Abstract
The effects of interleukin-8 (IL-8) on bovine mammary functions such as milk protein secretion and the blood-milk barrier during mammary involution were evaluated. Following the final milking, recombinant bovine (rb) IL-8 (5 or 25 μg) and a saline placebo were individually infused into the left- and right-front teat cisterns of 6 cows, respectively. Three cows without treatment at the final milking were also used as controls. Mammary secretions and blood were collected at −24, 0, 10, 24, 72, 168, 336, and 720 h after infusion. In the mammary glands infused with 25 μg of rbIL-8, the increases in somatic cell counts and in the concentrations of serum albumin, IgG1 and IgG2, and the decreases in the concentrations of α- and β-casein and β-lactoglobulin were greater than in the control glands. In the mammary glands infused with 5 μg of rbIL-8, compared to the glands infused with 25 μg of rbIL-8, these changes were moderate. These results indicate that rbIL-8 impairs the integrity of the blood-milk barrier and suppresses milk-specific protein secretions. In the cows infused with 25 μg of rbIL-8, the rectal temperature and serum haptoglobin level were transiently elevated after the infusion, showing that intramammary infusion of rbIL-8 could elicit systemic inflammation.
Résumé
Les effets de l’interleukine 8 (IL-8) sur les fonctions de la glande mammaire bovine, telles que la sécrétion de protéines du lait et la barrière lait-sang, durant l’involution mammaire ont été évalués. Suite à la dernière traite, de l’IL-8 bovin recombinant (rb) (5 ou 25 μg) et un placebo (de la saline) ont été infusés individuellement dans les citernes des trayons avant gauches et droits de 6 vaches, respectivement. Trois vaches sans traitement à la dernière traite ont également été utilisées comme témoins. Des sécrétions mammaires et du sans ont été prélevés à −24, 0, 10, 24, 72, 168, 336 et 720 h après l’infusion. Dans les glandes mammaires infusées avec 25 μgd’IL-8rb, les augmentations notées dans le dénombrement des cellules somatiques et dans les concentrations d’albumine sérique, d’IgG1 et IgG2, et les réductions de concentrations d’α et de β-caséine et de β-lactoglobuline étaient supérieures à celles des glandes témoins. Dans les glandes mammaires infusées avec 5 μg d’IL-8rb ces changements étaient modérés par rapport à ceux observés dans les glandes infusées avec 25 μg. Ces résultats indiquent que l’IL-8rb altère l’intégrité de la barrière lait-sang et supprime la sécrétion de protéines spécifiques du lait. Chez les vaches infusées avec 25 μg d’IL-8rb, la température rectale et le niveau d’haptoglobine sérique étaient augmentés de manière transitoire après l’infusion, démontrant ainsi que l’infusion intra-mammaire d’IL-8rb pourrait éliciter une inflammation systémique.
(Traduit par Docteur Serge Messier)
Interleukin-8 (IL-8) is an inflammatory cytokine that is produced by various cell types such as lymphocytes, neutrophils, monocytes, macrophages, and epithelial cells (1), including bovine mammary epithelial cells (2,3). At the site of inflammation, IL-8 plays a role in recruiting and activating neutrophils (1,4,5). During the acute phase of coliform mastitis, the concentration of IL-8 is greatly increased in mastitic milk (6,7). Chemotactic activities of IL-8 were detected in mastitic mammary secretions during intramammary infection with Staphylococcus aureus, but were not found in nonmastitic mammary secretions (8). Interleukin-8 is therefore considered to be involved in the infiltration of neutrophils into mammary secretions during mastitis.
Interleukin-8 is reported to affect not only neutrophils, but also airway and gastric epithelia by impairing their barrier functions (9,10). An influx of serum proteins into mammary secretions is one of the features of mastitic milk, and is a sign of impairment of the blood-milk barrier (11). It is possible that IL-8 is involved in the infiltration of serum proteins into mammary secretions. The physiological role of IL-8 in the regulation of mammary functions in vivo, however, has not been well demonstrated.
In this study, an attempt was made to obtain a better understanding of the mechanisms of mastitic changes in the mammary gland, especially regarding the role of IL-8 in mammary physiological functions. The present study was designed to evaluate the effect of recombinant bovine (rb) IL-8 on the functions of the bovine mammary gland such as maintaining the integrity of the blood-milk barrier and the secretion of milk-specific proteins. An attempt was also made to determine the effect of intramammary infusion of rbIL-8 on the systemic inflammatory response by examining rectal temperature (RT) and the concentration of haptoglobin (Hp), a principal acute-phase protein in bovines (12), in serum.
Recombinant bovine (rb) IL-8, produced in a Brevibacillus chosinensis expression system, was donated (Higeta Shoyu, Choshi, Japan). Experiments were conducted on 9 lactating cows (5- to 6-years-old). The cows were cared for according to the Guide for the Care and Use of Experimental Animals in the National Institute of Animal Health (National Institute of Animal Health). The 9 cows were milked twice daily (8:00 and 16:00) until they reached the 225th to 232nd day of lactation. Following the final milking (at 8:00), 3 cows were infused with rbIL-8 (5 μg in 10 mL of endotoxin-free sterile saline) into the left-front teat cistern, and the same volume of a saline placebo was infused into the right-front teat cistern via a papillary duct catheter. In another 3 cows, 25 μg of rbIL-8 and the saline placebo were similarly infused into the left- and right- front teat cisterns, respectively. The mammary glands infused with saline placebo were regarded as controls with respect to the rbIL-8-infused glands, because local intramammary inflammatory reactions in cows occur separately at each quarter level (6,13). Milking of the remaining 3 cows was also ceased, but no treatment was performed. Both mammary secretions (4 mL) and blood were sampled at −24, 0, 10, 24, 72, 168, 336, and 720 h after the infusion. Bacteriology yielded negative cultures for all samples and no bacteria could be detected.
Mammary secretions were filtered with 2 layers of surgical gauze. Somatic cell counts (SCC) were determined by direct microscopic examination, and smear specimens of the cells (200 cells/specimen) were examined to classify polymorphonuclear leukocytes (PMN) or others (14). The mammary secretions were centrifuged at 1700 μg for 20 min (0°C), and fat and cells were removed (defatted milk). Serum albumin (Alb), α-casein (α-CN), β-CN, and β-lactoglobulin (β-LG) in the defatted milk were separated by sodium dodecyl sulfate-urea-polyacrylamide gel electrophoresis and then quantified by scanning densitometry of the electrophoretogram stained with Coomasie brilliant blue-R250 (Nacalai tesque, Kyoto, Japan) (14). This quantification was calibrated with each of the purified materials. Concentrations of IgG1, IgG2, and Hp were determined by enzyme-linked immunosorbent assay (ELISA) (14).
The SCC was logarithmically transformed to maintain a normal distribution prior to analysis. For comparison with the data before the infusion of rbIL-8 and saline, the data were analyzed using a paired “t” test. To determine the difference between the effects caused by different treatments, the data were analyzed by two-way repeated-measures analysis of variance and Tukey’s multiple comparison. The data are presented as means ± SEM and a difference of P < 0.05 was judged to be significant.
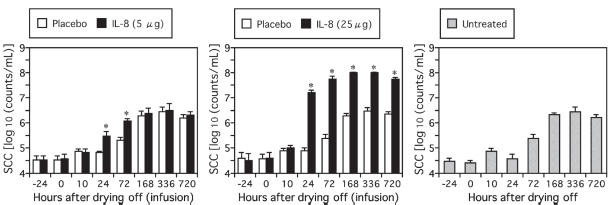
In mammary glands infused with 25 μg of rbIL-8, significant increases of SCC were detected from 24 to 720 h after the infusion (Figure 1). In glands infused with 5 μg of rbIL-8, the increases of SCC were smaller than in those infused with 25 μg of rbIL-8, but they were significant at 24 h and 72 h after the infusion. The increases in SCC were estimated to be mainly due to the infiltration of PMN leukocytes (Table I). These results showed that the rbIL-8 used in this study was biologically active. In all of the placebo-infused mammary glands, SCC and the population of PMN were changed according to the progress of mammary involution and were similar to those in the mammary glands of the untreated cows (Figure 1, Table I).
Figure 1.
Changes in somatic cell counts (SCC) in mammary secretions from cows infused with 5 μg (A) or 25 μg (B) of rbIL-8 at drying off. Changes in SCC in mammary secretions from rbIL-8 (closed bars) and saline-infused glands (open bars) are shown. Changes in SCC in mammary secretions from cows without any infusions into the mammary glands at drying off are also shown (shaded bars) (C). An asterisk (*) indicates a significant difference from both the saline-infused mammary glands and the mammary glands of untreated cows at the same time point.
Table I.
Percentage of polymorphonuclear neutrophil (PMN) in somatic cell counts (SCC) in the mammary secretions from the mammary glands infused with rbIL-8, placebo saline, or untreated
| PMN (%) (mean ± Sx̄)
|
|||||
|---|---|---|---|---|---|
| Time (h) | Placebo salinea | rbIL-8 (5 μg)a | Placebo salineb | rbIL-8 (25 μg)b | Untreatedc |
| −24 | 38.8 ± 3.0 | 40.2 ± 3.9 | 40.3 ± 2.5 | 38.7 ± 3.6 | 38.5 ± 1.2 |
| 0 | 40.5 ± 3.4 | 39.0 ± 2.8 | 39.5 ± 3.1 | 40.8 ± 4.8 | 38.2 ± 0.9 |
| 10 | 39.7 ± 2.5 | 42.3 ± 1.2 | 40.3 ± 4.2 | 53.0 ± 8.8 | 38.8 ± 1.5 |
| 24 | 38.2 ± 3.3 | 70.0 ± 6.4 | 38.7 ± 3.9 | 90.8 ± 1.4 | 37.7 ± 1.3 |
| 72 | 35.7 ± 2.4 | 73.5 ± 5.5 | 33.8 ± 2.5 | 92.0 ± 1.2 | 34.5 ± 1.4 |
| 168 | 30.3 ± 0.4 | 58.2 ± 5.3 | 30.8 ± 1.9 | 90.5 ± 0.6 | 29.2 ± 2.2 |
| 336 | 26.8 ± 1.3 | 45.8 ± 4.0 | 28.0 ± 1.4 | 87.8 ± 2.2 | 24.8 ± 1.5 |
| 720 | 23.3 ± 2.0 | 34.8 ± 5.2 | 24.0 ± 3.1 | 78.2 ± 4.1 | 21.5 ± 2.9 |
rbIL-8 (5 μg in 10 mL of saline) and placebo saline were individually infused into the left- and right-front mammary glands, respectively, of 3 cows following the final milking.
rbIL-8 (25 μg in 10 mL of saline) and placebo saline were individually infused into the left- and right-front mammary glands, respectively, of 3 cows following the final milking.
No infusion was done in the udders of 3 cows at drying off.
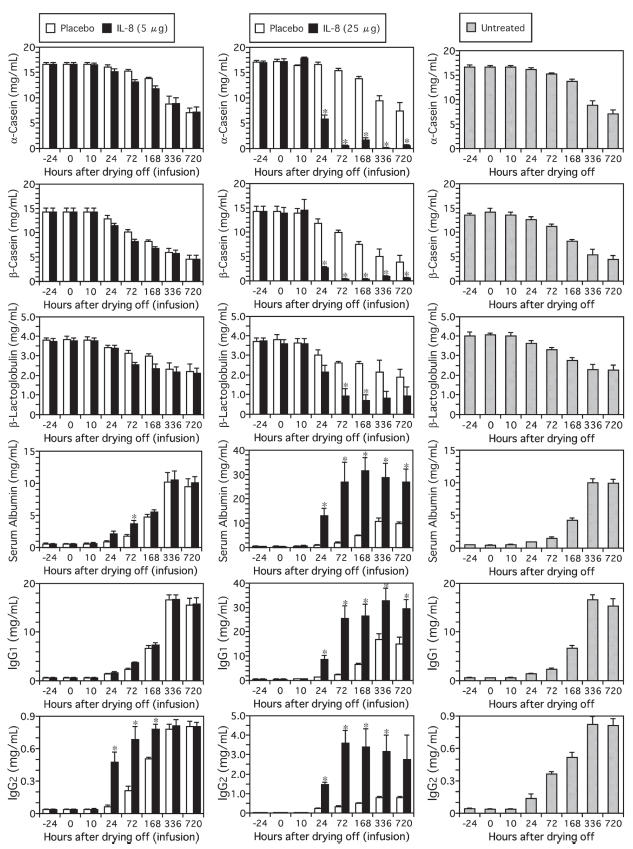
In the mammary glands that were infused with 25 μg of rbIL-8, the decreases in the concentrations of α- and β-CN and the increases in the concentrations of Alb, IgG1 and IgG2 were significant from 24 h after the infusion until 336 (IgG2 concentrations) or 720 h (α-CN, β-CN, Alb, and IgG1 concentrations) after the infusion (Figure 2). A decrease of β-LG concentration was also detected at 72 h and 168 h after the infusion. These changes in the glands infused with 5 μg of rbIL-8 were moderate and were not different from those in the control glands with the exception of the changes in the Alb and IgG2 concentrations. In all placebo-infused mammary glands, the changes in concentrations of the proteins examined were similar to those in the mammary glands of the untreated cows.
Figure 2.
Changes in concentrations of α-casein, β-casein, β-lactoglobulin, serum albumin, IgG1 and IgG2 in mammary secretions from cows infused with 5 μg (A) or 25 μg (B) of rbIL-8 at drying off. The data from rbIL-8- and saline-infused udders are shown using closed and open bars, respectively. The data from the untreated cows are shown using the shaded bars (C). An asterisk (*) indicates a significant difference from both the saline-infused mammary gland and the mammary gland of untreated cows at the same time point.
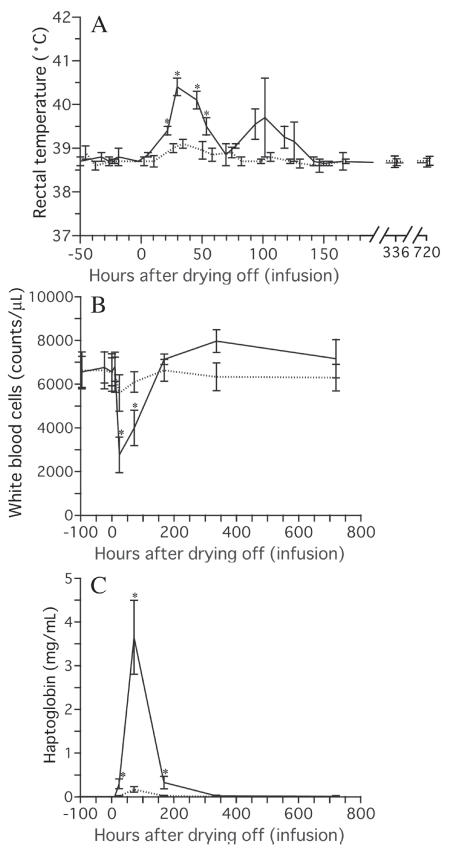
In cows infused with 25 μg of rbIL-8, a significant increase in RT was observed from 24 h to 48 h after the infusion, and a significant increase in serum Hp level was observed from 24 h to 168 h after the infusion (Figure 3). In addition to those changes, decreases in white blood cell counts were observed from 24 h to 72 h after the infusion, suggesting that the infiltration of leukocytes into mammary secretions from blood was most active at this time. In cows infused with 5 μg of rbIL-8, the decrease in the white blood cell count and the increases in RT and serum Hp concentration did not reach significant values. In the untreated cows, no significant changes in the white blood cell count, RT, or serum Hp level were detected (data not shown).
Figure 3.
Changes in rectal temperature (A), white blood cell count (B) and haptoglobin concentration in the serum (C) following intramammary infusion of rbIL-8. The data from the cows infused with 5 μg of rbIL-8 are shown using broken lines, and the data from the cows infused with 25 μg of rbIL-8 are shown using solid lines. An asterisk (*) indicates a significant difference from the data recorded before the infusion of rbIL-8.
In the mammary glands infused with rbIL-8, decreased concentrations of milk-specific proteins and increased concentrations of serum proteins were detected. These observations indicated that rbIL-8 affected mammary epithelial functions, such as the secretion of milk proteins and the maintenance of the integrity of the milk-blood barrier. Interleukin-8 has been reported to impair the barrier functions of airway and gastric epithelia (9,10). Interleukin-8 may impair the epithelial barrier function by regulating the tight junctions between epithelial cells, as has been suggested for several inflammatory cytokines (15). Decreased concentrations of milk-specific proteins and increases in serum protein concentrations have been observed in mastitic milk (11). Increased concentrations of IL-8 have been detected in mastitic mammary secretions (6–8). Interleukin-8 is assumed to be involved in mastitic changes in the milk protein composition via the suppression of milk-specific protein secretion and the influx of serum proteins.
Some of the changes in protein composition in the mammary secretions reported here appeared to be advantageous for the defense system against intramammary infections. These included: increased concentration of IgG2, which usually contains opsonic antibodies (16); and the decreased concentration of CN, which inhibits myeloperoxidase (MPO)-mediated oxygen-dependent bactericidal activity of neutrophils at the concentrations contained in normal milk (17). In the mammary gland, IL-8 might arrange the composition of mammary secretions to conform to the appropriate conditions for bactericidal activities of phagocytic cells, in addition to recruiting neutrophils. In the mammary gland during mastitis, mammary epithelial cells have been proposed to be a main source of IL-8 because the level of IL-8 was higher in milk than in lymph during the early stage of LPS-induced mastitis (18). Mammary epithelial cells might be involved in the defense system against intramammary infections via the secretion of IL-8.
A substantial elevation of RT was observed in the cows infused with 25 μg of rbIL-8. Furthermore, the serum concentration of Hp, a principal acute-phase protein in the bovine (12), was greatly increased. These findings indicate that the rbIL-8 infused into the teat cistern could elicit systemic inflammatory responses according to the dosage. At present, it is unknown if these responses were due to the direct effects of rbIL-8. Although IL-8 is a pyrogen in rodents (19), it has not been confirmed to act as a pyrogen in bovine. It was also reported that IL-8 mediates acute-phase protein production by isolated human hepatocytes (20), but whether it directly induces acute-phase proteins has not been demonstrated in bovine. The involvement of IL-8 in elevating RT and serum acute-phase protein levels in bovine still needs to be investigated. In addition to the elevation of RT and serum Hp, a significant decrease of the white blood cell count was also observed from 24 h to 72 h after the infusion of 25 μg of rbIL-8. An elevation of RT and a decrease of the white blood cell count in peripheral blood are systemic signs often observed in mastitis, especially in coliform mastitis (21,22). The elevation of the plasma Hp level is more obvious during intra-mammary infection with Escherichia coli than with S. aureus (7). The concentration of IL-8 is known to increase greatly in mammary secretions during coliform mastitis (6,7). Because intramammary infusion of rbIL-8 produced local and systemic symptoms similar to those during coliform mastitis, IL-8 is considered to be a factor in controlling the pathological status during mastitis, especially coliform mastitis.
In conclusion, intramammary infusion of rbIL-8 affected the bovine mammary gland by impairing the integrity of the blood-milk barrier and suppressing the milk-specific protein secretion as well as recruiting leukocytes. Functional regulation of the mammary gland by IL-8 was assumed to be implicated in mastitic changes in milk protein composition. The changes in milk protein composition elicited by rbIL-8 may contribute to the host defense system against intramammary infection, because these changes included an increase in the concentration of IgG2 containing opsonic antibodies, and a decrease in the concentration of CN, which inhibits the MPO-mediated bactericidal activity of neutrophils at the level of normal milk. Intramammary infusion of rbIL-8 induced not only local responses but also several systemic inflammatory responses such as increases in RT and the serum Hp level.
Acknowledgments
The authors acknowledge the excellent technical assistance provided by Yukio Chikayama and Rie Nunome.
References
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Barber MR, Pantschenko AG, Hinckley LS, Yang TJ. Inducible and constitutive in vitro neutrophil chemokine expression by mammary epithelial and myoepithelial cells. Clin Diagn Lab Immunol. 1999;6:791–798. doi: 10.1128/cdli.6.6.791-798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudjellab N, Chan-Tang HS, Li X, Zhao X. Interleukin 8 response by bovine mammary epithelial cells to lipopolysaccharide stimulation. Am J Vet Res. 1998;59:1563–1567. [PubMed] [Google Scholar]
- 4.Caswell JL, Middleton DM, Gordon JR. Production and functional characterization of recombinant bovine interleukin-8 as a specific neutrophil activator and chemoattractant. Vet Immunol Immunopathol. 1999;67:327–340. doi: 10.1016/s0165-2427(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 5.Mukaida N, Harada A, Matsushima K. Interleukin-8 and monocyte chemotactic and activating factor (MCAF/MCP-1), chemok-ines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 6.Bannerman DD, Paape MJ, Lee J-W, Zhao X, Hope JC, Rainard P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin Diagn Lab Immunol. 2004;11:463–472. doi: 10.1128/CDLI.11.3.463-472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riollet C, Rainard P, Poutrel B. Differential induction of complement fragment C5a and inflammatory cytokines during intra-mammary infections with Escherichia coli and Staphylococcus aureus. Clin Diagn Lab Immunol. 2000;7:161–167. doi: 10.1128/cdli.7.2.161-167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber MR, Yang TJ. Chemotactic activities in nonmastitic and mastitic mammary secretions: Presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin Diagn Lab Immunol. 1998;5:82–86. doi: 10.1128/cdli.5.1.82-86.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiwara Y, Arakawa T, Fukuda T, et al. Interleukin-8 stimulates leukocyte migration across a monolayer of cultured rabbit gastric epithelial cells. Effect associated with the impairment of gastric epithelial barrier function. Dig Dis Sci. 1997;42:1210–1215. doi: 10.1023/a:1018850006714. [DOI] [PubMed] [Google Scholar]
- 10.Laffon M, Pittet J-F, Modelska K, Matthay MA, Young DM. Interleukin-8 mediates injury from smoke inhalation to both the lung endothelial and the alveolar epithelial barriers in rabbits. Am J Respir Crit Care Med. 1999;160:1443–1449. doi: 10.1164/ajrccm.160.5.9901097. [DOI] [PubMed] [Google Scholar]
- 11.Sordillo LM, Nickerson SC, Akers RM, Oliver SP. Secretion composition during bovine mammary involution and the relationship with mastitis. Int J Biochem. 1987;19:1165–1172. doi: 10.1016/0020-711x(87)90098-x. [DOI] [PubMed] [Google Scholar]
- 12.Eckersall PD, Conner JG. Bovine and canine acute phase proteins. Vet Res Commun. 1988;12:169–178. doi: 10.1007/BF00362798. [DOI] [PubMed] [Google Scholar]
- 13.Wedlock DN, McCarthy AR, Doolin EE, Lacy-Hulbert SJ, Woolford MW, Buddle BM. Effect of recombinant cytokines on leucocytes and physiological changes in bovine mammary glands during early involution. J Dairy Res. 2004;71:154–161. doi: 10.1017/s0022029904000111. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe A, Yagi Y, Shiono H, Yokomizo Y. Effect of intra-mammary infusion of tumor necrosis factor-α on milk protein composition and induction of acute-phase protein in the lactating cow. J Vet Med B. 2000;47:653–662. doi: 10.1046/j.1439-0450.2000.00400.x. [DOI] [PubMed] [Google Scholar]
- 15.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson RG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rainard P, Lautrou Y, Poutrel B. Ingestion and killing of Streptococcus agalactiae by bovine granulocytes in the presence of natural opsonins. Vet Microbiol. 1988;18:41–50. doi: 10.1016/0378-1135(88)90114-9. [DOI] [PubMed] [Google Scholar]
- 17.Cooray R. Casein effects on the myeloperoxidase-mediated oxygen-dependent bactericidal activity of bovine neutrophils. Vet Immunol Immunopathol. 1996;51:55–65. doi: 10.1016/0165-2427(95)05496-0. [DOI] [PubMed] [Google Scholar]
- 18.Persson Waller K, Colditz IG, Lun S, Ostensson K. Cytokines in mammary lymph and milk during endotoxin-induced bovine mastitis. Res Vet Sci. 2003;74:31–36. doi: 10.1016/s0034-5288(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 19.Zampronio AR, Souza GE, Silva CA, Cunha FQ, Ferreira SH. Interleukin-8 induces fever by a prostaglandin-independent mechanism. Am J Physiol. 1994;266:R1670–R1674. doi: 10.1152/ajpregu.1994.266.5.R1670. [DOI] [PubMed] [Google Scholar]
- 20.Wigmore SJ, Fearon KCH, Maingay JP, Lai PBS, Ross JA. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol. 1997;273:E720–E726. doi: 10.1152/ajpendo.1997.273.4.E720. [DOI] [PubMed] [Google Scholar]
- 21.Mehrzad J, Dosogne H, Meyer E, Burvenich C. Local and systemic effects of endotoxin mastitis on the chemiluminescence of milk and blood neutrophils in dairy cows. Vet Res. 2001;32:131–144. doi: 10.1051/vetres:2001100. [DOI] [PubMed] [Google Scholar]
- 22.Wenz JR, Barrington GM, Garry FB, Dinsmore RP, Callan RJ. Use of systemic disease signs to assess disease severity in dairy cows with acute coliform mastitis. J Am Vet Med Assoc. 2001;218:567–572. doi: 10.2460/javma.2001.218.567. [DOI] [PubMed] [Google Scholar]