1996;64:2381. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orange JS, Salazar-mather TP, Opal SM, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun WH, Burkholder JK, Sun J, et al. In vivo cytokine transfer by gene gun reduces tumor growth in mice. Proc Natl Acad Sci USA. 1995;92:2889. doi: 10.1073/pnas.92.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD. DNA-based immunization by in vivo transfection of dendritic cells. Nature Med. 1996;2:1122. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 40.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan J, Newton CA, Djeu JY, et al. Injection of complementary DNA encoding interleukin-12 inhibits tumor establishment at a distant site in a murine renal carcinoma model. Cancer Res. 1996;56:3399. [PubMed] [Google Scholar]
- 42.Nitta Y, Tashiro F, Tokui M, et al. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum Gen Ther. 1998;10:1701. doi: 10.1089/hum.1998.9.12-1701. [DOI] [PubMed] [Google Scholar]
- 43.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945. [PubMed] [Google Scholar]
- 44.Manickan E, Daheshia M, Kuklin N, Chun S, Rouse BT. Modulation of virus-induced delayed-type hypersensitivity by plasmid DNA encoding the cytokine interleukin-10. Immunology. 1998;94:129. doi: 10.1046/j.1365-2567.1998.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]