Abstract
The purpose of this study was to investigate and compare the effects of medetomidine and xylazine on some neurohormonal and metabolic variables in healthy cats. Five cats were used repeatedly in each of 11 groups, which were injected intramuscularly with physiological saline solution (control), 20, 40, 80, 160, and 320 μg/kg of medetomidine, and 0.5, 1, 2, 4, and 8 mg/kg of xylazine. Blood samples were taken over 24 h from the jugular vein for determination of plasma glucose, insulin, cortisol, epinephrine, norepinephrine, glucagon, and nonesterified fatty acid concentrations. Both medetomidine and xylazine induced remarkable hyperglycemia that was dose-dependent except for the response to medetomidine from 0 to 3 h. Both agents suppressed epinephrine and norepinephrine release but not in a dose-dependent manner at the tested dosages. Both agents inhibited insulin release and lipolysis, with similar potency, and tended to suppress cortisol release. The glucagon levels did not change significantly in any of the groups. These results suggest that the effects of medetomidine and xylazine on glucose metabolism and catecholamine release may not be due only to the actions mediated by α2-adrenoceptors.
Résumé
Cette étude avait pour but d’étudier et de comparer les effets de la medetomidine et de la xylazine sur quelques variables neurohormonales et métaboliques chez des chats en santé. Cinq chats ont été utilisés de manière répétée dans chacun des 11 groupes de traitement et ont reçu par injection intramusculaire une solution de saline physiologique (témoin), 20, 40, 80, 160 et 320 μg/kg de medetomidine et 0,5, 1, 2, 4 et 8 mg/kg de xylazine. Des échantillons sanguins ont été prélevés sur une période de 24 h à partir de la veine jugulaire pour déterminer les concentrations plasmatiques de glucose, insuline, cortisol, adrénaline, noradrénaline, glucagon et acides gras non-estérifiés. La medetomidine et la xylazine ont toutes deux induit une hyperglycémie qui était dose-dépendante sauf pour la réponse à la medetomidine de 0 à 3 h. Les deux agents ont empêché la relâche d’adrénaline et de noradrénaline mais pas d’une manière dose-dépendante avec les concentrations testées. Les deux agents ont inhibé la relâche d’insuline et la lipolyse avec une efficacité similaire et avaient tendance à supprimer la relâche de cortisol. Les niveaux de glucagon n’ont pas changé de manière significative dans aucun des groupes. Ces résultats suggèrent que les effets de la medetomidine et de la xylazine sur le métabolisme du glucose et la relâche de la catécholamine pourraient ne pas être dus seulement aux actions à médiation par les adrénorécepteurs-α2.
(Traduit par Docteur Serge Messier)
Introduction
In veterinary practice, the α2-adrenoceptor agonists medetomidine and xylazine are used mainly for sedative, muscle relaxant, and analgesic purposes (1). These agents are also effective as emetics in small animal practice (2). In addition, xylazine is used to stimulate the release of growth hormone for the diagnosis of congenital or acquired growth hormone deficiency (3). Medetomidine is a more selective and specific α2-adrenoceptor agonist than xylazine: the α2/α1 selectivity ratios are 1620 and 160, respectively (4). In spite of this difference, the 2 agents may be used similarly in practice.
Surgical stressors such as pain, blood loss, excitement, and underlying pathological conditions are well known to induce neurohormonal and metabolic changes in animals that are characterized by increases in blood levels of glucose, cortisol, catecholamines, and nonesterified fatty acids (NEFAs) and a decrease in blood insulin levels (5). Since α2-adrenoceptor-mediated actions are closely coordinated with these events, medetomidine and xylazine may interfere with the neurohormonal and metabolic response induced by stressors during and after anesthesia and surgery. For appropriate use, veterinarians need to know the neurohormonal and metabolic effects of medetomidine and xylazine in animals. Both agents induce hyperglycemia, hypoinsulinemia, inhibition of catecholamine release, and lipolysis in beagle dogs, but the hyperglycemic effect of medetomidine, in contrast to that of xylazine, is not dose-dependent (6).
There are limited reports as to the effects of xylazine on the blood glucose and insulin levels in cats (7), and specific time-dependent and dose-dependent data on the neurohormonal and metabolic effects of medetomidine and xylazine are lacking in cats, to our knowledge. Therefore, our study aimed to investigate and compare the effects of these 2 agents on the blood levels of epinephrine, norepinephrine, cortisol, glucose, insulin, glucagon, and NEFAs in cats.
Materials and methods
Animals
Five healthy mixed-breed cats of both sexes, of mean age 3.4 (standard deviation 1.34) y, and of mean weight 5.05 (0.23) kg were used. The cats were housed in our laboratory for at least 1 mo before the experiment and fed a standard commercial dry cat food. Routine hematologic examination was performed before the experiment; all values were within normal physiological ranges (8). The experimental protocols were approved by the Animal Research Committee of Tottori University, Tottori, Japan.
Experimental protocols
The experiment involved 11 treatment groups in which each cat was given an intramuscular injection of physiological saline solution (2.0 mL/animal) as the control agent, 20, 40, 80, 160, or 320 μg/kg of a 1% solution of medetomidine hydrochloride (Domitor; Meiji Seika, Tokyo, Japan), or 0.5, 1, 2, 4, or 8 mg/kg of a 2% solution of xylazine hydrochloride (Celactal; Bayer, Tokyo, Japan). The groups will be referred to as control, MED-20, -40, -80, -160, and -320, and XYL-0.5, -1, -2, -4, and -8. Five cats were used repeatedly, with at least 1 wk between treatments, in each of the 11 groups, according to a modified randomized design, as follows: cat 1, control, XYL-0.5, MED-20, XYL-4, MED-160, XYL-2, MED-80, XYL-8, MED-320, XYL-1, and MED-40; cat 2, XYL-1, MED-320, XYL-8, MED-160, XYL-4, MED-20, XYL-0.5, MED-80, control, MED-40, and XYL-2; cat 3, MED-20, XYL-0.5, control, XYL-8, MED-320, XYL-1, MED-40, XYL-4, MED-160, XYL-2, and MED-80; cat 4, XYL-1, MED-40, control, MED-20, XYL-0.5, MED-80, XYL-2, MED-320, XYL-8, MED-160, and XYL-4; and cat 5, MED-80, XYL-2, MED-40, XYL-1, MED-160, XYL-4, control, XYL-0.5, MED-20, XYL-8, and MED-320. The cats were fasted for 12 h before injection. Food and water were also withheld during the experiment and offered again 1 h after the last blood sampling of the day. The experiments were performed in a room with air temperature set at 25°C.
Sample collection
Blood samples (2.0 mL) were collected from the jugular vein by means of a 23-gauge needle with a 2.5-mL disposable syringe at the following 10 times: time 0 (before injection of the agent) and 0.25, 0.5, 1, 2, 3, 4, 5, 6, and 24 h after injection. An aliquot of 0.5 mL from each sample was mixed with aprotinin (Trasylol; Bayer, Leverksen, Germany) for glucagon measurement; the remaining 1.5 mL was mixed with ethylene diamine tetraacetic acid. Both samples were centrifuged immediately at 4°C, and then the plasma was separated and kept frozen at −80°C until analyzed for concentrations of catecholamines (epinephrine and norepinephrine), cortisol, glucose, insulin, glucagon, and NEFAs.
Analytical methods
Glucose and NEFA concentrations were determined with the use of commercially available kits (Glucose CII-test Wako and NEFA C-test Wako; Wako Junyakukogyo, Osaka, Japan). Glucose was analyzed by the mutarotase–glucose oxidase method, and NEFAs were analyzed by the acyl-coenzyme A (CoA) synthetase–acyl-CoA oxidase method. The intra-assay coefficients of variation (CVs) were < 2% and < 3% and the limits of quantification 700 mg/dL and 2 mmol/L, respectively. The glucose and NEFA concentrations were measured with a spectrophotometer (Auto Sipper Photometer U-1080; Hitachi, Tokyo, Japan).
Insulin and glucagon concentrations were measured by double-antibody radioimmunoassay (RIA) with the use of the commercially available kits I-AJ16 (Eiken Chemical Company, Tokyo, Japan) and Glucagon kit Daiichi (TFB Stock Company, Tokyo, Japan), respectively. The intra-assay CVs were < 10% and 2.6% to 5.3%, respectively; the interassay CV with the glucagon kit was 2.4% to 3.6%. The limits of detection and quantification were 5 to 320 μU/mL for insulin and 15.6 to 4000 pg/mL for glucagon.
Cortisol was measured by single-antibody RIA with the use of a commercially available kit (Gamma Coat Cortisol; Nihon Sheering, Chiba, Japan). The intra-assay CV was 3.5% to 5.0% and the inter-assay CV 4.2% to 8.7%. The limits of detection and quantification were 0.23 to 60 μg/dL.
Catecholamines were extracted on activated alumina according to the method described by Bouloux et al (9) and measured by means of high-performance liquid chromatography (LaChrom; Hitachi) and an electrochemical detector (Coulochem II; ESA, Chelmsford, Massachusetts, USA). As an internal standard, 3,4-dihydroxybenzylamine (DHBA; Sigma Chemical Company, St. Louis, Missouri, USA) was used. The percentage recovery of authentic DHBA standard was 64% to 77%.
Data evaluation
All data obtained were analyzed together with Prism statistical software (version 4; GraphPad Software, San Diego, California, USA). One-way analysis of variance for repeated measures was used to examine the time effect within each group and the group effect at each time point. When a significant difference was found, the Tukey test was used to compare the means.
The normalized area under the curve (AUC) was calculated for each biochemical variable. The AUC was measured by calculating the sum of the trapezoids formed by the data points and the x-axis from 0 to 6 h. The difference between the mean AUC of the control group and the AUC of a certain individual was defined as the normalized AUC. The normalized AUC data were plotted against dose of medetomidine or xylazine, and simple linear regression analysis was applied. When a significant difference was found, the effect of the drug on the plasma level of the examined biochemical was claimed to be dose-related.
Mean values are presented with standard deviation in parenthesis. The level of significance in all tests was set at P < 0.05.
Results
For all of the variables, there were no significant differences between the groups at baseline (time 0).
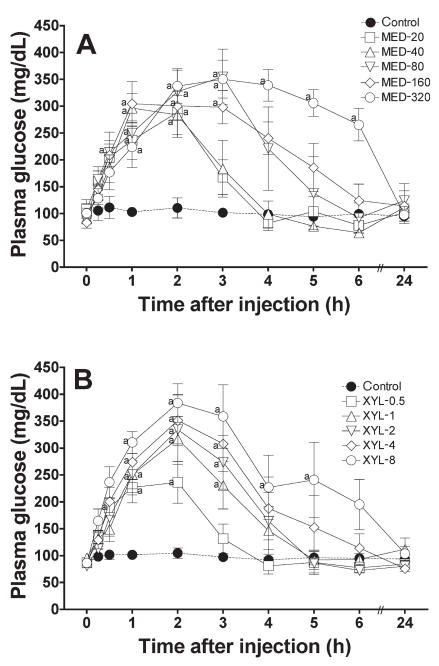
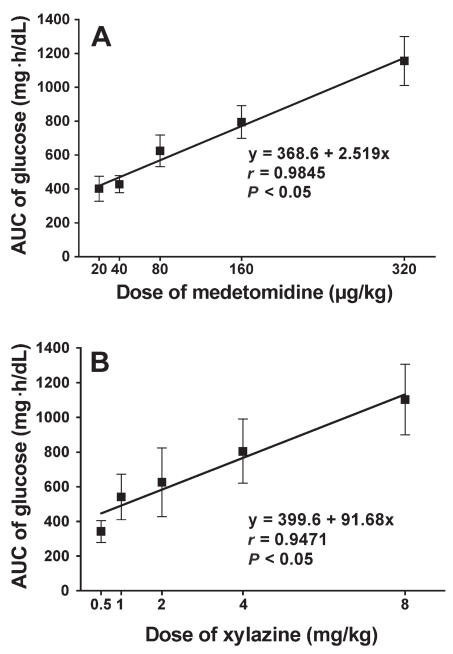
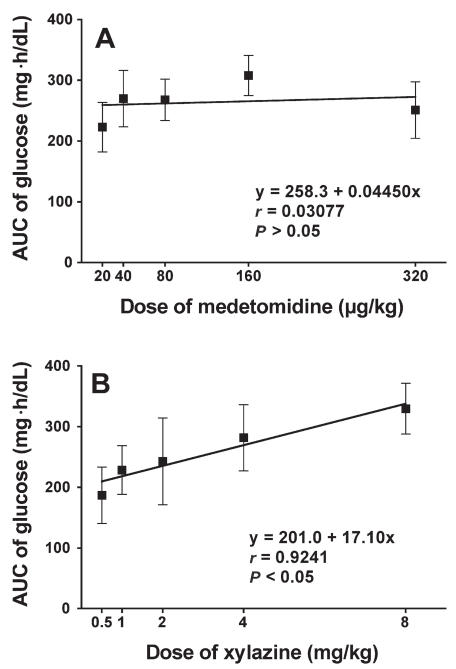
Glucose values increased greatly after administration in all groups except the control group (Figure 1). A dose-dependent peak response was found in the XYL groups at 2 h after administration. The maximum mean value was 383 (80.4) mg/dL with XYL-8 and 371 (61.8) mg/dL with MED-320. The linear regression of the normalized AUC data from 0 to 6 h was significant in both groups (Figure 2), indicating that both drugs induced hyperglycemia in a dose-dependent manner. However, time-related changes differed: the glucose values in the XYL groups returned to baseline gradually after the peak at 2 h, whereas the values in the MED groups tended to plateau near the peak and then return gradually to baseline in a dose-dependent manner. The linear regression of the normalized AUC data from 0 to 2 h was significant in the XYL groups but not in the MED groups, indicating that medetomidine, in contrast to xylazine, did not cause a dose-related increase in plasma glucose concentration, especially during the early phase after administration (Figure 3). Similar results were obtained with linear regression of the normalized AUC data from 0 to 3 h.
Figure 1.
Plasma glucose concentrations after the administration of various doses of A) medetomidine (MED; μg/kg) or B) xylazine (XYL; mg/kg) to cats. Each point and vertical bar represent the mean and standard error (n = 5); a — significantly different (P < 0.05) from the value at time 0 (before drug administration).
Figure 2.
Normalized area-under-the-curve (AUC) data from 0 to 6 h for the plasma glucose concentration after administration of A) medetomidine or B) xylazine. Simple linear regression analysis was applied.
Figure 3.
Normalized AUC data from 0 to 2 h for the plasma glucose concentration after administration of A) medetomidine or B) xylazine. Simple linear regression analysis was applied.
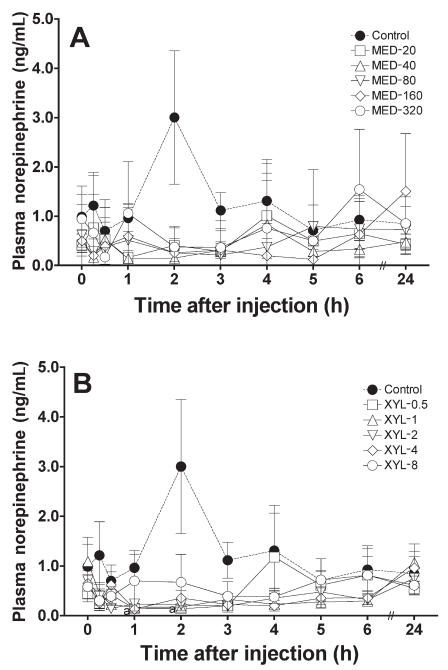
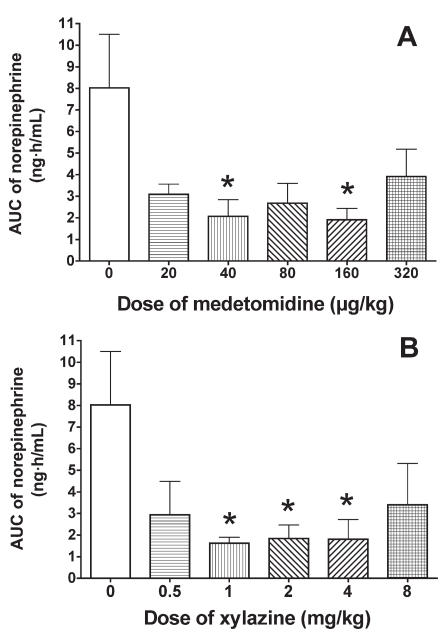
Compared with the baseline value, the mean concentration of norepinephrine was increased, but not significantly, in the control group 2 h after saline administration. The mean concentration was significantly decreased in the XYL-1 group 1 and 2 h after drug administration and tended to decrease in the other XYL and MED groups (Figure 4). The normalized AUC data from 0 to 6 h were lower in the MED and XYL groups than in the control group (Figure 5), significantly so in the MED-40, MED-160, XYL-1, XYL-2, and XYL-4 groups. However, the AUC data for the highest-dosage groups (MED-320 and XYL-8) were not further reduced when compared with those for the MED-160 and XYL-4 groups, respectively. The linear regression of the normalized AUC data was not significant for either treatment group, indicating that neither medetomidine nor xylazine induced a dose-dependent suppression of norepinephrine release within the tested dosages.
Figure 4.
Plasma norepinephrine concentrations after the administration of A) medetomidine or B) xylazine to the cats. Meanings of points, bars, and “a” as for Figure 1.
Figure 5.
Normalized AUC data from 0 to 6 h for the plasma norepinephrine concentration after administration of A) medetomidine or B) xylazine. Asterisks indicate a significant difference (P < 0.05) from the value at time 0.
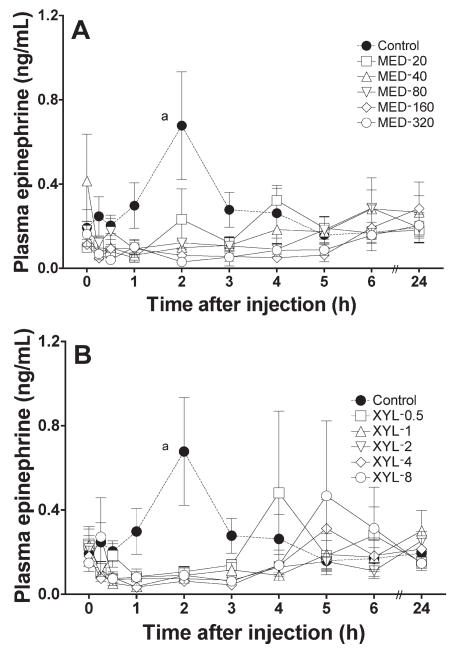
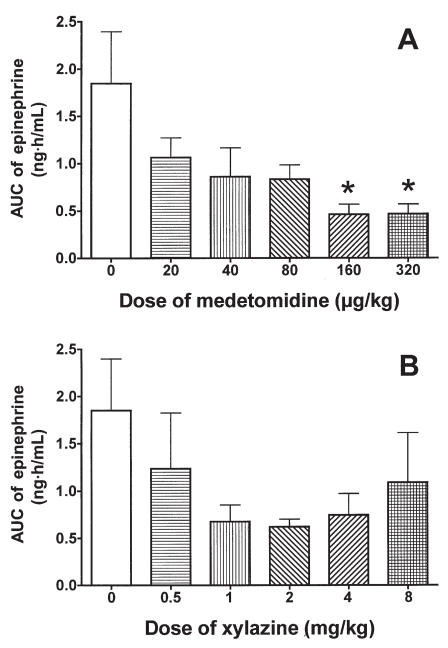
Compared with the baseline value, the mean concentration of epinephrine was significantly increased in the control group 2 h after saline administration. The mean concentration tended to decrease in all MED and XYL groups (Figure 6). The normalized AUC data from 0 to 6 h tended to be lower in the MED and XYL groups than in the control group (Figure 7) and were significantly lower in the MED-160 and MED-320 groups. The linear regression of the normalized AUC data was not significant for either treatment group, indicating that neither medetomidine nor xylazine induced a dose-dependent suppression of epinephrine release within the tested dosages.
Figure 6.
Plasma epinephrine concentrations after the administration of A) medetomidine or B) xylazine to the cats. Meanings of points, bars, and “a” as for Figure 1.
Figure 7.
Normalized AUC data from 0 to 6 h for the plasma epinephrine concentration after administration of A) medetomidine or B) xylazine. Asterisks indicate a significant difference (P < 0.05) from the value at time 0.
The mean concentration of cortisol had increased, though not significantly, from 1.42 (1.37) to 3.40 (2.79) μg/dL 0.5 h after administration of saline; it then returned to baseline (data not shown). In all MED groups, the concentration tended to decrease by 1 h after administration (for example, from 1.40 [0.75] to 0.28 [0.13] μg/dL in the MED-80 group). In the XYL-2, -4, and -8 groups, the concentration tended to decrease by 15 min to 1 h after administration (for example, from 1.24 [1.03] to 0.64 [0.43] μg/dL at 0.5 h in the XYL-2 group), whereas in the XYL-0.5 and -1 groups the concentration initially increased and then gradually returned to baseline. However, overall, there were no significant differences between the control and MED groups or between the control and XYL groups.
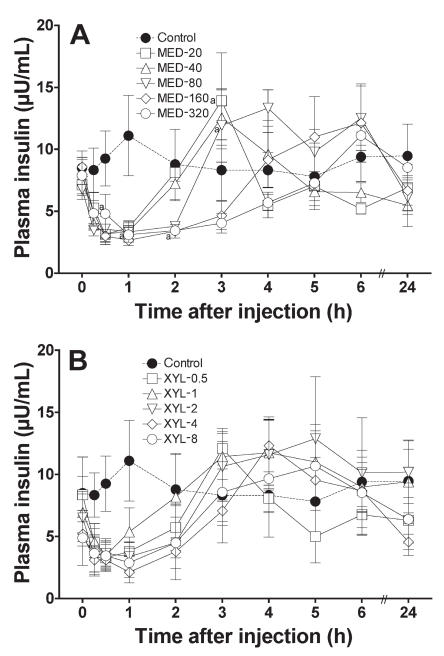
In both drug groups, the insulin concentration decreased immediately after administration of medetomidine or xylazine and gradually returned to baseline (Figure 8). The decrease was significant (P < 0.05) for 2 h in the MED-320 group. The slopes of the recovery phases indicated that medetomidine suppressed the plasma insulin concentration in a dose-dependent manner. The higher doses of xylazine delayed recovery from the insulin suppression. However, the linear regression of the normalized AUC data from 0 to 6 h was not significant for either treatment group, indicating that neither medetomidine nor xylazine induced a dose-dependent inhibition of insulin release within the tested dosages.
Figure 8.
Plasma insulin concentrations after the administration of A) medetomidine or B) xylazine to the cats. Meanings of points, bars, and “a” as for Figure 1.
The NEFA concentrations decreased after administration of both drugs and then gradually returned to baseline (data not shown). Higher doses of medetomidine and xylazine tended to delay recovery from the NEFA suppression. The linear regression of the normalized AUC data from 0 to 6 h was not significant for either treatment group.
The glucagon concentration did not significantly change during the experiments in any of the groups treated with either drug (data not shown). The glucagon/insulin ratio increased and then returned to baseline with both drugs (data not shown). These findings depended on the changes in plasma insulin concentration.
Discussion
Induction of hyperglycemia by medetomidine or xylazine has been reported in various animals, including dogs (6,10,11). In this study, we found that the hyperglycemia induced in cats by these drugs was greater than the previously reported values for dogs (6). Hyperglycemia was reported to occur easily in cats as a result of acute stress, such as with restraint (12), and to be due to elevation of plasma concentrations of stress-related hormones, such as cortisol and catecholamines. However, in our experiment, only a slight, nonsignificant increase in cortisol level was observed in the control group, and it apparently was insufficient to cause an increase in glucose level. In addition, we did not find elevations of cortisol and catecholamine levels in either drug-treated group, in spite of the remarkable hyperglycemia.
The mechanism of hyperglycemia induction by α2-adrenoceptor agonists is understood to be mainly via inhibition of insulin secretion through actions of the agonists on α2-adrenoceptors in the β cells of the pancreas (13,14). Our results in cats showed that, although the suppression of the plasma insulin concentration induced by medetomidine and xylazine was to the same degree as in dogs, the elevation of the plasma glucose concentration was much greater than in dogs. The increases in glucose level were also higher in cats than in dogs soon after drug administration. These findings suggest that factors in addition to inhibition of insulin secretion may be involved in α2-adrenoceptor-induced hyperglycemia in cats.
The effects of α2-adrenoceptor agonists on plasma glucose and insulin concentrations have been reported in a variety of animals (6,15–20). Clonidine and medetomidine were reported to increase plasma glucose levels in a dose-dependent manner in cattle (15,16) and rats (17,18). Furthermore, 2.2 mg/kg of xylazine administered intramuscularly to dogs caused an increase in plasma glucose concentration and a decrease in insulin concentration (19). The results of these studies were similar to our results.
When 10 and 20 μg/kg of medetomidine was administered intravenously to beagle dogs, the plasma glucose concentration tended to increase, but not significantly, and it remained within the normal physiological range (20); the investigators observed a peak of about 90 mg/dL 3 h after administration of 20 μg/kg. In another study in beagle dogs, intramuscular injections of 10 to 80 μg/kg induced an increase in plasma glucose concentration and a peak of 122 mg/dL at 3 h after administration of 20 μg/kg (6). In these 2 studies, the plasma insulin concentration decreased similarly. However, the hyperglycemic response was not dose-dependent (6). In the present study, although the decrease in the plasma insulin concentration in cats was similar to that reported in dogs (6), the elevation in the plasma glucose concentration was remarkably greater in the cats than in dogs. Some factors besides the decrease in the plasma insulin concentration may be responsible for the difference between dogs and cats in the degree of hyperglycemia. It has been suggested that glucose production with high activity of rate-limiting glycogenic enzymes was greater in feline liver than in canine liver, although the plasma glucose concentrations under physiologically normal conditions were similar (21). It is also possible that the increase in the plasma glucose concentration related to the decrease in the plasma insulin level is greater in cats than in dogs. This may be one of the reasons for the remarkable hyperglycemia induced by α2-adrenoceptor agonists in cats.
A previous study found that in beagle dogs the hyperglycemic effect was dose-dependent with xylazine but not with medetomidine (6). In the present study in cats, we found that both drugs induced remarkable hyperglycemia in a dose-dependent manner. However, the hyperglycemic response in the early phase (up to 3 h after injection) was not dose-dependent with medetomidine but was with xylazine. To our knowledge, this is the first report of a difference in hyperglycemic response between medetomidine and xylazine in cats. The decreases in insulin concentration after administration of medetomidine and xylazine were similar in this study. Although the changes were not dose-dependent, high doses of both drugs tended to prolong the inhibition of insulin release. Therefore, our results in cats suggest that the difference between the 2 drugs in the hyperglycemic response cannot be explained only by α2-adrenoceptor-mediated inhibition of insulin release.
The increase in plasma glucose concentration was reported to be more significant when xylazine was administered via a peripheral vein rather than the lateral ventricle, indicating that xylazine-induced hyperglycemia is mediated through actions on peripheral sites rather than the central nervous system (7). Furthermore, those investigators found that xylazine acted more rapidly and induced greater hyperglycemia when it was infused into the femoral rather than the portal vein in cats. They suggested that xylazine-induced hyperglycemia was not produced by a direct action on the liver. Glucose metabolism in the liver is regulated by both anabolic action, which is accelerated by insulin, and catabolic action, which is accelerated by glucagon. The glucagon/insulin ratio is an indication of glucose metabolism in the liver. The ratio was found to increase after xylazine administration in cats, which meant relative acceleration of gluconeogenesis over glycolysis (7). We obtained similar results in cats given either xylazine or medetomidine.
Other have reported that higher doses of clonidine increased glucose release from bovine and canine liver slices in vitro (15,22). The subtype of α-adrenoceptors mediating this action seemed to be α1 rather than α2, because prazosin, a specific α1-adrenoceptor antagonist, blocked the release of glucose more effectively than yohimbine, an α2-antagonist (22). Both medetomidine and xylazine also have an effect on α1-adrenoceptors, xylazine more so than medetomidine. Imidazoline-receptor agonists were reported to increase the secretion of insulin (23). Medetomidine has an affinity for the imidazoline receptors, unlike xylazine (2). However, the decrease in plasma insulin concentration was similar with medetomidine and xylazine in this study. Therefore, some of the differences between medetomidine and xylazine in their direct actions in the liver or in their actions that are mediated by α1-adrenoceptors, imidazoline receptors, or both may be partially involved in the hyperglycemic responses to the 2 agents in cats.
The elevation in plasma glucose concentration was apparent 15 min after drug administration in this study. We assumed that this acute hyperglycemia in cats might be partially due to the changing cortisol concentration. The cortisol concentration is regulated by both the peripheral system, in the adrenal cortex, and the central nervous system, through the release of corticotropin-releasing hormone and adrenocorticotropic hormone (ACTH). The effect of α2-adrenoceptor agonists on the secretion of cortisol has been reported for various animals. In dogs given medetomidine or xylazine intramuscularly, there was no significant change in the plasma cortisol concentration (6). In calves given 40 μg/kg of medetomidine, there was a slight, nonsignificant elevation in plasma cortisol concentration, whereas in cows and sheep given the same dosages there were increases of approximately 4 times and 6 to 8 times, respectively (11). In humans, oral administration of clonidine, 0.45 mg/d for 3 d, decreased the plasma cortisol level (24), whereas 0.1 to 0.2 mg/kg for 4 d did not cause a significant decrease (25). In dogs, premedication with medetomidine reduced or delayed the elevation in plasma cortisol concentration induced by ovariohysterectomy in dogs (26), and sedation with xylazine diminished the increase after intradermal testing (27).
These studies indicate that α2-adrenoceptor agonists such as medetomidine, xylazine, and clonidine can inhibit cortisol secretion. However, it is unclear whether this effect is specific for α2-adrenoceptors or other receptors. Recent studies suggest that imidazoline receptors, but not α2-adrenoceptors, may be involved in the inhibition of cortisol secretion from the adrenal cortex. An in vitro study revealed that the imidazoline–α2-adrenergic agents medetomidine, detomidine, and atipamezole all inhibited the secretion of cortisol from porcine adrenocortical cells (28). As medetomidine and detomidine are selective α2-adrenoceptor agonists and atipamezole is a selective α2-adrenoceptor antagonist, this effect is unrelated to these agents’ actions on α2-adrenoceptors. The selective α2-adrenoceptor agonist D-medetomidine and its enantiomer, L-medetomidine, which is ineffective clinically, were found to be equally effective in inhibiting ACTH-stimulated corticosterone secretion from adrenocortical cells of rats (29). These findings also indicate that imidazoline receptors may be involved in the suppression of cortisol secretion. Our experiments in cats revealed that the plasma cortisol concentration 15 min to 1 h after administration of either medetomidine or xylazine tended to be suppressed, whereas it tended to be increased 0.5 h after administration of saline. However, these findings were not significant. Therefore, our results suggest the possibility that both medetomidine and xylazine have an inhibitory effect on the elevation of the plasma cortisol concentration in cats. These effects would be useful for relief from a variety of stressors.
The α2-adrenoceptor agonists are well known to inhibit sympathetic outflow in the central nervous system through their actions on α2-adrenoceptors, hence decreasing the level of circulating catecholamines. A reduction in plasma catecholamine levels associated with the use of α2-adrenoceptor agonists has been reported for dogs (6), humans (30,31), horses (32,33), and goats (34). However, there were no previous reports about the effects of medetomidine and xylazine on plasma catecholamine levels in cats. Our study in cats revealed that both drugs reduced the plasma epinephrine and norepinephrine concentrations. A previous report in dogs described a dose-dependent decrease in plasma epinephrine and norepinephrine concentrations with both drugs (6). In the cats in our study, however, the suppression of catecholamine release was not dose-dependent for either drug. In the XYL-8 group, the reductions tended to be smaller than in the other XYL groups. As xylazine has a low α2/α1 selectivity ratio of 160 (5), a high dose of xylazine (as in the XYL-8 group) may act on α1-adrenoceptors that mediate the stimulation of sympathetic outflow and thus be less effective in reducing the plasma epinephrine and norepinephrine concentrations. In contrast, since the α2/α1 selectivity ratio of medetomidine is 1620, its action on α1-adrenoceptors may not have been present in the MED groups in our study. However, medetomidine also has an affinity for imidazoline receptors. Imidazoline-related drugs are able not only to inhibit norepinephrine release through the α2-adrenoceptor-mediated mechanism but also to induce norepinephrine release through indirect mechanisms related to I1 imidazoline receptors (35). Therefore, actions of medetomidine on both α2-adrenoceptors and imidazoline receptors may have been involved in the changes in plasma epinephrine and norepinephrine concentrations in the present study.
It is also possible that the action of α2-adrenoceptor agonists on the cardiovascular system may be responsible for the smaller suppression of catecholamine release with high doses of medetomidine and xylazine. The α2-adrenoceptor agonists can produce hypotensive and bradycardic effects through the central nervous system, whereas they induce vasoconstriction via peripheral α2-adrenoceptors in both the arterial and venous vasculature. Thus, α2-adrenoceptor agonists show a biphasic effect on blood pressure. Hypotension activates the sympathetic system through the arterial baroreceptor reflex, causing an increase in epinephrine and norepinephrine concentrations (36). In humans, an overdose of xylazine can induce hypotension (37). Such differences in the sympathetic system among the dosages of medetomidine and xylazine might influence the suppression of epinephrine and norepinephrine release. The precise mechanisms by which the higher doses of medetomidine and xylazine did not further reduce the catecholamine concentrations are not clear.
In the present study, the plasma glucagon level did not change significantly after administration of either medetomidine or xylazine to the cats, indicating that it is not related to the hyperglycemic effects of these agents. This finding is in agreement with a previous report on dogs (6).
The plasma NEFA concentration decreased similarly after administration of medetomidine and xylazine in this study. To our knowledge, this is the first report outlining the effect of these drugs on the plasma NEFA concentration in cats. The suppression was similar to that previously reported for dogs (6) and cattle (38). Suppression of lipolysis in cats may be mediated by both central and peripheral α2-adrenoceptors, as has previously been reported for dogs (39) and humans (40).
In conclusion, both medetomidine and xylazine induced remarkable hyperglycemia in cats compared with that reported for dogs. The hyperglycemic response to medetomidine during the initial 3 h was not dose-dependent, in contrast to the response to xylazine. Both drugs suppressed epinephrine and norepinephrine release, but the suppression was also not dose-dependent at the tested dosages. Both agents inhibited insulin release and lipolysis, with similar potency, and tended to suppress cortisol release. The glucagon level did not change significantly in any treatment group. These results suggest that the effects of medetomidine and xylazine on glucose metabolism and catecholamine release may not be due only to the actions mediated by α2-adrenoceptors.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (C) from the Japanese Ministry of Education, Science, Sports and Culture (18580316) and by the Scholarship Donation Fund 2005 from Meiji Seika, Tokyo, Japan. The authors thank Dr. Kota Sato for his valuable suggestions.
References
- 1.Greene SA. Pros and cons of using alpha-2 agonists in small animal anesthesia practice. Clin Tech Small Anim Pract. 1999;14:10–14. doi: 10.1016/s1096-2867(99)80022-x. [DOI] [PubMed] [Google Scholar]
- 2.Hikasa Y, Ogasawara S, Takase K. Alpha adrenoceptor subtypes involved in the emetic action in dogs. J Pharmacol Exp Ther. 1992;261:746–754. [PubMed] [Google Scholar]
- 3.Arce V, Cella SG, Loche S, Ghigo E, Devesa J, Müller EE. Synergistic effect of growth hormone-releasing hormone (GHRH) and clonidine in stimulating GH release in young and old dogs. Brain Res. 1990;537:359–362. doi: 10.1016/0006-8993(90)90386-p. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen R. Pharmacological profiles of medetomidine and its antagonist, atipamezole. Acta Vet Scand Suppl. 1989;85:29–37. [PubMed] [Google Scholar]
- 5.Ambrisko TD, Hikasa Y, Sato K. Influence of medetomidine on stress-related neurohormonal and metabolic effects caused by butorphanol, fentanyl, and ketamine administration in dogs. Am J Vet Res. 2005;66:406–412. doi: 10.2460/ajvr.2005.66.406. [DOI] [PubMed] [Google Scholar]
- 6.Ambrisko TD, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res. 2002;66:42–49. [PMC free article] [PubMed] [Google Scholar]
- 7.Feldberg W, Symonds HW. Hyperglycaemic effect of xylazine. J Vet Pharmacol Ther. 1980;3:197–202. [Google Scholar]
- 8.Jain NC. The cat: normal hematology with comments on response to disease. In: Jain NC, editor. Schalm’s Veterinary Hematology. 4. Philadelphia: Lea & Febiger; 1986. pp. 126–139. [Google Scholar]
- 9.Bouloux P, Perrett D, Besser GM. Methodological considerations in the determination of plasma catecholamines by high- performance liquid chromatography with electrochemical detection. Ann Clin Biochem. 1985;22:194–203. doi: 10.1177/000456328502200217. [DOI] [PubMed] [Google Scholar]
- 10.Brockman RP. Effect of xylazine on plasma glucose, glucagon and insulin concentrations in sheep. Res Vet Sci. 1981;30:383–384. [PubMed] [Google Scholar]
- 11.Ranheim B, Horsberg TE, Søli NE, Ryeng KA, Arnemo JM. The effects of medetomidine and its reversal with atipamezole on plasma glucose, cortisol and noradrenaline in cattle and sheep. J Vet Pharmacol Ther. 2000;23:379–387. doi: 10.1046/j.1365-2885.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 12.Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med. 2002;16:123–132. doi: 10.1892/0891-6640(2002)016<0123:ashici>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Hillaire-Buys D, Gross R, Blayac JP, Ribes G, Loubatières-Mariani MM. Effects of alpha-adrenoceptor agonists and antagonists on insulin secreting cells and pancreatic blood vessels: comparative study. Eur J Pharmacol. 1985;117:253–257. doi: 10.1016/0014-2999(85)90610-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki S, Katada T, Ui M. Alpha 2-adrenergic inhibition of insulin secretion via interference with cyclic AMP generation in rat pancreatic islets. Mol Pharmacol. 1982;21:648–653. [PubMed] [Google Scholar]
- 15.Gorewit RC. Effects of clonidine on glucose production and insulin secretion of cattle. Am J Vet Res. 1980;41:1769–1772. [PubMed] [Google Scholar]
- 16.Hsu WH, Hummel SK. Xylazine-induced hyperglycemia in cattle: a possible involvement of alpha 2-adrenergic receptors regulating insulin release. Endocrinology. 1981;109:825–829. doi: 10.1210/endo-109-3-825. [DOI] [PubMed] [Google Scholar]
- 17.DiTullio NW, Cieslinski L, Matthews WD, Storer B. Mechanisms involved in the hyperglycemic response induced by clonidine and other alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther. 1984;228:168–173. [PubMed] [Google Scholar]
- 18.Gotoh M, Iguchi A, Sakamoto N. Central versus peripheral effect of clonidine on hepatic venous plasma glucose concentrations in fasted rats. Diabetes. 1988;37:44–49. doi: 10.2337/diab.37.1.44. [DOI] [PubMed] [Google Scholar]
- 19.Benson GJ, Thurmon JC, Neff CA. Effect of xylazine hydrochloride upon plasma glucose and serum insulin concentrations in adult pointer dogs. J Am Anim Hosp Assoc. 1984;20:791–794. [Google Scholar]
- 20.Burton SA, Lemke KA, Ihle SL, Mackenzie AL. Effects of medeto-midine on serum insulin and plasma glucose concentrations in clinically normal dogs. Am J Vet Res. 1997;58:1440–1442. [PubMed] [Google Scholar]
- 21.Washizu T, Tanaka A, Sako T, Washizu M, Arai T. Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci. 1999;67:205–206. doi: 10.1053/rvsc.1998.0305. [DOI] [PubMed] [Google Scholar]
- 22.Maroto R, Calvo S, Sancho C, Esquerro E. Alpha- and beta- adrenoceptor cross-talk in the regulation of glycogenolysis in dog and guinea-pig liver. Arch Int Pharmacodyn Ther. 1992;317:35–46. [PubMed] [Google Scholar]
- 23.Hirose H, Seto Y, Maruyama H, Dan K, Nakamura K, Saruta T. Effects of alpha 2-adrenergic agonism, imidazolines, and G-protein on insulin secretion in beta cells. Metabolism. 1997;46:1146–1149. doi: 10.1016/s0026-0495(97)90207-9. [DOI] [PubMed] [Google Scholar]
- 24.Slowinska-Srzednicka J, Zgliczynski S, Soszynski P, Pucilowska J, Wierzbicki M, Jeske W. Effect of clonidine on beta-endorphin, ACTH and cortisol secretion in essential hypertension and obesity. Eur J Clin Pharmacol. 1988;35:115–121. doi: 10.1007/BF00609239. [DOI] [PubMed] [Google Scholar]
- 25.Grossman A, Weerasuriya K, Al-Damluji S, Turner P, Besser GM. Alpha 2-adrenoceptor agonists stimulate growth hormone secretion but have no acute effects on plasma cortisol under basal conditions. Horm Res. 1987;25:65–71. doi: 10.1159/000180635. [DOI] [PubMed] [Google Scholar]
- 26.Benson GJ, Grubb TL, Neff-Davis C, et al. Perioperative stress response in the dog: effect of pre-emptive administration of medetomidine. Vet Surg. 2000;29:85–91. doi: 10.1111/j.1532-950x.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 27.Frank LA, Kunkle GA, Beale KM. Comparison of serum cortisol concentration before and after intradermal testing in sedated and nonsedated dogs. J Am Vet Med Assoc. 1992;200:507–510. [PubMed] [Google Scholar]
- 28.Jager LP, De Graaf GJ, Widjaja-Greefkes HC. Effects of atipamezole, detomidine and medetomidine on release of steroid hormones by porcine adrenocortical cells in vitro. Eur J Pharmacol. 1998;346:71–76. doi: 10.1016/s0014-2999(98)00044-2. [DOI] [PubMed] [Google Scholar]
- 29.Maze M, Virtanen R, Daunt D, Banks SJ, Stover EP, Feldman D. Effects of dexmedetomidine, a novel imidazole sedative– anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg. 1991;73:204–208. doi: 10.1213/00000539-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Scheinin H, Kallio A, Koulu M, Scheinin M. Pharmacological effects of medetomidine in humans. Acta Vet Scand Suppl. 1989;85:145–147. [PubMed] [Google Scholar]
- 31.Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89:574–584. doi: 10.1097/00000542-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Raekallio M, Vainio O, Scheinin M. Detomidine reduces the plasma catecholamine, but not cortisol concentrations in horses. Zentralbl Veterinarmed A. 1991;38:153–156. doi: 10.1111/j.1439-0442.1991.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 33.Carroll GL, Matthews NS, Hartsfield SM, Slater MR, Champney TH, Erickson SW. The effect of detomidine and its antagonism with tolazoline on stress-related hormones, metabolites, physiologic responses, and behavior in awake ponies. Vet Surg. 1997;26:69–77. doi: 10.1111/j.1532-950x.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 34.Carroll GL, Hartsfield SM, Champney TH, Geller SC, Martinez EA, Haley EL. Effect of medetomidine and its antagonism with atipamezole on stress-related hormones, metabolites, physiologic responses, sedation, and mechanical threshold in goats. Vet Anaesth Analg. 2005;32:147–157. doi: 10.1111/j.1467-2995.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 35.Meana JJ, Herrera-Marschitz M, Goiny M, Silveira R. Modulation of catecholamine release by alpha 2-adrenoceptors and I1-imidazoline receptors in rat brain. Brain Res. 1997;744:216–226. doi: 10.1016/s0006-8993(96)01080-3. [DOI] [PubMed] [Google Scholar]
- 36.Medvedev OS, Kuz’min AI, Iashina LP, Rozin BD, Maĭorov DN. [Analysis of the baroreflex activation of the sympathetic system in waking cats induced by urapidil and sodium nitroprusside] Farmakol Toksikol. 1988;51:53–56. [PubMed] [Google Scholar]
- 37.Spoerke DG, Hall AH, Grimes MJ, Honea BN, Rumack BH. Human overdose with the veterinary tranquilizer xylazine. Am J Emerg Med. 1986;4:222–224. doi: 10.1016/0735-6757(86)90070-7. [DOI] [PubMed] [Google Scholar]
- 38.Scholtysik G, Regli F, Bruckmaier RM, Blum JW. The alpha2-adrenoceptor agonists xylazine and guanfacine exert different central nervous system, but comparable peripheral effects in calves. J Vet Pharmacol Ther. 1998;21:477–484. doi: 10.1046/j.1365-2885.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 39.Taouis M, Berlan M, Montastruc P, Lafontan M. Mechanism of the lipid-mobilizing effect of alpha-2 adrenergic antagonists in the dog. J Pharmacol Exp Ther. 1988;247:1172–1180. [PubMed] [Google Scholar]
- 40.Vikman HL, Savola JM, Raasmaja A, Ohisalo JJ. Alpha 2A-adrenergic regulation of cyclic AMP accumulation and lipolysis in human omental and subcutaneous adipocytes. Int J Obes Relat Metab Disord. 1996;20:185–189. [PubMed] [Google Scholar]