Abstract
Previous investigations have shown that a subset of inferior colliculus neurons, which have been designated type O units, respond selectively to isolated features of the cat’s head-related transfer functions (HRTFs: the directional transformation of a free-field sound as it propagates from the head to the eardrum). Based on those results, it was hypothesized that type O units would show enhanced spatial tuning in a virtual sound field that conveyed the full complement of HRTF-based localization cues. As anticipated, a number of neurons produced representations of virtual sound source locations that were spatially tuned, level tolerant, and effective under monaural conditions. Preferred locations were associated with spectral cues that complemented the highly individualized broadband inhibitory patterns of tuned neurons. That is, higher response magnitudes were achieved when spectral peaks coincided with excitatory influences at best frequency (BF: the most sensitive frequency) and spectral notches fell within flanking inhibitory regions. The directionally dependent modulation of narrowband ON-BF excitation by broadband OFF-BF inhibition was not a unique property of type O units.
Keywords: head-related transfer function, spatial tuning, spectral integration, level tolerance, monaural hearing
1. Introduction
The neural representation of sensory information undergoes a series of fundamental transformations as it ascends the central nervous system (Eggermont, 2001; Somjen, 1967). At the early stages of auditory processing, populations of neurons in the auditory nerve and ventral cochlear nucleus encode elemental properties of sound such as the amplitude or timing of frequency components (Kiang, 1980; Kim and Molnar, 1979; Sachs and Young, 1979; Young and Sachs, 1979). This initial Fourier-like analysis is largely linear and a great deal may be learned about the information content of the neural response by parametric manipulations of simple auditory stimuli (Le Prell et al., 1996; May and Huang, 1997; Sachs and Young, 1980).
When the distributed representation of the sound’s physical characteristics reaches higher levels of processing, unique patterns of convergence establish neural receptive fields that are tuned to discrete acoustic features (Bullock, 1986; Kolomiets et al., 2001; Majorossy and Kiss, 1994; Oliver, 1987). For example, bilateral projections from the ventral cochlear nuclei merge in the superior olive to endow local neurons with a sensitivity for interaural level or time disparities (ILDs and ITDs)(Glendenning et al., 1985; Oliver et al., 2003; Tsuchitani, 1988). These binaural pathways are critical for the accurate localization of sound sources in the horizontal plane (Casseday and Neff, 1975; Furst et al., 2000; Neff, 1977).
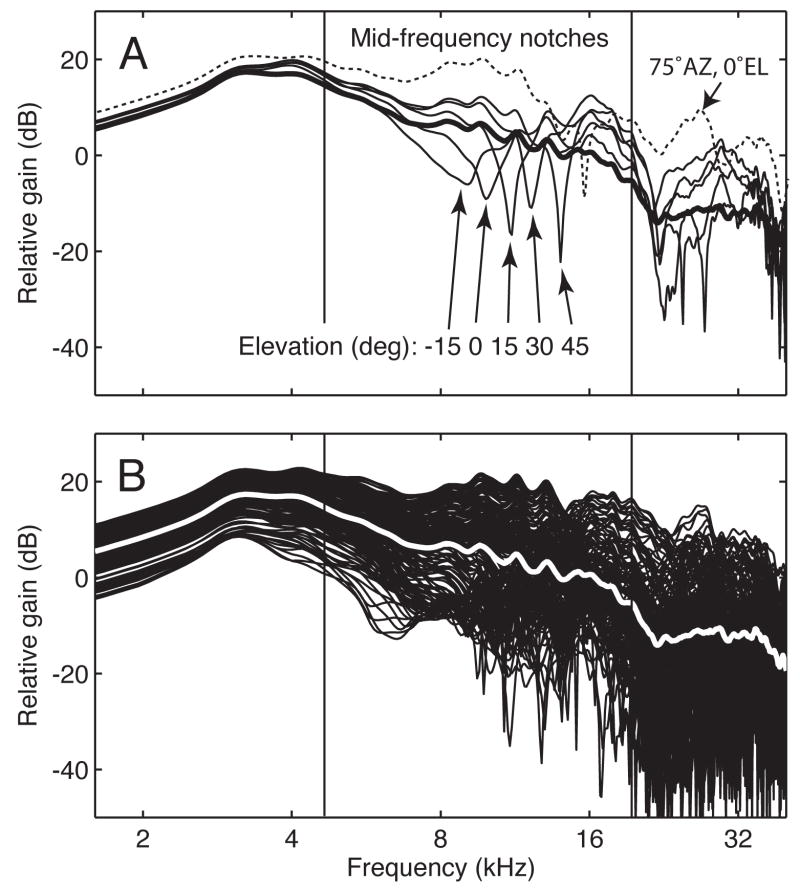
Parallel pathways linking the dorsal cochlear nuclei and inferior colliculus play an equally important role in vertical localization in cats (Davis et al., 2003; May, 2000; Sutherland et al., 1998). The elevation of a sound source is conveyed by monaural spectral cues that are superimposed on complex sounds by directional filtering properties of the head and outer ear (Butler and Belendiuk, 1977; Hebrank and Wright, 1974; Middlebrooks and Green, 1991). Figure 1 summarizes the general acoustic features of the domestic cat’s head-related transfer functions (HRTFs) (Phillips et al., 1982; Rice et al., 1992; Xu and Middlebrooks, 2000). The measures were made by implanting a probe tube microphone in the ear canal and recording the spectrum that propagated to the tympanic membrane from fixed locations in the frontal field. A relative gain of 0 dB indicates no change between the remote spectrum and sound levels in the ear canal.
Figure 1.
Directional filtering properties of the cat’s outer ear. A. Head-related transfer functions (HRTFs) at five elevations in the median vertical plane (numerical labels indicate elevation) and one lateralized location in the interaural horizontal plane (dashed line). B. A larger collection of HRTFs for locations encompassing the frontal sound field. Bold lines in A and B indicate the average spectrum of all HRTFs. These recording were originally reported by Rice et al., 1992.
The HRTFs in Figure 1A show how spectral energy in the ear canal is altered when the source of a broadband sound moves in the median vertical and interaural horizontal planes. A prominent feature of the HRTFs is a spectral notch at frequencies between 8 to 16 kHz. Localization deficits are observed if these directional cues are removed from broadband sounds (Huang and May, 1996a; Huang and May, 1996b; Middlebrooks and Green, 1991).
As originally noted by Rice et al. (1992), general properties of the cat’s HRTF suggest three domains of localization cues. These directional characteristics are revealed by superimposing a large collection of HRTFs in Figure 1B. The measures represent a sampling grid that stretches across azimuths of ±75 degrees and elevations of −30 to +90 degrees. Frequencies below 5 kHz display a broad resonance that varies in level with sound source azimuth. This azimuth dependence is only seen for the lateralized sound source in Figure 1A (dashed line) because it is created by the sound shadow of the head at locations outside the median plane. Frequencies between 5 and 20 kHz exhibit directionally dependent notches that are systematically related to the elevation and azimuth of the sound source. These mid-frequency localization cues are a dominant feature of the HRTFs in Figure 1A. Frequencies above 20 kHz reveal complex patterns of peaks and notches that are communicated with low relative gains. The high-frequency spectrum does not support accurate localization when presented in isolation (Huang and May, 1996a; Huang and May, 1996b).
Unlike the auditory nerve and ventral cochlear nucleus, the spectral integration properties of the dorsal cochlear nucleus (DCN) and inferior colliculus are highly nonlinear (Nelken et al., 1997; Young et al., 2005; Yu and Young, 2000). This complexity makes it impossible to extrapolate the neural representations of HRTF-based spectral features from responses to narrowband tones or unshaped noise. More sophisticated analyses have attempted to define the neural tuning of auditory neurons with reverse correlation (Carney and Friedman, 1998; Eggermont et al., 1983a), spectral temporal receptive fields (Aertsen and Johannesma, 1981; Eggermont et al., 1983b; Kim and Young, 1994), and weighting function models (Young et al., 2005). In particular, estimates of spectral weights based on responses to random spectral shapes (RSS) have proven useful in defining the receptive field properties of DCN neurons (Kim and Young, 1994; Reiss et al., 2007; Yu and Young, 2000). The predictive power of these models is improved by incorporating higher-order weights that describe the interactions between frequency terms. Despite these enhancements, weighting models fail to predict the coding properties of nonlinear neurons in the spectral processing pathway with consistent accuracy. A potential limitation of any parametric approach to sensory coding is the need to sample an adequate range of deterministic stimulus conditions. The present study avoided the pitfalls of parameter estimation by measuring the responses of inferior colliculus neurons to naturally occurring HRTF spectra.
Results from our previous studies (Davis et al., 1999; Ramachandran et al., 1999) suggest that single-unit responses in the inferior colliculus of decerebrate cats can be classified according to the frequency distribution of their inhibitory inputs, which are revealed by frequency response maps (FRMs: 3-dimensional plots of tone-driven activity in coordinates of frequency and level). In particular, type O responses show highly nonlinear spectral integration properties that enhance their ability to encode sharp spectral features (Davis, 2005; Davis et al., 2003). Based on those observations, it was hypothesized that type O neurons would provide the most sensitive representation of HRTF-based localization cues. As predicted, our tests of that hypothesis revealed neurons with receptive fields that matched spatially tuned HRTF shapes. This spectral selectivity persisted across a wide range of sound levels and under monaural testing conditions. The patterns of bandband inhibition that direct these localization processes were not a unique property of type O neurons. Consequently, the following descriptions relate our findings to a number of alternative classification systems that have been applied to the inferior colliculus.
2. Methods
2.1. Subjects
Single-unit responses were recorded in the inferior colliculus of 16 adult male cats (3 – 4 kg). The cats were procured from Liberty Labs (Liberty Corners, NJ) and housed in institutional facilities for approximately one week. Prior to experimentation, otoscopic examinations confirmed that all subjects had clean external ears and clear tympanic membranes. The cats participated in acute recording sessions that were terminated by barbiturate overdose (sodium pentobarbital, 100 mg/kg i.v.) after approximately 24 hours. All procedures were performed under the guidelines of the Institutional Animal Care and Use Committee of Johns Hopkins University.
2.2. Surgical procedures
Surgical procedures for single-unit recording in the inferior colliculus of decerebrate cats are routine in our laboratory and have been described in detail in previous publications (Davis et al., 1999; Ramachandran et al., 1999). Prior to recording, the animal was anesthetized with xylazine (0.1 mg, i.m.) and ketamine (initial dose 40 mg/kg, i.m.; supplemental doses 15 mg/kg, i.v.). The skull over the left parietal cortex was opened and a transverse section of brainstem was aspirated between the superior colliculus and thalamus to eliminate motor control and pain reception from the cerebral cortex (Flecknell and Silverman, 2000). Anesthesia was discontinued at the conclusion of decerebration.
Preparation for single-unit recording included catheterization of the cephalic vein to allow intravenous injections of anesthetic agents that were required by initial surgical procedures. A tracheotomy was performed to facilitate quiet breathing. The tympanic bullae were ventilated to equalize middle ear pressure. The head was secured in a stereotaxic apparatus by inserting hollow ear bars into both ear canals, which were transected near the ear drum. The ear bars served as specula for delivering acoustic stimuli in closed field. The left inferior colliculus was exposed by aspirating occipital cortex and partial removal of the overlying tentorium.
2.3. Recording procedures
Platinum-iridium microelectrodes were advanced through the inferior colliculus with a hydraulic micromanipulator. The electrode signal was amplified (10,000–30,000×) and low-pass filtered at 5 kHz. A Schmitt trigger was used to record the timing of action potentials that were well isolated from background activity. The BF and threshold of auditory units were determined by manually sweeping the frequency and level of tone bursts in the contralateral ear. Rate-level functions for BF tones and noise bursts were collected to characterize the dynamic range of rate representations under narrowband and broadband conditions.
Results are initially presented using the type I – V response classes of Evans and Nelson (Evans and Nelson, 1973). They are later related to our own type V/I/O classification system (Davis et al., 1999; Ramachandran et al., 1999). Although the type I – V nomenclature was originally defined in the context of frequency response maps (FRMs), these designations have been extended to refer to the degree of non-monotonicity for pure-tone rate-level functions under monaural conditions (Young et al., 1988). Because rate-level functions also may be obtained with complex binaural sounds, these descriptions are a useful metric for investigating the effects of stimulus bandwidth and binaurality on HRTF-based discharge rates. Details of the type V/I/O nomenclatures, and how these classifications relate to the type I – V system, are presented later. In essence, type IV and V responses are associated with the type O neurons upon which our hypothesis is based.
Type I and III units show exclusively excitatory responses at BF. The rate-level functions of type I units are monotonic, while type III units are non-monotonic. Type IV and V units are inhibited at high BF levels. Type IV units are excited at low BF levels, while type V units are exclusively inhibited. The latter classification is rarely encountered in the inferior colliculus. Type II units lack spontaneous activity and do not respond to noise. This unit type is associated with inhibitory interneurons found in the DCN (Young and Voigt, 1982). It is not observed in the inferior colliculus.
Descriptions of group classifications combine type I and III neurons (type III/I) and type IV and V units (type IV/V) to distinguish the strength of ON-BF inhibition and putative sources of input. The less inhibited type III/I response patterns in the inferior colliculus are assumed to arise from ascending projections from the ventral cochlear nucleus, while the more inhibited type IV/V responses have been linked to the dorsal cochlear nucleus (Young et al., 1988).
Spectral selectivity was assessed by presenting 99 HRTF-shaped noise bursts at multiple presentation levels, and under binaural and monaural conditions. Spatial tuning was evaluated by plotting discharge rates in coordinates that reflected the free-field locations of the HRTFs. Non-directional components of the neural responses were removed from each test result by subtracting the average rate for all 99 HRTFs from responses to individual stimuli. The distribution of ‘directional rates’ identified preferred locations without changing relative response magnitudes. A similar analysis has been used to describe directional acoustic properties of the HRTF (Middlebrooks, 1992; Middlebrooks and Green, 1990).
2.4. Acoustic stimuli
HRTF-shaped noise bursts were 330 ms in duration with 10 ms rise/fall times and a 1-s repetition period. The directional properties of the cat’s outer ear were previously recorded by surgically implanting a probe microphone near the ear drum of anesthetized cats and recording the sound energy that propagated to the ear canal from fixed locations in the frontal sound field (Rice et al., 1992). The 99 HRTFs represented a region of space from −60 to 60° azimuth (increments of 15°) and −30 to 45° elevation (increments of 7.5°). Gaussian noise was filtered through the HRTF spectra and digitally recorded at a 100-kHz sampling rate. Virtual space (VS) was simulated by simultaneous playback of paired HRTF stimuli. For example, a sound localization of 45° azimuth (AZ), 0° elevation (EL) was produced by presenting the 45° AZ, 0° EL HRTF to the right (contralateral) ear and the −45° AZ, 0° EL HRTF to the left (ipsilateral) ear. In addition to monaural spectral cues, the VS stimuli also conveyed veridical interaural level differences (ILDs) and interaural time differences (ITDs).
The frequency response of the closed-field sound system was measured at the beginning of each experiment by inserting a probe tube microphone into calibration bores on the ear bars. Once positioned, the tip of the probe tube was approximately 3 mm from the eardrum. Microphone voltages were measured with a lock-in amplifier while sweeping tones across frequencies from 0.04 to 40 kHz. Voltages were converted to dB SPL based on microphone sensitivity. These calibrations varied less than ±5 dB across the range of unit BFs in the current study.
3. Results
Physiological measures were performed on 231 neurons in the central nucleus of the inferior colliculus. Although BFs and thresholds of this sample spanned most of the audible frequency range of the domestic cat (0.3 – 39 kHz), electrode penetrations were intentionally biased toward regions of the central nucleus where BFs fell between 5 and 20 kHz (N = 144) because monaural spectral cues in this frequency range dictate the accuracy of directional hearing in the median plane (Huang and May, 1996a; Huang and May, 1996b). Rate-level classifications were completed with tones versus noise, or monaural versus binaural stimulation on 183 neurons. HRTF sensitivity was measured in 169 neurons.
3.1. Limitations of physiological classification systems
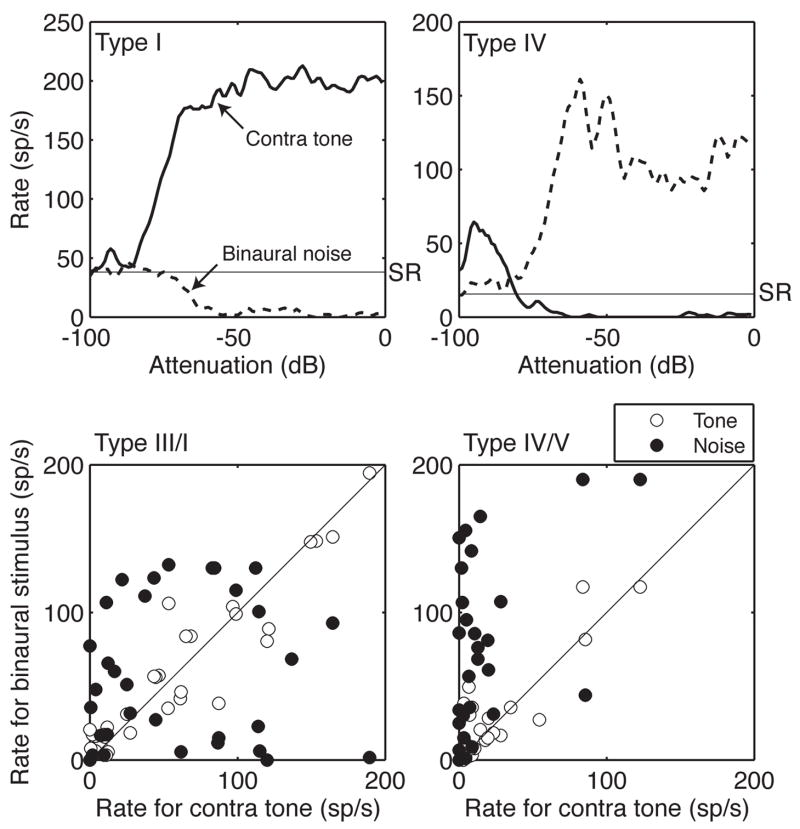
Inferior colliculus neurons have been classified by patterns of inhibition in rate-level functions and frequency response maps (Davis et al., 1999; Ramachandran et al., 1999). These measures are typically collected by presenting pure tones to the contralateral ear. The rate-level functions in Fig. 2 illustrate the effects of stimulus bandwidth and presentation mode on the inhibitory patterns of two common response types. The type I unit showed a strong monotonic excitation for monaural tones but was largely inhibited by binaural noise. Conversely, the type IV neuron was inhibited by tones but strongly driven by noise. This dissociation of response relationships for narrowband versus broadband stimuli is a well known property of the highly nonlinear integration properties of central auditory neurons (Escabi and Schreiner, 2002; Mayer-Kress, 1998; Young et al., 2005). This nonlinearity severely limits that ability of a pure-tone classification system to predict tuning in the context of sound localization where bandwidth, binaurality, and spectral shape are defining characteristics of natural stimuli.
Figure 2.
Effects of bandwidth and presentation mode on discharge rates in the inferior colliculus (IC). Representative rate-level functions of type I and IV units are compared in the upper panels. The binaural tone and noise responses of a broad sampling of type III/I and type IV/V units are contrasted with contralateral tone responses in the lower scatterplots. The discharge rates were sampled at a fixed 50-dB attenuation level. The responses of units with linear integration properties fall along the unity line.
The lack of correlation across bandwidth and presentation mode is evident for the contralateral tone versus binaural responses of a broad sampling of physiologically classified units in the lower panels of Fig. 2. Responses are compared at a fixed 50-dB attenuation, which has been selected to emphasize the non-linearity of spectral integration at levels within the dynamic range of most neurons in the sample. Neurons with type III/I designations were strictly excited by contralateral BF tones. The majority of the units showed similar discharge rates when tested with binaural tones (unfilled symbols near the unity line in the scatterplot). The effects of presentation mode were not statistically significant (paired sign test, P > 0.05). The binaural noise responses of type I and III units were poorly predicted by contralateral tone discharge rates (filled symbols). An approximately equal number of units showed higher and lower noise rates than tone rates; consequently, the combined effects of bandwidth and presentation mode failed to attain statistical significance. The effects of presentation mode on the tone-driven activity of type IV/V units also were not statistically significant. These units, however, showed significantly less inhibition when tested with binaural noise (P < 0.001).
3.2. Rate-level correlations
The bandwidth effects in Figure 2 suggest that the discharge rates of inferior colliculus neurons may be modulated by spectral energy that is remote to unit BF. Under normal free-field listening conditions, the spectral characteristics of broadband sounds are strongly shaped by directional properties of the HRTF. As a result, acoustic energy in the inhibitory or excitatory receptive field of an auditory neuron may be augmented or diminished depending on source location.
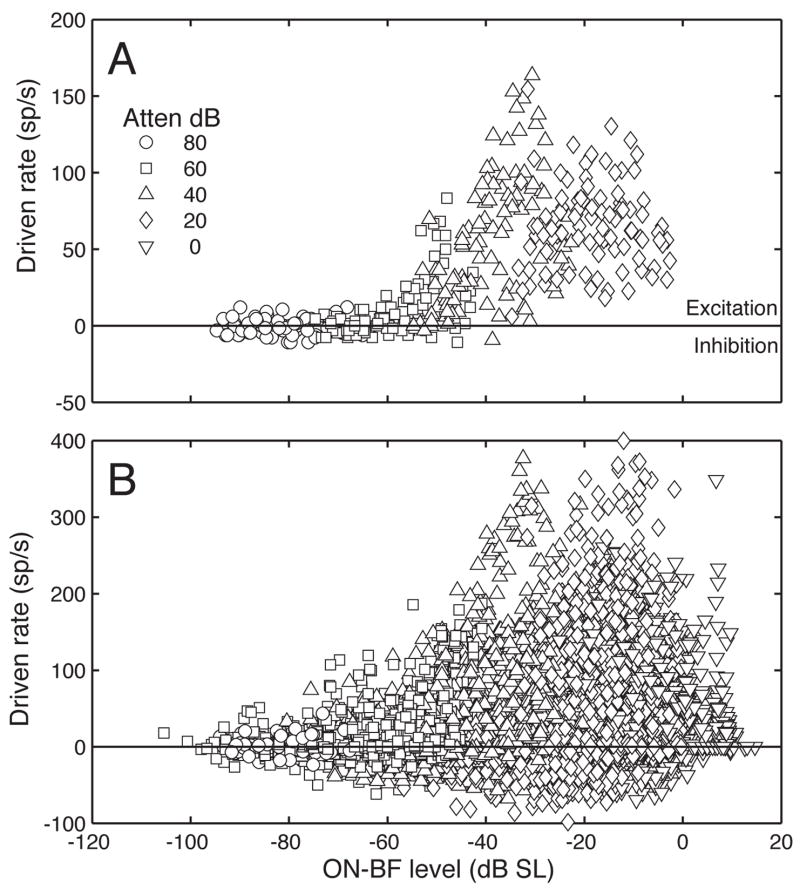
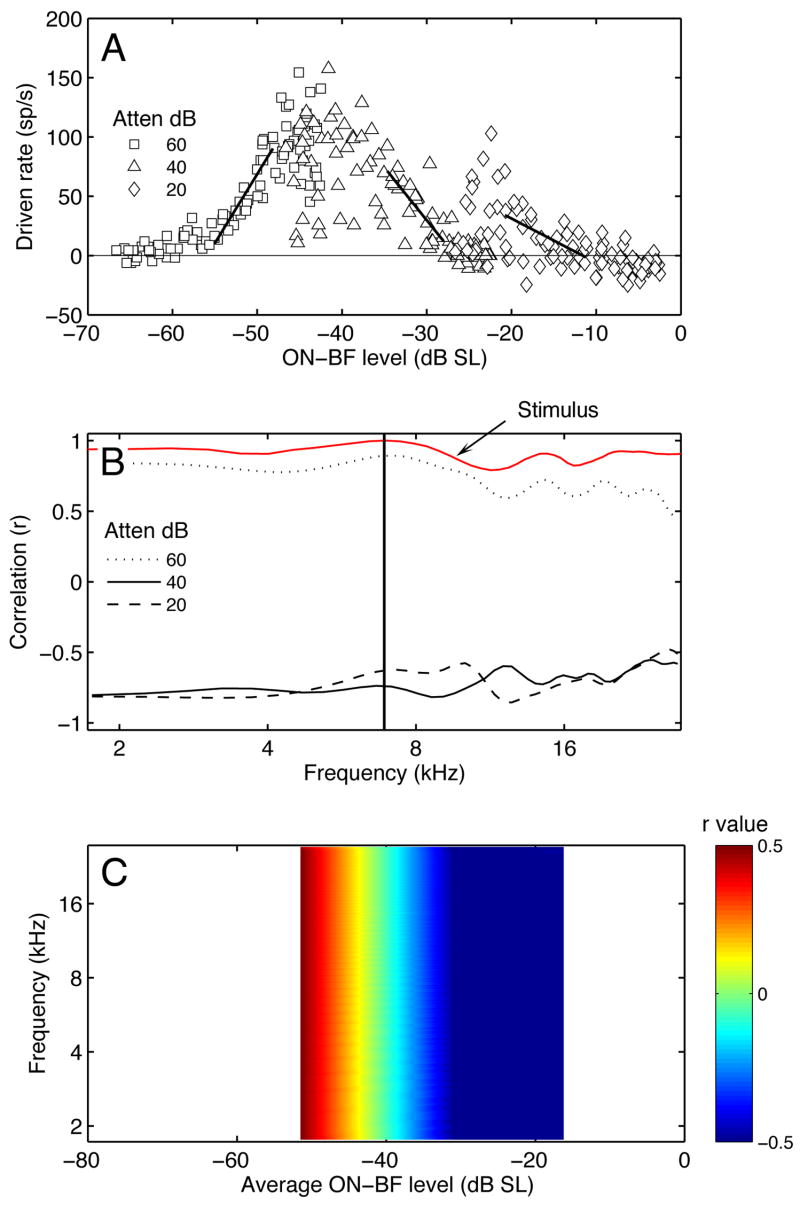
Figure 3A illustrates the effects of spectral shape on the discharge rates of a representative type III neuron. The unit’s driven rates for the 99 HRTF-shaped noise bursts are plotted in relation to ON-BF spectrum level (BF = 13.6 kHz). Replications at four attenuation settings are indicated by symbol type. Level variations within the same attenuation reflect the directional filtering effects in Figure 1. Driven rates were calculated by subtracting spontaneous activity in stimulus-off periods from discharge rates in stimulus-on periods. Positive rates mean the unit was excited by the stimulus; negative rates indicate inhibition. Rate variations at the same ON-BF level reflect the unit’s sensitivity to differences in OFF-BF energy.
Figure 3.
Effects of spectral shape on discharge rates in the inferior colliculus (IC). A. Responses of a representative type III neuron to 99 HRTF-shaped noise bursts. Driven rates are plotted relative to stimulus level at BF (13.6 kHz). Replications at different attenuation settings are distinguished by symbol type. B. Same plotting format showing the rate-level relationships of a population of IC neurons (N = 160 units).
Figure 3B extends the rate-level analysis to all IC neurons that were tested with binaural HRTF-shaped noise bursts (N = 160 units). A striking aspect of the scatterplot is the diversity of responses at the same ON-BF level. Although the lack of a systematic rate versus level relationship suggests poor spectral representation, the responses were highly reproducible across repetitions at the same presentation level and often showed the same relative magnitudes across presentation levels. ON-BF rate-level relationships inadequately describe the responses of certain inferior colliculus neurons because they are performing a broad bandwidth integration of the HRTF shape.
The spectral features that modulate HRTF-driven responses can be identified by correlating discharge rates to the acoustic variation at any frequency component of the complex stimulus. This process is introduced in Figure 4A, using the ON-BF rate-level functions from Figure 3A. The unit’s discharge rates have been sorted by the spectrum level at BF and presentation level. Piece-wise correlation coefficients (r) were obtained by applying multiple linear fits to continuous, overlapping segments of the rate-level functions. Each fit was applied to 33 contiguous points, starting with the lower limit of the first fit at the lowest ON-BF level and shifting by one point until the upper limit of the final fit reached the highest ON-BF level. Thus, a total of 67 fits were performed on each data set.
Figure 4.
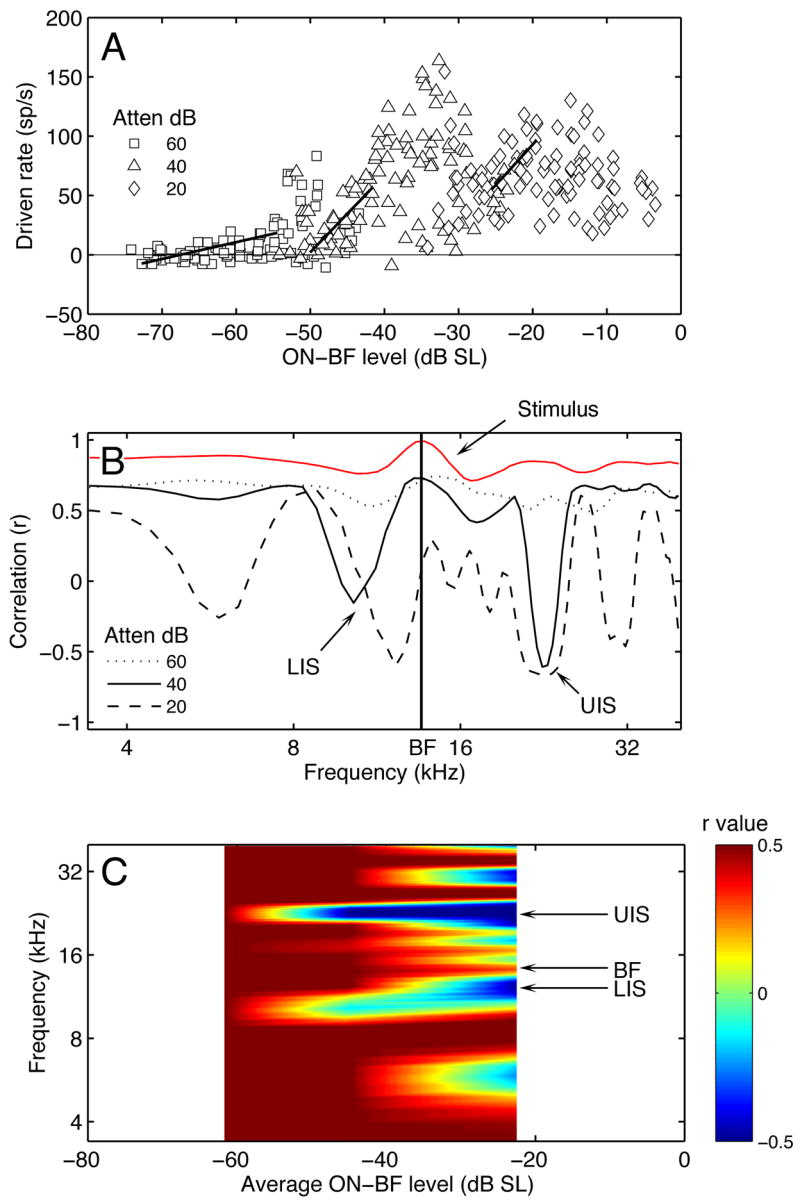
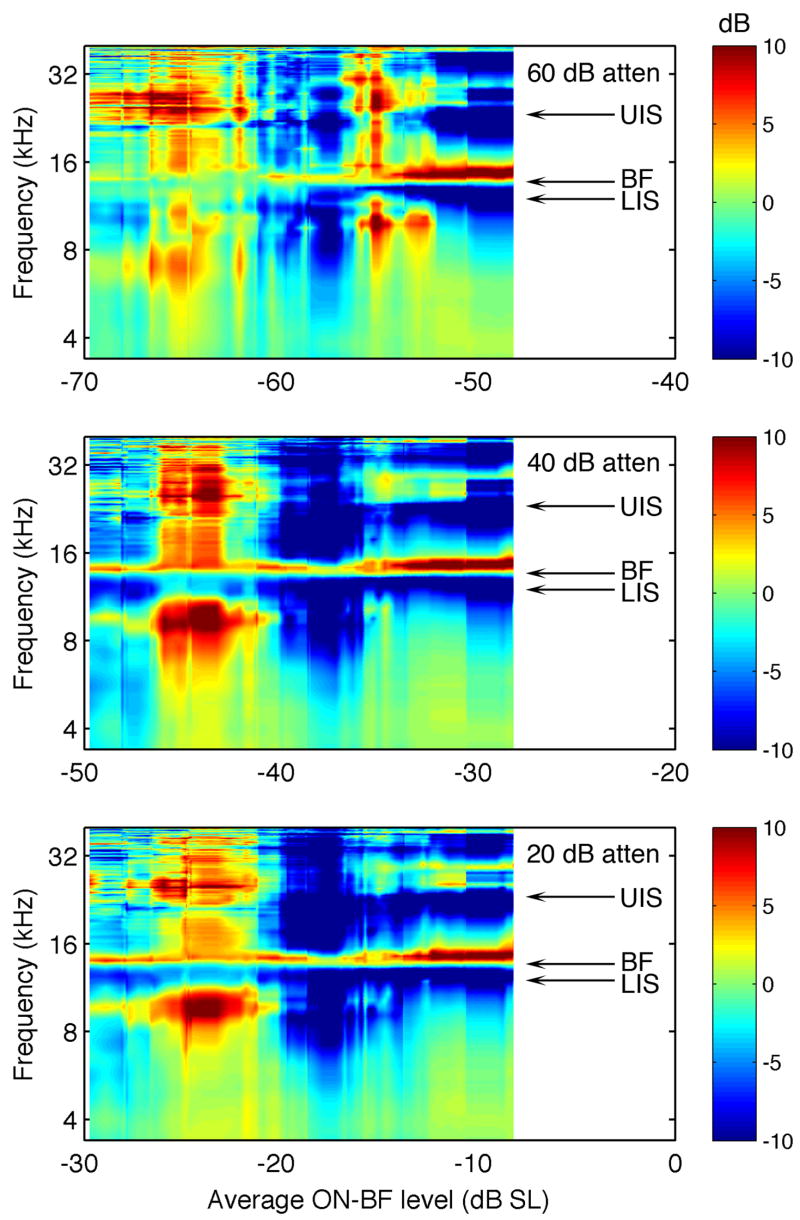
Spectral integration properties of the representative type III neuron (same unit from Figure 3). A. Rate-level functions showing the region of maximum linear correlation between ON-BF level and response magnitude (line segments). B. Maximum linear correlations (r) for rate-level functions derived at frequencies surrounding BF. Negative r values reveal lower and upper inhibitory sidebands (LIS, UIS). Stimulus-bound correlations between BF and OFF-BF components are indicated in red. C. Correlation map showing r in relation to frequencies in B and the average ON-BF levels in A.
The strongest stimulus effect was defined by the highest absolute correlation coefficient among the 67 fits. Positive correlations indicate an excitatory relationship between spectrum level and discharge rates, while negative correlations reveal inhibition. In Figure 4A, the 33 data points with the highest correlation are indicated for each presentation level by their corresponding linear fits. The positive slope of the line segments indicates that the most powerful effect of ON-BF energy was excitatory.
Additional sets of correlation coefficients were obtained to determine the effects of additional spectral components on discharge rates. OFF-BF fits were calculated by shuffling the rates in Figure 4A according to the spectrum level in 100-Hz frequency bins that ranged from ±2 octaves relative to BF. Figure 4B plots the maximum absolute correlation coefficient for each bin at the three presentation levels. A prominent feature of these frequency maps is the expression of strong negative correlations that suggest the presence of lower and upper inhibitory sidebands (LIS and UIS).
A concern with the correlation method is the lack of independence between the frequency components of HRTFs. When a sound source changes in horizontal location, the effective attenuation of the head’s sound shadow is simultaneously altered across a wide frequency range. For example, in Figure 1, the lateralized HRTF at 75° AZ, 0° EL shows higher than average gains at all but a few frequency bins. These universal shifts generate large positive correlations between ON-BF and OFF-BF gains. Consequently, discharge rates correlated to changes in ON-BF spectral energy will also show indirect, purely stimulus-bound correlations at OFF-BF frequencies.
The stimulus-bound correlations between spectral components were quantified with the same linear fitting algorithm that was applied to HRTF rate-level functions. Each HRTF produced an ON-BF level and a comparison level for the OFF-BF frequency bin. The data were sorted by ON-BF level and linear fits were applied to overlapping segments of the resulting level-level functions. The maximum absolute stimulus-bound correlation for each frequency bin is shown in Figure 4B (red line).
Paradoxically, the illusion of broadband sensitivity is exacerbated by the narrow tuning of most neurons near threshold because responses are dictated by a single frequency component. In this example, notice how stimulus-bound correlations dominate those derived from spectral effects at the 60-dB attenuation. At higher presentation levels, the stimulus correlations are offset by OFF-BF inhibitory effects.
The surface plots in Figure 4C combine the correlation frequency maps across level to illustrate the dynamic range of the LIS and UIS. Positive correlations (excitatory rate-level relationships) are indicated in red. Negative correlations (inhibitory relationships) are indicated in blue. This unit exhibited ON-BF excitation at low presentation levels, but was largely influenced by OFF-BF inhibition at high levels.
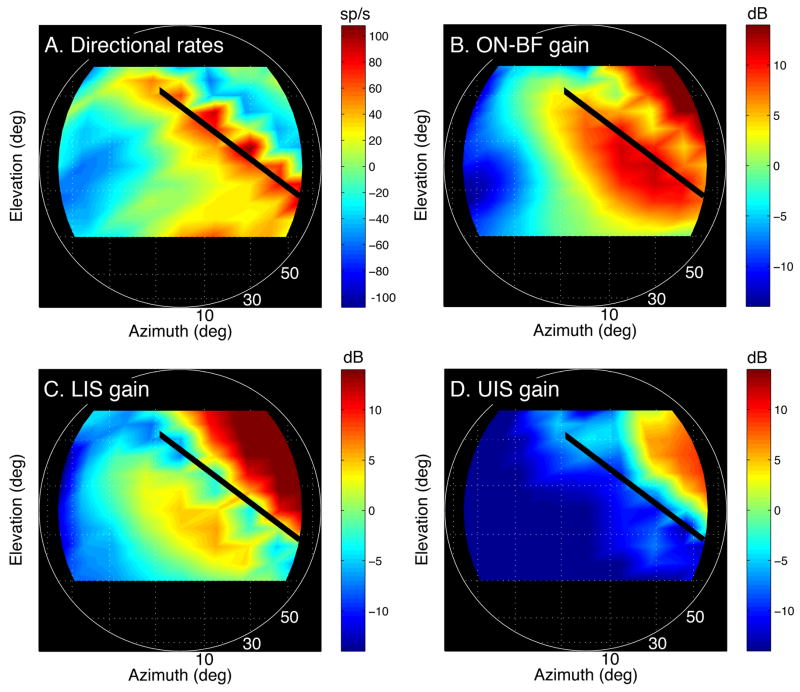
The effects of inhibitory sidebands on the spatial tuning of the neuron are summarized in Figure 5. Figure 5A re-plots the unit’s discharge rates at 40-dB attenuation in spatial coordinates that refer to the original free-field location of the HRTF shapes. Maximum rates demarcate a well-defined spatial receptive field that falls in elevation as the VS stimulus ‘moves’ toward contralateral azimuths (bold line). This tuning is not predicted by the more diffuse directionality of highest ON-BF gain in Figure 5B (red area).
Figure 5.
Importance of inhibitory sidebands on spatial tuning. A. Virtual space location of the representative unit’s responses to HRTF-shaped noise bursts. The colormap indicates directional rate, which was calculated from the HRTF-driven rates in Figure 3. B – D. The directional gain of the HRTFs at the unit’s BF, LIS and UIS plotted at the same spatial coordinates. Bold lines identify locations associated with maximum directional rates.
The spatial distribution of energy at the LIS and UIS is shown in Figures 5C and D. The tight coupling of maximum directional rates (bold line) to locations with low spectral energy (blue areas) suggests that the unit’s spatial tuning was derived by matching a subset HRTF templates to the frequency distribution of excitatory and inhibitory inputs. The unit was made responsive by ON-BF excitatory influences, but it was made directional by OFF-BF inhibition.
3.3. Spectral pattern matching
A post hoc analysis of the optimal spectral template for the representative unit is presented in Figure 6. The line segment in Figure 6A is fit to the 40-dB attenuation rate-level function. The segment indicates the 21 contiguous points with maximum rate variation. The 5 highest (lowest) rates within this constrained range of levels are indicated by filled symbols above (below) the line segment.
Figure 6.
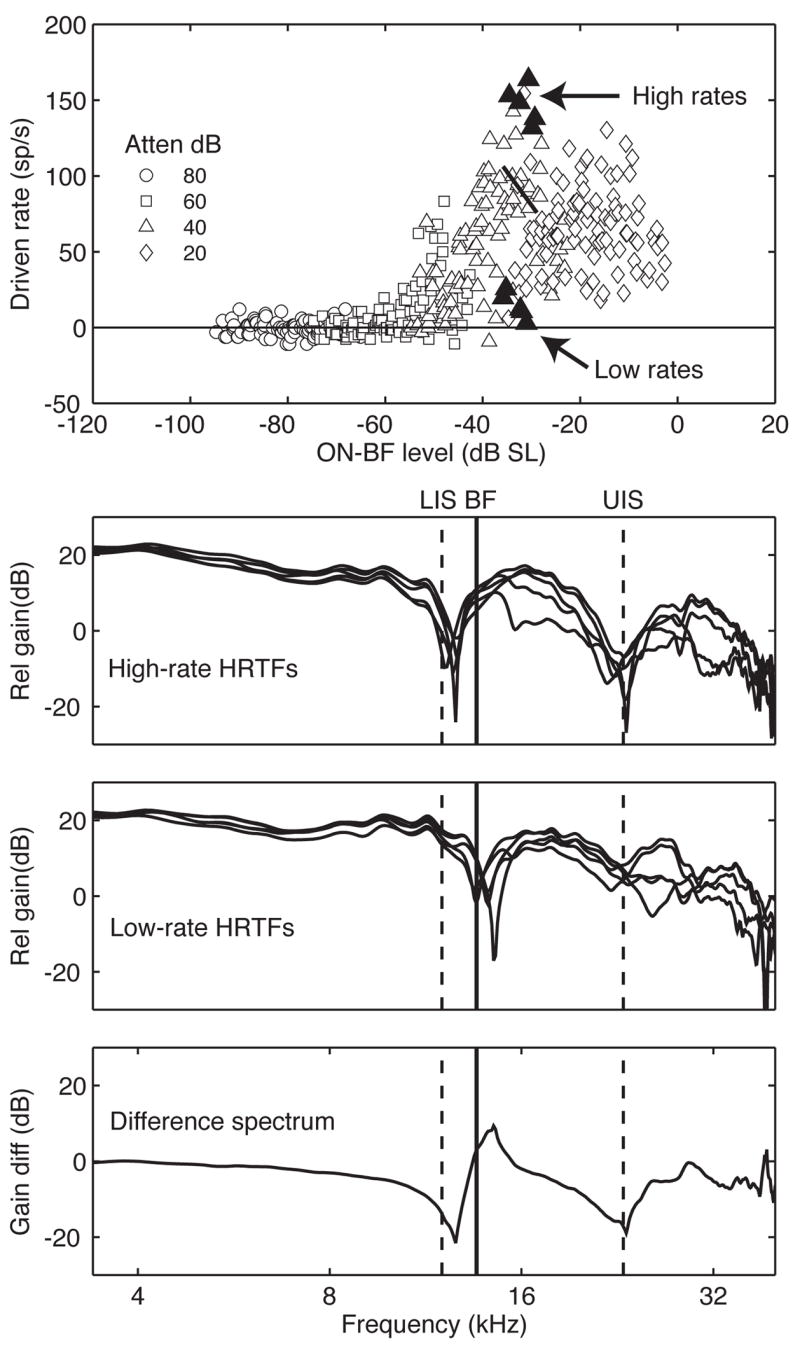
Deriving an optimal spectral shape from directional responses. A. Line segment identifying the region of maximum rate variation for a presentation level of 40-dB attenuation. Filled symbols identify the five highest and lowest rates. B, C. The HRTF shapes that elicited the five highest and lowest rates. D. Difference spectrum for the average shape of high versus low rate HRTFs.
The similarities between the HRTFs that produced either high or low driven rates are striking. The five high-rate HRTFs in Figure 6B maximize energy within the unit’s excitatory ON-BF receptive field (vertical solid line) and minimize energy in the LIS and UIS (vertical dashed lines). Conversely, in Figure 6C, the five low-rate HRTFs share spectral notches at BF and energy peaks at the inhibitory sidebands. Thus, the two groups of spectral shapes display diametrically opposed energy distributions within the unit’s inhibitory bandwidth.
The unit’s optimal stimulus was estimated by computing the difference spectrum for the average shape of high versus low rate HRTFs. This template is shown in Figure 6D. The preferred distribution of spectral energy corresponds well with the excitatory and inhibitory features of the rate-level correlations that are shown in Figure 4.
3.4. Level tolerance of broadband inhibition
Neural mechanisms for spectral pattern matching must persist over a wide range of stimulus conditions to support natural sound localization. The effect of sound level on optimal spectral shapes was investigated by calculating continuous overlapping difference spectra across all of the ON-BF levels in Figure 6A. The surface plots in Figure 7 represent the 78 difference spectra at one of three presentation levels. Positive energy bands (red) reveal the sharp frequency tuning of the unit’s excitatory inputs. Negative energy bands (blue) highlight the broadly distributed areas of inhibition that dominated the receptive field at higher ON-BF levels. Frequencies of the LIS and UIS are indicated to emphasize the similarity of inhibitory tuning for correlation and difference measures. These basic patterns of local excitation and global inhibition changed little across presentation level.
Figure 7.
Effects of level on optimal spectral shapes. Each surface plot illustrates the change in difference spectrum across a full range of ON-BF levels. Frequencies of the LIS and UIS are shown to emphasize the similarity of inhibitory tuning for correlation and difference measures.
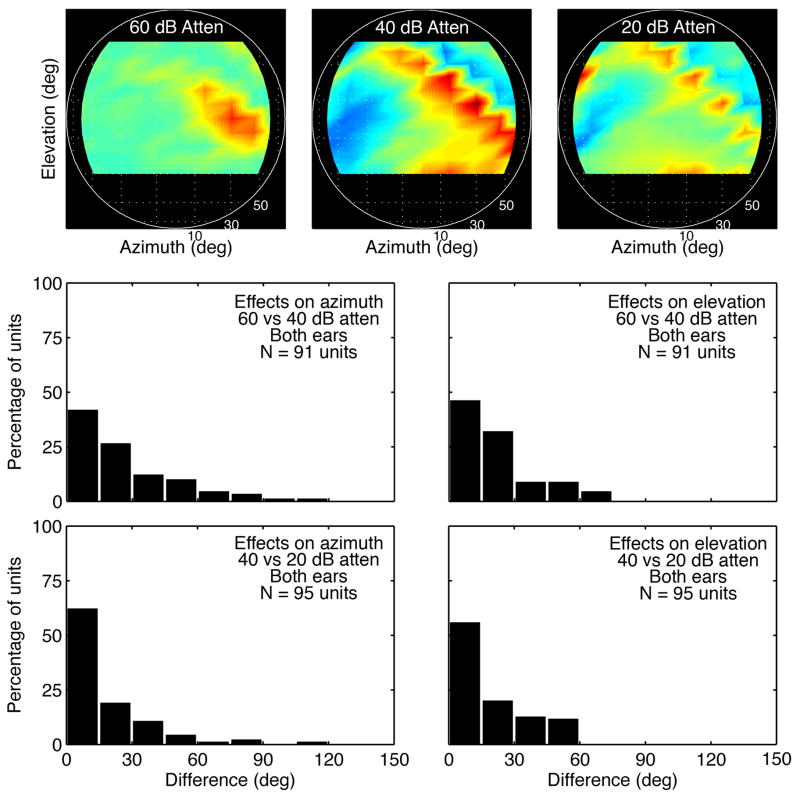
The stability of inhibitory tuning across presentation level, as noted with two independent methods of analyses, predicts that HRTF selectivity should also persist across presentation level. Figure 6 compares the spatial receptive fields of the representative unit at attenuations from 60 to 20 dB. Spatial tuning is well defined at the 60-dB attenuation because responses were evoked only by HRTFs with sufficient ON-BF gain to surpass the unit’s threshold for excitation. More importantly, the unit’s selectivity increased at higher levels because broadband inhibition suppressed responses to ON-BF energy in sub-optimal spectral contexts.
A more complete statistical description of level tolerance is presented in the lower panels of Figure 8. Each histogram describes the effect of attenuation on best azimuth or elevation, which is defined as the location producing the maximum directional rate. For most neurons, a 20-dB change in presentation level altered spatial tuning by less than 30 degrees.
Figure 8.
Effects of level on spatial tuning. Surface plots (upper panels) show the directional rates of the representative unit at three presentation levels. Histograms (lower panels) describe the difference in the azimuth and elevation of maximum directional rates at two sound levels for a population of IC neurons (lower panels).
3.5. Monaural versus binaural processing
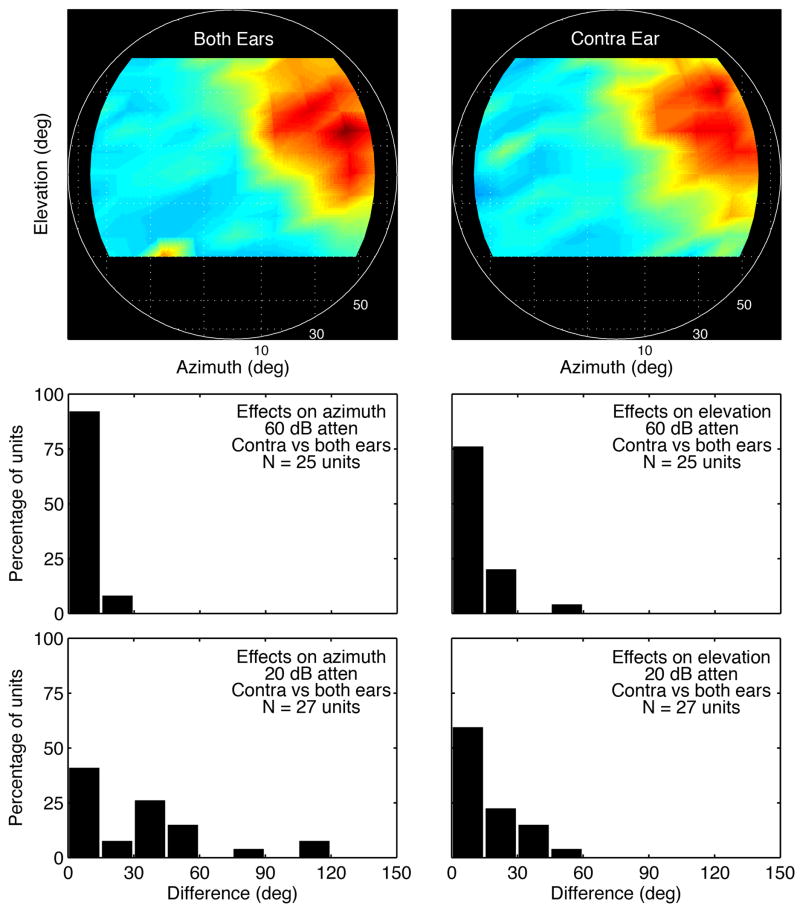
It may be inferred from the close correspondence between spectrum-based localization cues and directional rates that spatial tuning in the inferior colliculus is derived from monaural processing. This interpretation is partially supported by the effects of lesions on the brainstem’s monaural localization pathways (May, 2000; Sutherland et al., 1998). The importance of presentation mode was examined in the present study by collecting HRTF-driven rates with binaural versus monaural (contralateral) stimuli. Figure 9 shows the results of these measures for a representative type IV neuron (BF = 11.0 kHz). The unit’s preferred sound locations were virtually identical for binaural and monaural presentations.
Figure 9.
Effects of presentation mode on spatial tuning. Surface plots (upper panels) show the directional rates of a type IV neuron (BF = 11.0 kHz) for binaural versus contralateral stimuli. Histograms (lower panels) describe the difference in the azimuth and elevation of maximum directional rates under binaural versus contralateral conditions.
The histograms in Figure 9 describe the statistical distribution of the effects of presentation mode. The stability of best azimuth and elevation under monaural conditions was most evident at low levels (middle panels). Effects on elevation tuning also were minor at high levels (lower panels). Large changes in azimuth tuning, however, confirmed the importance of binaural processing for localization in the horizontal plane.
4. Discussion
The major finding of the present study was the essential role that OFF-BF inhibition played in the spectral selectivity, and therefore the spatial tuning, of inferior colliculus neurons. When characterized with rate-level correlations or template matching procedures, these inhibitory influences tailored spatial receptive fields in a manner that was well tuned and tolerant of changes in level. In most instances, spectral selectivity did not change when neurons were tested with monaural versus binaural stimuli.
4.1. Importance of nonlinearity
A longstanding obstacle for theoretical interpretations of auditory coding is the ‘dynamic range problem’ (Viemeister, 1988). At the heart of this controversy is the apparent mismatch between the limited dynamic range of neural discharge rates and the exceptional range of human hearing. The issue has been vigorously pursued in the context of level coding (Costalupes et al., 1984; Palmer and Evans, 1982) or the representation of complex spectral shapes such as human speech (Delgutte and Kiang, 1984; Kiang, 1980; Sachs and Young, 1979; Sinex, 1993; Young and Sachs, 1979). The product of that research has led to the advocacy of coding mechanisms that are based on the ability of auditory neurons to fire in a time-locked manner to the fine structure or temporal envelope of the stimulus (Batra et al., 1989; Eggermont, 1991; Joris, 1996; Rhode and Greenberg, 1994; Rose et al., 1967).
Alternatively, statistical descriptions of discharge rates in the auditory nerve and cochlear nucleus suggest that the dynamic range of rate-level coding is more robust than previously assumed (Dean et al., 2005; Smith, 1988). For simple sounds, operating characteristics are enhanced by onset effects, a diversity of absolute thresholds, and sensitivity to stimulus context. For complex sounds, particularly those with shaped spectra, they are also influenced by two-tone suppression (Sachs and Kiang, 1968). This potent nonlinearity was once considered a detriment to rate representation (Sachs and Young, 1980), but it is now clear that suppression can preserve vowel coding at high levels (Le Prell et al., 1996). The dynamic range of the neural response is extended because auditory-nerve fibers exhibit high saturation rates at spectral peaks, where suppression is weak, and low saturation rates at spectral troughs, where suppression is strong. Unlike the generalized nonlinearities that give rise to suppression effects in the auditory periphery (Hall, 1977; Le Page and Johnstone, 1980), our present results indicate that central inhibitory mechanisms vary greatly in frequency, threshold, and complexity and thus have the capacity to tune neural representations to any acoustic feature that communicates biological information. Such a pattern recognition process has long been proposed for the auditory coding of HRTF stimuli (Irvine, 1986).
The HRTF-driven rates of a type IV neuron are presented in Figures 10 and 11 to illustrate how spatial tuning degrades in the absence of OFF-BF inhibition. Extreme rate variations at the same ON-BF level, a defining characteristic of broadband inhibition (Figure 3), are less evident on the neuron’s rate-level functions (Figure 10A). Instead, discharge rates show the strong non-monotonicities that characterize the narrowband ON-BF responses of type IV neurons (see Figure 2). Maximum correlations (indicated by line segments) shift from excitation near threshold to inhibition at higher levels. When linear fits are derived at surrounding frequencies (Figure 10B), an illusion of broad correlation is created because OFF-BF levels were linked to ON-BF levels by global directional properties of the HRTF (red line). Stimulus-bound correlations also are noted near the narrowly tuned excitation thresholds of wideband neurons (Figure 4C), but they pervade all frequencies and presentation levels of the receptive field producing a correlation map that is devoid of tuned responses (Figure 10C).
Figure 10.
Spectral integration properties of a type IV neuron (BF = 6.9 kHz) that lacks OFF-BF inhibitory features. The balance of excitatory/inhibitory interactions at remote frequencies tracks changes at BF because stimulus components are correlated (red line). Plotting conventions are described in the legend of Figure 4.
Figure 11.
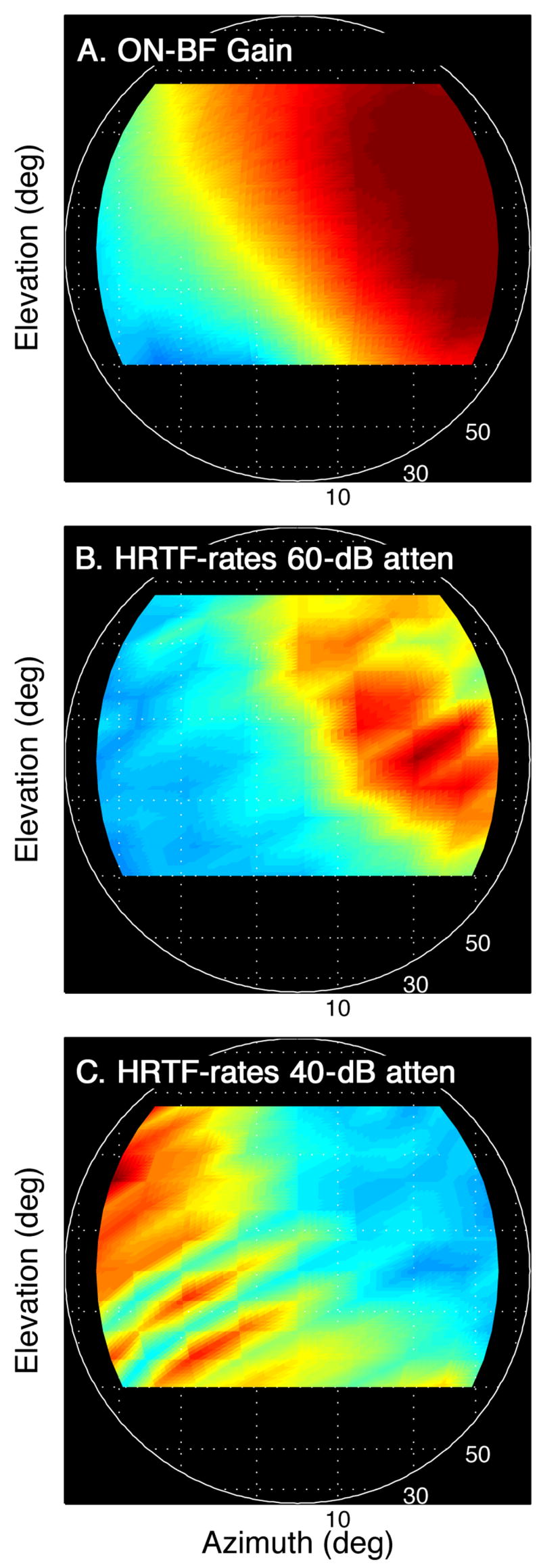
Spatial tuning of the narrowband type IV neuron in Figure 10. A. The directional gains of the HRTFs at the unit’s BF (6.9 kHz). B, C. The unit’s directional rates at presentation levels of 60 and 40 dB attenuation.
The spatial tuning and level tolerance characteristics of the narrowband type IV neuron are summarized in Figure 11. Directional gains at the unit’s BF favor the contralateral hemifield (Figure 11A). When the level of HRTF-shaped noise was near-threshold (Figure 11B), only a circumscribed area in this region of maximum gain delivered sufficient energy to elicit an excitatory response. This tuning was solely determined by energy at BF and it was very sensitive to changes in presentation level. A modest 20-dB increase drove contralateral responses into inhibition and shifted excitatory directional rates to the ipsilateral hemifield (Figure 11C). In cases where spectral tuning was narrow and rate-level functions were monotonic, spatial receptive fields expanded with increasing level eventually encompassing the entire sound field. Both outcomes provide an ambiguous representation of sound source location.
4.2. The spectral processing pathway
Our previous studies of the inferior colliculus (Davis et al., 1999; Ramachandran et al., 1999) have advanced a physiological classification system that is based on the inhibitory features of frequency response maps (FRMs). Type V units (pronounced as the English letter ‘v’) produce FRMs with a broad v-shaped excitatory area and no inhibition. Type I units (pronounced as the English letter ‘i’) have a narrowly tuned i-shaped excitatory area that is flanked by inhibition. Type O units (pronounced as the English letter ‘o’) show an o-shaped island of excitation at threshold but are inhibited by most other combinations of frequency and level.
Because they manifest common physiological properties of type IV projection neurons (Young et al., 1988), type O units are assumed to represent the inferior colliculus targets of monaural spectral representations ascending from the contralateral dorsal cochlear nucleus (DCN). In addition to sharing inhibitory FRMs, both neural populations are relatively insensitive to interaural level differences (Davis, 2005; Davis et al., 1999; but see Chase and Young, 2005) and interaural time differences (Ramachandran and May, 2002). They are silenced by blocking transmission in the output pathways of the DCN (Davis, 2002). By contrast, type V and I units display binaural responses that appear to reflect brainstem inputs from the medial and lateral superior olives (Chase and Young, 2005; Ramachandran and May, 2002).
The potential segregation of different forms of directional information within discrete neural populations has led to the conceptualization of a monaural sound localization pathway (Davis et al., 2003; May, 2007). The pathway is established by spectral processing non-linearities of the dorsal cochlear nucleus (Reiss et al., 2007; Young et al., 2005; Yu and Young, 2000) and then refined by the inhibitory properties of inferior colliculus type O units (Davis, 2002; Davis, 2005). The sensitivity of DCN projection neurons for directional properties of the HRTF has been simulated under closed-field conditions (Nelken et al., 1997; Reiss et al., 2007) and verified in free field (Imig et al., 2000).
Our present study was designed to extend functional validations of the hypothesized spectral pathway by confirming the predicted processing enhancements of type O units. Instead, our experimental results suggest that optimal representations of HRTF shapes are determined by broadband inhibitory interactions that are absent in many type O units (type IV/V units) and present in a high percentage of type I units (type III/I units). Figure 12 addresses this issue by comparing the excitatory/inhibitory features of the response types. Although our current classifications are based on rate-level functions, they capture ON-BF inhibitory patterns that are the essential defining properties of FRM classifications (Ramachandran et al., 1999). Type III/I and IV/V units in the present study (e.g.; Figures 4C and 10C) are unambiguously associated with type I and O designations, respectively.
Figure 12.
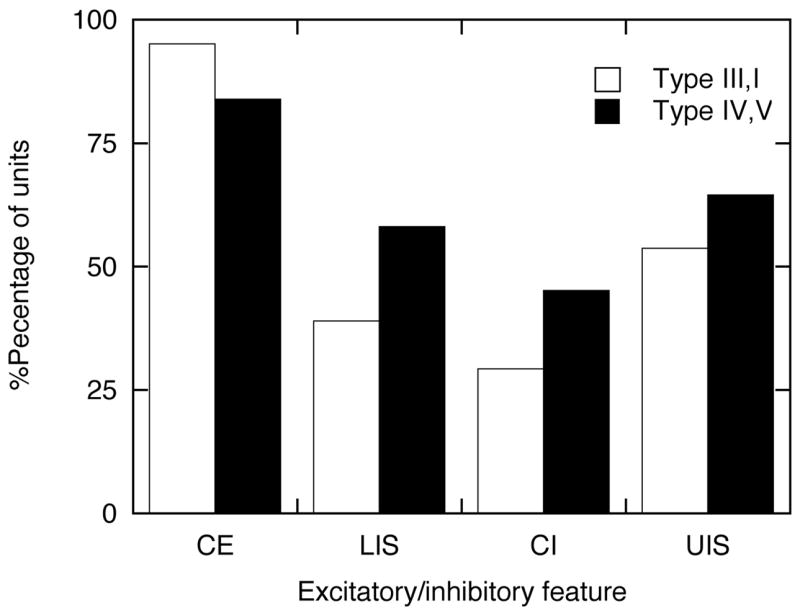
The distribution of excitatory/inhibitory features across unit types. Abbreviations: LIS, lower inhibitory sideband; OBE, ON-BF excitation; OBI, ON-BF inhibition; UIS, upper inhibitory sideband.
The excitatory/inhibitory properties of inferior colliculus neurons are taken from maximum correlation functions that were derived from HRTF-driven responses. ON-BF excitation (OBE) is defined as a maximum ON-BF correlation that was greater than 0.5 at any presentation level. The type III unit in Figure 4A achieved this criterion at attenuations of 60 and 40 dB. The type IV unit in Figure 10A was similarly excited only at 60-dB attenuation. With few exceptions, inferior colliculus neurons are excited by BF tones near threshold. Nevertheless, OBE remains an informative response metric because excitation must meet the following conditions: it must be generated by HRTF stimuli, it must represent the maximum correlation for the ON-BF rate-level function, and it must be strongly excitatory. Almost all neurons (>83%) showed ON-BF excitation.
ON-BF inhibition (OBI) was defined as a negative ON-BF correlation of less than −0.5 at any presentation level. By convention, type III/I units are never inhibited by BF tones, so it is not surprising that the representative type III neuron failed to show OBI when tested with HRTF-shaped noise (Figure 4A). Type IV/V units are inhibited by supra-threshold tones. The representative type IV unit also exhibited OBI at higher HRTF levels (Figure 10A). These examples convey the impression that inhibitory relationships were consistent for narrowband tones and broadband HRTFs, but many inferior colliculus neurons displayed clear differences. The percentage of type IV/V units with OBI (45%) was only slightly higher than the percentage of type III/I units (29%). The units also showed less inhibition when tested with broadband noise (Figure 2).
Broadband inhibitory effects were most prevalent in type IV/V units (LIS = 58% and UIS = 65%). They were, however, frequently observed in type III/I units (LIS = 39% and UIS = 54%). Thus, there was a high probability for observing narrowband type IV/V units with broad spectral tuning for HRTF features (Figure 11) and broadband type III/I units with excellent selectivity (Figure 5). It is interesting that type III/I units tended to show less inhibition at frequencies below BF. These asymmetries are also evident in the inhibitory features of first-order weighting functions in the ventral cochlear nucleus (Yu and Young, 2000), which is further evidence for the assumed anatomical connections between the two neural populations.
The representation of localization cues by type V, I, and O units has been explored with an information theory approach (Chase and Young, 2005). This method isolates the quality of neural coding for ITD, ILD, and mid-frequency spectral notches by independently manipulating directional parameters under closed-field conditions. Rather than the segregation of localization cues by unit type, as predicted by Ramachandran et al. (1999), type I and O units were sensitive to all three acoustic variables and conveyed information that was of similar quality.
Despite similarities in the information content of type I and O responses, the results of Chase and Young (2005) support our observation that representations of HRTF spectra may be driven by different integration patterns. Their type I units exhibited narrowband behavior; that is, information was closely linked to the effects of the cue manipulation on spectrum levels at BF. The responses of their type O units were diverse. Some neurons mimicked the narrowband properties of type I units, while others produced discharge rates with no systematic relationship to the isolated parameter. In the context of information theory paradigms, these latter neurons may provide less information during parametric testing because they are not universally driven by the stimulus set. In the context of sound localization, however, HRTF-selective responses have the capacity to provide a spatially tuned and level tolerant representation of a preferred source location. It is clear from our results, and those of Chase and Young (2005), that the requisite broadband inhibitory interactions for these processes only loosely segregate within type O versus type I classifications.
Because our correlation measures examine the systematic relationships between discharge rates and HRTF spectra, they are most reliable at frequencies between 5 – 20 kHz where the acoustic differences between HRTFs can be related to the shape, depth, and frequency of a single prominent spectral notch. These differences are small in magnitude at frequencies below 5 kHz, and complex at frequencies about 20 kHz. Parametric information theoretic approaches, which are not constrained by natural patterns of variation, have shown that optimal representations of spectral notches are provided by IC neurons with higher BFs (Chase and Young, 2005; Young et al., 2005). These results suggest that the neurons may be physiologically tuned to extract spectral features where they are most likely to occur. This observation establishes the potential importance of individualized HRTFs in future studies of the spectral templates of IC neurons.
4.3. Sources of broadband inhibition
Type IV/V units were expected to show good selectivity for HRTF-shaped noise because they are known to receive inputs from DCN neurons with integration properties that are broadband (Nelken and Young, 1994; Spirou and Young, 1991) and non-linear (Nelken et al., 1997; Young et al., 2005; Yu and Young, 2000). A surprising observation was that a good percentage of type III/I units showed equivalent spectral selectivity. These results suggest that narrowband and linear inputs from the ventral cochlear nucleus (VCN) (May et al., 1998; Yu and Young, 2000) are altered by the convergence of broadband inhibitory sources at intermediate processing levels or within the inferior colliculus.
Quantitative immunocytochemical studies have confirmed the pervasive GABAergic innervation of the central nucleus of the inferior colliculus (Merchan et al., 2005). The effects of this inhibition on sound-driven activity have been evaluated in a number of species by delivering GABA to the recording site (Faingold et al., 1989) or blocking its action with bicuculline (Davis et al., 2003; LeBeau et al., 2001; Palombi and Caspary, 1996; Yang et al., 1992). Inhibitory blockade increases tone-driven activity and expands the excitatory receptive fields of neurons with non-V type FRMs (LeBeau et al., 2001; Yang et al., 1992). The majority of type O units are not affected by pharmacological manipulations, although a subpopulation of bicuculline sensitive type O units has been identified (Davis et al., 2003). These demonstrations suggest that broadband inhibition, a critical component of spectral representation in the inferior colliculus, is created remotely in the DCN, and locally by acting upon other ascending pathways.
The major sources of extrinsic inhibition have been determined by combining retrograde tracers with immunocytochemistry. Histological verification of double-labeled neurons reveals significant GABAergic input from the dorsal and ventral nuclei of the lateral lemniscus (Zhang et al., 1998) and the contralateral central nucleus via commissural connections (Gonzalez-Hernandez et al., 1996; Hernandez et al., 2006). The functional significance of these discrete sources of inhibition may be isolated by recording inferior colliculus activity during their reversible inactivation with kynurenic acid. Silencing any of these structures uncovers a mixture of excitatory and inhibitory inputs. Assuming the inactivation of a direct monosynaptic connection, the former is associated with decreases in the inferior colliculus, while the latter is indicated by rate increases.
Dense commissural projections from the opposite inferior colliculus modify the responsiveness of virtually all neurons in the central nucleus (Malmierca et al., 2005; Malmierca et al., 2003). Discharge rates elicited by monaural BF tones may either increase (48% of neurons) or decrease (35% of neurons). The effects are most pronounced within 15 dB of threshold. The discharge rates of binaurally sensitive neurons (7 out of 11 units) increase under ILD conditions without altering the slope of input-output relationships. These commissural influences appear to modulate the gain of the rate representation without improving directional sensitivity.
The OFF-BF inhibitory characteristics of commissural projections also have been investigated (Malmierca et al., 2003). Contraction or expansion of FRMs during application of kynurenic acid suggest a mixture of excitatory and inhibitory influences that are not segregated by unit type. Given the nonlinear spectral integration properties of the inferior colliculus, it is impossible to predict how these changes impact the representation of complex spectra. In lieu of the requisite HRTF measures, the strong bias of the influences of the pathway toward near-threshold excitation and ON-BF frequencies implies a limited role in the pattern recognition processes that are inferred by our observations.
The dorsal nucleus of the inferior colliculus (DNLL) is the principal source of bilateral GABAergic projections to the central nucleus (Adams and Mugnaini, 1984; Shneiderman et al., 1988). This inhibition has been most extensively examined in the context of binaural processing (Faingold et al., 1993; Li and Kelly, 1992). Pharmacological inactivation of the pathway reduces the ipsilateral inhibition of neurons with EI binaural sensitivity (i.e.; contralateral excitation, ipsilateral inhibition), which is an important component for the coding of ILD-based localization cues. Although ILD-sensitive neurons are first observed in the lateral superior olive (Tsuchitani, 1988), these higher-order inputs have the capacity to create EI responses de novo or to modify the balance and timing of existing inhibition. Such interactions may enhance echolocation, aid in the pursuit of moving sounds, or allow the listener to attend to multiple competing sources (Burger and Pollak, 2001).
The ipsilateral inhibition that defines EI neurons is a property of type I and O units in the inferior colliculus (Davis et al., 1999). Changes in the shape of these non-V-shaped FRMs are observed during temporary disruption of DNLL inputs (Thornton and Rees, 2001). As noted with direct application of bicuculline, sound-driven discharge rates increase and the bandwidth of excitatory receptive fields expand when kynurenic acid is injected into the ipsilateral DNLL. Inactivation of the contralateral DNLL usually produces expansion (release from inhibition) but contraction (loss of excitation) is also seen. The diversity of these effects suggests that DNLL influences are more complex than simple monosynaptic inhibition.
The major output pathway of the ventral nucleus of the lateral lemniscus (VNLL) is an inhibitory projection to the ipsilateral inferior colliculus (Adams, 1979). Although the majority of VNLL neurons are immunoreactive for glycine, this input source may also transmit GABAergic inhibition. Many of its glycinergic neurons appear to co-localize GABA (Saint Marie et al., 1997), while a small population of multipolar neurons appears to be strictly GABAergic (Winer et al., 1995).
In addition to having monaural output properties, the input properties of the VNLL are particularly interesting from the standpoint of monaural sound localization. The major source of ascending input to the VNLL is a direct projection from stellate cells in the contralateral cochlear nucleus (Cant, 1981). Chopper units, the physiological counterpart of stellate cells, exhibit high discharge rates and exceptional inter-spike regularity when responding to narrowband signals (Blackburn and Sachs, 1989) and thus are able to encode small perturbations of stimulus level with high resolution (Young et al., 1988). Although chopper units are narrowly tuned in frequency, the topography of their input to the VNLL suggests a discontinuous clustering of frequency bands that creates the opportunity for wideband cross-frequency integration (Malmierca et al., 1998). This circuitry been hypothesized to play a role in analyzing temporal acoustic features (Covey and Casseday, 1991) or recognizing temporal patterns in natural communication sounds (Oertel and Wickesberg, 2002). Our observations suggest that these properties also are ideal for pattern recognition in the spectral domain. To date, the effects of VNLL inactivation on spectral selectivity have not been measured. Given our previous findings in the DCN (May, 2000), it is intriguing to speculate that loss of these processes would impede the auditory processing of spectral cues for sound localization.
Acknowledgments
These findings were originally presented at the Satellite Meeting on The Auditory Brain – a Tribute to Dexter Irvine, IBRO 2007. The authors thank the organizers of that meeting and participants who have contributed to our current views of spectral integration in the inferior colliculus. We gratefully acknowledge Dexter Irvine’s lifetime of collegial support for students of the auditory neurosciences. This research was supported by NIDCD grant R01 DC00954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC. Ascending projections to the inferior colliculus. The Journal of comparative neurology. 1979;183:519–38. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain research bulletin. 1984;13:585–90. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Aertsen AM, Johannesma PI. The spectro-temporal receptive field. A functional characteristic of auditory neurons. Biological cybernetics. 1981;42:133–43. doi: 10.1007/BF00336731. [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Stanford TR. Temporal coding of envelopes and their interaural delays in the inferior colliculus of the unanesthetized rabbit. Journal of neurophysiology. 1989;61:257–68. doi: 10.1152/jn.1989.61.2.257. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. Journal of neurophysiology. 1989;62:1303–29. doi: 10.1152/jn.1989.62.6.1303. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Some principles in the brain analysis of important signals: mapping and stimulus recognition. Brain, behavior and evolution. 1986;28:145–56. doi: 10.1159/000118699. [DOI] [PubMed] [Google Scholar]
- Burger RM, Pollak GD. Reversible inactivation of the dorsal nucleus of the lateral lemniscus reveals its role in the processing of multiple sound sources in the inferior colliculus of bats. J Neurosci. 2001;21:4830–43. doi: 10.1523/JNEUROSCI.21-13-04830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RA, Belendiuk K. Spectral cues utilized in the localization of sound in the median sagittal plane. The Journal of the Acoustical Society of America. 1977;61:1264–9. doi: 10.1121/1.381427. [DOI] [PubMed] [Google Scholar]
- Cant NB. The fine structure of two types of stellate cells in the anterior division of the anteroventral cochlear nucleus of the cat. Neuroscience. 1981;6:2643–55. doi: 10.1016/0306-4522(81)90109-3. [DOI] [PubMed] [Google Scholar]
- Carney LH, Friedman M. Spatiotemporal tuning of low-frequency cells in the anteroventral cochlear nucleus. J Neurosci. 1998;18:1096–104. doi: 10.1523/JNEUROSCI.18-03-01096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Neff WD. Auditory localization: role of auditory pathways in brain stem of the cat. Journal of neurophysiology. 1975;38:842–58. doi: 10.1152/jn.1975.38.4.842. [DOI] [PubMed] [Google Scholar]
- Chase SM, Young ED. Limited segregation of different types of sound localization information among classes of units in the inferior colliculus. J Neurosci. 2005;25:7575–85. doi: 10.1523/JNEUROSCI.0915-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. Journal of neurophysiology. 1984;51:1326–44. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Covey E, Casseday JH. The monaural nuclei of the lateral lemniscus in an echolocating bat: parallel pathways for analyzing temporal features of sound. J Neurosci. 1991;11:3456–70. doi: 10.1523/JNEUROSCI.11-11-03456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA. Evidence of a functionally segregated pathway from dorsal cochlear nucleus to inferior colliculus. Journal of neurophysiology. 2002;87:1824–35. doi: 10.1152/jn.00769.2001. [DOI] [PubMed] [Google Scholar]
- Davis KA. Spectral processing in the inferior colliculus. International review of neurobiology. 2005;70:169–205. doi: 10.1016/S0074-7742(05)70006-4. [DOI] [PubMed] [Google Scholar]
- Davis KA, Ramachandran R, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. II. Sensitivity to interaural level differences. Journal of neurophysiology. 1999;82:164–75. doi: 10.1152/jn.1999.82.1.164. [DOI] [PubMed] [Google Scholar]
- Davis KA, Ramachandran R, May BJ. Auditory processing of spectral cues for sound localization in the inferior colliculus. J Assoc Res Otolaryngol. 2003;4:148–63. doi: 10.1007/s10162-002-2002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nature neuroscience. 2005;8:1684–9. doi: 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- Delgutte B, Kiang NY. Speech coding in the auditory nerve: I. Vowel-like sounds. The Journal of the Acoustical Society of America. 1984;75:866–78. doi: 10.1121/1.390596. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Rate and synchronization measures of periodicity coding in cat primary auditory cortex. Hearing research. 1991;56:153–67. doi: 10.1016/0378-5955(91)90165-6. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hearing research. 2001;157:1–42. doi: 10.1016/s0378-5955(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Johannesma PM, Aertsen AM. Reverse-correlation methods in auditory research. Quarterly reviews of biophysics. 1983a;16:341–414. doi: 10.1017/s0033583500005126. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Aertsen AM, Johannesma PI. Quantitative characterisation procedure for auditory neurons based on the spectro-temporal receptive field. Hearing research. 1983b;10:167–90. doi: 10.1016/0378-5955(83)90052-7. [DOI] [PubMed] [Google Scholar]
- Escabi MA, Schreiner CE. Nonlinear spectrotemporal sound analysis by neurons in the auditory midbrain. J Neurosci. 2002;22:4114–31. doi: 10.1523/JNEUROSCI.22-10-04114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EF, Nelson PG. The responses of single neurones in the cochlear nucleus of the cat as a function of their location and the anaesthetic state. Experimental brain research. Experimentelle Hirnforschung. 1973;17:402–27. doi: 10.1007/BF00234103. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Gehlbach G, Caspary DM. On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies. Brain research. 1989;500:302–12. doi: 10.1016/0006-8993(89)90326-0. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Anderson CA, Randall ME. Stimulation or blockade of the dorsal nucleus of the lateral lemniscus alters binaural and tonic inhibition in contralateral inferior colliculus neurons. Hearing research. 1993;69:98–106. doi: 10.1016/0378-5955(93)90097-k. [DOI] [PubMed] [Google Scholar]
- Flecknell P, Silverman J. Pain and Distress. In: Silverman J, Suckow MA, Murthy S, editors. The IACUC Handbook. CRC Press; Boca Raton: 2000. pp. 221–249. [Google Scholar]
- Furst M, Aharonson V, Levine RA, Fullerton BC, Tadmor R, Pratt H, Polyakov A, Korczyn AD. Sound lateralization and interaural discrimination. Effects of brainstem infarcts and multiple sclerosis lesions. Hearing research. 2000;143:29–42. doi: 10.1016/s0378-5955(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Hutson KA, Nudo RJ, Masterton RB. Acoustic chiasm II: Anatomical basis of binaurality in lateral superior olive of cat. The Journal of comparative neurology. 1985;232:261–85. doi: 10.1002/cne.902320210. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. The Journal of comparative neurology. 1996;372:309–26. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hall JL. Two-tone suppression in a nonlinear model of the basilar membrane. The Journal of the Acoustical Society of America. 1977;61:802–10. doi: 10.1121/1.381369. [DOI] [PubMed] [Google Scholar]
- Hebrank J, Wright D. Spectral cues used in the localization of sound sources on the median plane. The Journal of the Acoustical Society of America. 1974;56:1829–34. doi: 10.1121/1.1903520. [DOI] [PubMed] [Google Scholar]
- Hernandez O, Rees A, Malmierca MS. A GABAergic component in the commissure of the inferior colliculus in rat. Neuroreport. 2006;17:1611–4. doi: 10.1097/01.wnr.0000236857.70715.be. [DOI] [PubMed] [Google Scholar]
- Huang AY, May BJ. Sound orientation behavior in cats. II. Mid-frequency spectral cues for sound localization. The Journal of the Acoustical Society of America. 1996a;100:1070–80. doi: 10.1121/1.416293. [DOI] [PubMed] [Google Scholar]
- Huang AY, May BJ. Spectral cues for sound localization in cats: effects of frequency domain on minimum audible angles in the median and horizontal planes. The Journal of the Acoustical Society of America. 1996b;100:2341–8. doi: 10.1121/1.417943. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Bibikov NG, Poirier P, Samson FK. Directionality derived from pinna-cue spectral notches in cat dorsal cochlear nucleus. Journal of neurophysiology. 2000;83:907–25. doi: 10.1152/jn.2000.83.2.907. [DOI] [PubMed] [Google Scholar]
- Irvine DRF. The Auditory Brainstem. Springer-Verlag; Berlin: 1986. [Google Scholar]
- Joris PX. Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive. Journal of neurophysiology. 1996;76:2137–56. doi: 10.1152/jn.1996.76.4.2137. [DOI] [PubMed] [Google Scholar]
- Kiang NY. Processing of speech by the auditory nervous system. The Journal of the Acoustical Society of America. 1980;68:830–5. doi: 10.1121/1.384822. [DOI] [PubMed] [Google Scholar]
- Kim DO, Molnar CE. A population study of cochlear nerve fibers: comparison of spatial distributions of average-rate and phase-locking measures of responses to single tones. Journal of neurophysiology. 1979;42:16–30. doi: 10.1152/jn.1979.42.1.16. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Young ED. Comparative analysis of spectro-temporal receptive fields, reverse correlation functions, and frequency tuning curves of auditory-nerve fibers. The Journal of the Acoustical Society of America. 1994;95:410–22. doi: 10.1121/1.408335. [DOI] [PubMed] [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Menetrey A, Glowinski J, Thierry AM. Segregation and convergence of information flow through the cortico-subthalamic pathways. J Neurosci. 2001;21:5764–72. doi: 10.1523/JNEUROSCI.21-15-05764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page EL, Johnstone BM. Nonlinear mechanical behaviour of the basilar membrane in the basal turn of the guinea pig cochlea. Hearing research. 1980;2:183–189. doi: 10.1016/0378-5955(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Le Prell GS, Sachs MB, May BJ. Representation of vowel-like spectra by discharge rate responses of individual auditory-nerve fibers. Aud Neurosci. 1996;2:275–288. [PMC free article] [PubMed] [Google Scholar]
- LeBeau FE, Malmierca MS, Rees A. Iontophoresis in vivo demonstrates a key role for GABA(A) and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci. 2001;21:7303–12. doi: 10.1523/JNEUROSCI.21-18-07303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kelly JB. Inhibitory influence of the dorsal nucleus of the lateral lemniscus onbinaural responses in the rat’s inferior colliculus. J Neurosci. 1992;12:4530–9. doi: 10.1523/JNEUROSCI.12-11-04530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majorossy K, Kiss A. Convergence of topographic projections to the inferior colliculus from the auditory subcollicular nuclei. Acta biologica Hungarica. 1994;45:347–59. [PubMed] [Google Scholar]
- Malmierca MS, Hernandez O, Rees A. Intercollicular commissural projections modulate neuronal responses in the inferior colliculus. The European journal of neuroscience. 2005;21:2701–10. doi: 10.1111/j.1460-9568.2005.04103.x. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Leergaard TB, Bajo VM, Bjaalie JG, Merchan MA. Anatomic evidence of a three-dimensional mosaic pattern of tonotopic organization in the ventral complex of the lateral lemniscus in cat. J Neurosci. 1998;18:10603–18. doi: 10.1523/JNEUROSCI.18-24-10603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Hernandez O, Falconi A, Lopez-Poveda EA, Merchan M, Rees A. The commissure of the inferior colliculus shapes frequency response areas in rat: an in vivo study using reversible blockade with microinjection of kynurenic acid. Experimental brain research. Experimentelle Hirnforschung. 2003;153:522–9. doi: 10.1007/s00221-003-1615-1. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hearing research. 2000;148:74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- May BJ. Monaural Sound Localization Using Spectral Cues. In: Dallos P, Oertel D, editors. The Senses: A Comprehensive Reference. Academic Press; London: 2007. [Google Scholar]
- May BJ, Huang AY. Spectral cues for sound localization in cats: a model for discharge rate representations in the auditory nerve. The Journal of the Acoustical Society of America. 1997;101:2705–19. doi: 10.1121/1.418559. [DOI] [PubMed] [Google Scholar]
- May BJ, Prell GS, Sachs MB. Vowel representations in the ventral cochlear nucleus of the cat: effects of level, background noise, and behavioral state. Journal of neurophysiology. 1998;79:1755–67. doi: 10.1152/jn.1998.79.4.1755. [DOI] [PubMed] [Google Scholar]
- Mayer-Kress G. Non-linear mechanisms in the brain. Zeitschrift fur Naturforschung. 1998;53:677–85. doi: 10.1515/znc-1998-7-820. [DOI] [PubMed] [Google Scholar]
- Merchan M, Aguilar LA, Lopez-Poveda EA, Malmierca MS. The inferior colliculus of the rat: quantitative immunocytochemical study of GABA and glycine. Neuroscience. 2005;136:907–25. doi: 10.1016/j.neuroscience.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Narrow-band sound localization related to external ear acoustics. The Journal of the Acoustical Society of America. 1992;92:2607–24. doi: 10.1121/1.404400. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Directional dependence of interaural envelope delays. The Journal of the Acoustical Society of America. 1990;87:2149–62. doi: 10.1121/1.399183. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annual review of psychology. 1991;42:135–59. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Neff WD. The brain and hearing: auditory discriminations affected by brain lesions. The Annals of otology, rhinology, and laryngology. 1977;86:500–6. doi: 10.1177/000348947708600409. [DOI] [PubMed] [Google Scholar]
- Nelken I, Young ED. Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus type IV units to narrowband and wideband stimuli. Journal of neurophysiology. 1994;71:2446–62. doi: 10.1152/jn.1994.71.6.2446. [DOI] [PubMed] [Google Scholar]
- Nelken I, Kim PJ, Young ED. Linear and nonlinear spectral integration in type IV neurons of the dorsal cochlear nucleus. II. Predicting responses with the use of nonlinear models. Journal of neurophysiology. 1997;78:800–11. doi: 10.1152/jn.1997.78.2.800. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wickesberg RE. Ascending pathways through ventral nuclei of the lateral lemniscus and their possible role in pattern recognition in natural sounds. In: Oertel D, Fay RR, Popper AN, editors. Integrative Functions of the Mammalian Auditory Pathway. Springer-Verlag; New York: 2002. pp. 207–237. [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. The Journal of comparative neurology. 1987;264:24–46. doi: 10.1002/cne.902640104. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Loftus WC, Batra R. Topography of interaural temporal disparity coding in projections of medial superior olive to inferior colliculus. J Neurosci. 2003;23:7438–49. doi: 10.1523/JNEUROSCI.23-19-07438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR, Evans EF. Intensity coding in the auditory periphery of the cat: responses of cochlear nerve and cochlear nucleus neurons to signals in the presence of bandstop masking noise. Hearing research. 1982;7:305–23. doi: 10.1016/0378-5955(82)90042-9. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. Journal of neurophysiology. 1996;75:2211–9. doi: 10.1152/jn.1996.75.6.2211. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Calford MB, Pettigrew JD, Aitkin LM, Semple MN. Directionality of sound pressure transformation at the cat’s pinna. Hearing research. 1982;8:13–28. doi: 10.1016/0378-5955(82)90031-4. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, May BJ. Functional segregation of ITD sensitivity in the inferior colliculus of decerebrate cats. Journal of neurophysiology. 2002;88:2251–61. doi: 10.1152/jn.00356.2002. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Davis KA, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. I. Classification based on frequency response maps. Journal of neurophysiology. 1999;82:152–63. doi: 10.1152/jn.1999.82.1.152. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Bandyopadhyay S, Young ED. Effects of stimulus spectral contrast on receptive fields of dorsal cochlear nucleus neurons. Journal of neurophysiology. 2007 doi: 10.1152/jn.01239.2006. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Greenberg S. Encoding of amplitude modulation in the cochlear nucleus of the cat. Journal of neurophysiology. 1994;71:1797–825. doi: 10.1152/jn.1994.71.5.1797. [DOI] [PubMed] [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hearing research. 1992;58:132–52. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. Journal of neurophysiology. 1967;30:769–93. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Kiang NY. Two-tone inhibition in auditory-nerve fibers. The Journal of the Acoustical Society of America. 1968;43:1120–8. doi: 10.1121/1.1910947. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED. Encoding of steady-state vowels in the auditory nerve: representation in terms of discharge rate. The Journal of the Acoustical Society of America. 1979;66:470–9. doi: 10.1121/1.383098. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED. Effects of nonlinearities on speech encoding in the auditory nerve. The Journal of the Acoustical Society of America. 1980;68:858–75. doi: 10.1121/1.384825. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Shneiderman A, Stanforth DA. Patterns of gamma-aminobutyric acid and glycine immunoreactivities reflect structural and functional differences of the cat lateral lemniscal nuclei. The Journal of comparative neurology. 1997;389:264–76. doi: 10.1002/(sici)1096-9861(19971215)389:2<264::aid-cne6>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? The Journal of comparative neurology. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Sinex DG. Auditory nerve fiber representation of cues to voicing in syllable-final stop consonants. The Journal of the Acoustical Society of America. 1993;94:1351–62. doi: 10.1121/1.408163. [DOI] [PubMed] [Google Scholar]
- Smith RL. Encoding of sound intensity by auditory neurons. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function: Neurobiological Bases of Hearing. John Wiley and Sons; New York: 1988. [Google Scholar]
- Somjen GG. Science. Vol. 158. New York, NY: 1967. Sensory coding; pp. 399–405. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Young ED. Organization of dorsal cochlear nucleus type IV unit response maps and their relationship to activation by bandlimited noise. Journal of neurophysiology. 1991;66:1750–68. doi: 10.1152/jn.1991.66.5.1750. [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK. Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behavioural brain research. 1998;97:1–12. doi: 10.1016/s0166-4328(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Thornton SK, Rees A. The dorsal nucleus of the lateral lemniscus shapes auditory responses in the inferior colliculus. In: Breebaart DJ, Houstma AJM, Kohlrausch A, Prijs VF, Schoonhoven R, editors. Physiological and Psychophysical Bases of Auditory Function. Shaker Publishing; Maastricht, The Netherlands: 2001. pp. 298–305. [Google Scholar]
- Tsuchitani C. The inhibition of cat lateral superior olive unit excitatory responses to binaural tone bursts. I. The transient chopper response. Journal of neurophysiology. 1988;59:164–83. doi: 10.1152/jn.1988.59.1.164. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Intensity coding and the dynamic range problem. Hearing research. 1988;34:267–74. doi: 10.1016/0378-5955(88)90007-x. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Pollak GD. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. The Journal of comparative neurology. 1995;355:317–53. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- Xu L, Middlebrooks JC. Individual differences in external-ear transfer functions of cats. The Journal of the Acoustical Society of America. 2000;107:1451–9. doi: 10.1121/1.428432. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. Journal of neurophysiology. 1992;68:1760–74. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. The Journal of the Acoustical Society of America. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- Young ED, Voigt HF. Response properties of type II and type III units in dorsal cochlear nucleus. Hearing research. 1982;6:153–69. doi: 10.1016/0378-5955(82)90051-x. [DOI] [PubMed] [Google Scholar]
- Young ED, Yu JJ, Reiss LA. Non-linearities and the representation of auditory spectra. International review of neurobiology. 2005;70:135–68. doi: 10.1016/S0074-7742(05)70005-2. [DOI] [PubMed] [Google Scholar]
- Young ED, Shofner WP, White JA, Robert J-M, Voight HF. Response properties of cochlear nucleus neurons in relationship to physiological mechanisms. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function: Neurobiological Bases of Hearing. John Wiley & Sons; New York: 1988. pp. 277–312. [Google Scholar]
- Yu JJ, Young ED. Linear and nonlinear pathways of spectral information transmission in the cochlear nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11780–6. doi: 10.1073/pnas.97.22.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Li L, Kelly JB, Wu SH. GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hearing research. 1998;117:1–12. doi: 10.1016/s0378-5955(97)00202-5. [DOI] [PubMed] [Google Scholar]