Abstract
Fowl adenoviruses (FAdV) are generally considered ubiquitous, but certain serotypes and strains are known to be associated with primary diseases, such as inclusion body hepatitis (IBH). Fifty-two FAdV isolates were collected from the provinces of Ontario and Quebec over a 4-year period. These 2 provinces have the largest poultry industries in Canada. Except for one virus, which originated from a guinea fowl, all other viruses were isolated from chicken samples. Most of these were from broilers, although some were from broiler breeders, and one was from layer pullets. Thirty-four isolates were from clinical IBH cases with the final laboratory diagnosis of IBH; however, for 18 isolates, the varied case diagnosis was seemingly unrelated to FAdV. All IBH-associated viruses had deoxyribonucleic acid (DNA) profiles compatible with FAdV species E (28 cases) or species D (6 cases), and the DNA fragment profiles of 26 species E viruses were indicative of serotype 8. Two viruses were serotype 6, as confirmed by virus neutralization. All species D viruses had a DNA profile similar to that of FAdV-2. The number of serotype 8 virus isolations has increased over the years, and by 2001 serotype 8 had become the dominant serotype in Ontario, and continues to be so. Moreover, this virus (FAdV-8) has shown a strong association with IBH.
Résumé
Les adénovirus aviaires (FAdV) sont généralement considérés comme ubiquitaires, mais certains sérotypes et souches sont connus pour être associés à des maladies primaires, telle que l’hépatite à corps d’inclusions (IBH). Cinquante-deux isolats de FAdV ont été amassés de l’Ontario et du Québec durant une période de quatre ans. Ces 2 provinces possèdent les plus grosses industries avicoles au Canada. À l’exception d’un virus, provenant d’une pintade, tous les autres virus ont été isolés d’échantillons provenant de poulet. La plupart de ces derniers provenaient de poulets à griller, bien que certains proviennent de reproducteurs de poulets à griller, et un isolat venait d’une poulette pondeuse. Trente-quatre isolats étaient associés à des cas cliniques d’IBH avec un diagnostic de laboratoire final d’IBH; mais, pour 18 isolats, le diagnostic semblait non-relié au FAdV. Tous les virus associés à IBH avaient un profil d’ADN compatible avec l’espèce E du FAdV (28 cas) ou l’espèce D (6 cas), et les profils des fragments d’ADN de 26 virus de l’espèce E étaient indicatifs du sérotype 8. Deux virus étaient de sérotype 6, tel que confirmé par neutralisation virale. Tous les virus de l’espèce D avaient un profil d’ADN similaire à celui du FAdV-2. Le nombre d’isolats de virus de sérotype 8 a augmenté au fil des ans, et en 2001 le sérotype 8 est devenu, et demeure, le sérotype prédominant en Ontario. De plus, ce virus (FAdV-8) a démontré une forte association avec l’IBH.
(Traduit par Docteur Serge Messier)
Introduction
Fowl adenovirus (FAdV) is in the genus Aviadenovirus and is a member of the family Adenoviridae (1). An earlier study (2) on the restriction fragment length polymorphism (RFLP) of the genomic deoxyribonucleic acid (DNA) with restriction endonucleases BamHI and HindIII provided the basis for the grouping of FAdVs into 5 species. The Fowl adenovirus species A comprises serotype 1 (FAdV-1, or CELO virus), while species B, C, D, and E are composed of serotype 5 (FAdV-5), serotypes 4 and 10 (FAdV-4 and -10), serotypes 2, 3, 9 and 11 (FAdV-2, -3, -9 and -11), and serotypes 6, 7, 8a, and 8b (FAdV-6, -7, -8a and -8b), respectively (1).
Fowl adenoviruses have a worldwide distribution and appear to be ubiquitous in poultry farms (3). However, some FAdV isolates can cause clinical diseases such as inclusion body hepatitis (IBH), hydropericardium syndrome, respiratory disease, tenosynovitis, and other symptoms in chickens and other birds (4–7). Fowl adenoviruses are easily transmitted both horizontally and vertically (7,8).
Fowl adenovirus infections are routinely diagnosed by virus isolation in embryonated eggs or cell culture and by electron microscopy, or more recently by polymerase chain reaction (PCR) (9). Polymerase chain reaction, followed by restriction enzyme digestion of the products as described by Meulemans et al (10) allows the differentiation of field isolates to species and presumptive serotypes; this has been more recently supported by sequencing data (11). The agar gel immunodiffusion (AGID) serological test is still widely used for detecting FAdV antibodies (Ab); however, application of the more sensitive enzyme-linked immunosorbent assay (ELISA) has been considered for detecting group and type-specific Abs and for introduction to diagnostic laboratories (12,13).
Since 2001, the number of IBH outbreaks associated with FAdVs has increased in Canada causing considerable economic losses to the poultry industry (14). Although FAdVs are regularly isolated from IBH cases, most field isolates have not been fully analyzed. The present study describes the characterization of FAdV isolates collected in the provinces of Ontario and Quebec between 1998 and 2002. The restriction fragment length polymorphism (RFLP) of the viral DNA and virus neutralization test was used to determine the species and serotypes of these viruses and to establish the dominant serotype in Ontario. The electropherotypes were also considered in order to assess the temporal and geographic distribution of viruses.
Materials and methods
Viruses and virus propagation
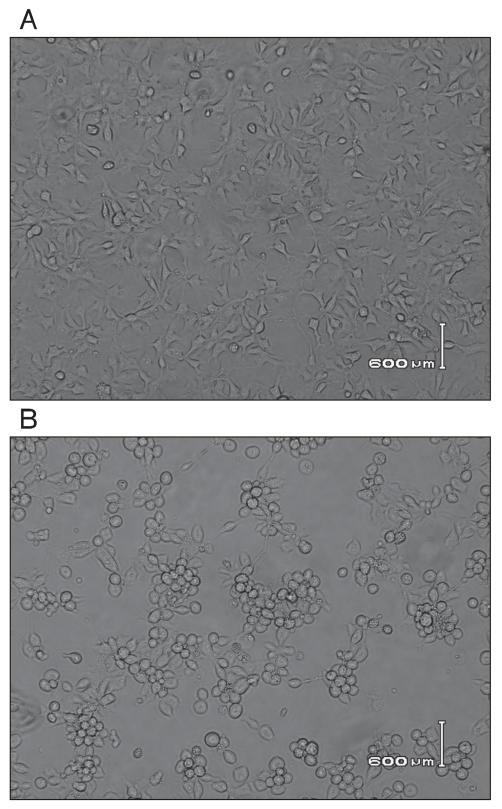
Fowl adenoviruses (Table I) were isolated from diagnostic materials submitted to the Animal Health Laboratory (AHL) of the University of Guelph, Guelph, Ontario, or from samples submitted directly to our research laboratory. Strains FAdV-9 (strain A-2A) and FAdV-1 (strain Phelps) were obtained from the American Type Culture Collection (ATCC). Virus isolation was done in 9- to 11-day-old embryonated chicken eggs, primary chicken liver cells, or in hepatoma cells [a CH-SAH cell line; (15)]. All viruses were propagated in hepatoma cells. For analysis, the cells together with the supernatant were collected when extensive cytopathic effect (CPE) was seen (Figure 1).
Table I.
Summary of fowl adenoviruses by year of isolation, type of the operation where the virus was isolated from, age of the flock, and the tissues used for isolation. The DNA pattern for each virus isolate for different restriction endonucleases and the serotype of some of the viruses were determined. Whether or not the final diagnosis reported by the diagnostic laboratory for the case (flock) was inclusion body hepatitis is also included
| DNA pattern
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Virus (year) | Source | Age | Tissue | Eco | Bam | Hin | Serotype | IBH |
| AV-2 (1998) | broiler | 38 days | trachea | A | A | —a | 1 | — |
| AV-3 (1998) | broiler | 14 days | bursa | A | A | — | ND (1)m | — |
| AV-5 (1998) | broiler | NR | kidney, lung, trachea | A | A | — | 1 | — |
| AV-6 (1998) | broiler breeder | NR | c. tonsil | A | A | — | ND (1) | — |
| AV-7 (1998) | chickenb | 41 days | NR | A | A | — | ND (1) | — |
| AV-8 (1998) | broiler | 34 days | bursa | A | A | — | ND (1) | — |
| AV-10 (1998) | guinea fowl | 57 weeks | c. tonsil | A | A | — | ND (1) | — |
| AV-11 (1998) | chicken | NR | NR | E | E | E | 8 | — |
| AV-12 (1998) | chicken | NR | lung, trachea | A | A | A | ND (1) | — |
| AV-13 (1998) | chicken | NR | trachea | A | A | A | ND (1) | — |
| AV-14 (1998) | broiler | 16 days | liver, spleen, bursa | D | D | D | 2 | + |
| AV-15 (1999) | broiler breeder | 231 days | c. tonsil | C | C | C | 4 | — |
| AV-16 (1999) | broiler breeder | 45 days | trachea | C | C | C | 4 | — |
| AV-17 (1999) | layer | 21 days | liver | E | E | E | 8 | + |
| AV-18 (1998) | broiler | 42 days | lung, trachea | A | A | A | 1 | — |
| AV-19 (1999) | broiler breeder | 56 days | liver, spleen | unique | no neutr. | — | ||
| AV-20 (1999) | chicken | 23 days | liver, thymus, bursa | E | E | E | 8 | + |
| AV-23 (1999) | broiler breeder | 22 weeks | liver, spleen, c. tonsil | C | C | C | 4 | — |
| AV-24 (1999) | chicken | 28 days | c. tonsil | A | A | — | ND (1) | — |
| AV-25 (1999) | broiler | 29 days | liver, kidney, spleen | E | E | E | 8 | + |
| AV-26 (2000) | chicken | 19 days | liver | E | E | E | 8 | + |
| AV-27 (2000) | chicken | 21 days | liver | D | D | D | ND (2) | + |
| AV-29 (2000) | broiler | 19 days | liver | E | E | — | ND (8) | + |
| AV-30 (2000) | broiler | 28 days | liver | — | E | — | ND (8) | + |
| AV-31 (2000) | broiler | 28 days | liver | — | E | — | ND (8) | + |
| AV-32 (2000) | broiler | 35 days | liver | — | E | — | ND (8) | + |
| AV-33 (2000) | broiler | 36 days | liver | — | E | — | ND (8) | + |
| AV-34 (2000) | broiler | 33 days | liver | — | E | — | ND (8) | + |
| AV-35 (2000) | chicken | 28 days | liver | E | E | — | ND (8) | + |
| AV-37 (2001) | broiler | 21 days | liver | E | E | — | 8 | + |
| AV-38 (2001) | broiler | 14 days | liver | E | E | — | 8 | + |
| AV-39 (2001) | broiler | 21 days | liver | E | E | — | ND (8) | + |
| AV-40 (2001) | chicken | 35 days | lung, trachea | A | A | — | 1 | — |
| AV-41 (2001) | chicken | 35 days | liver, lung, trachea | E | E | 6 | — | |
| AV-42 (2001) | chicken | 32 days | liver | E | E | — | ND (8) | + |
| AV-43 (2001) | broiler breeder | 10 days | liver | D | D | — | 2 | + |
| AV-44 (2001) | chicken | 12 days | liver | E | E | — | 8 | + |
| AV-45 (2001) | broiler | 14 days | liver, kidney | E | E | — | ND (8) | + |
| AV-46 (2001) | broiler | 16 days | liver | E | E | — | 8 | + |
| AV-47 (2001) | broiler | 16 days | liver, bursa | E | E | E | 8 | + |
| AV-48 (2001) | broiler | 14 days | liver | — | E | — | ND (8) | + |
| AV-49 (2001) | broiler | 14 days | liver | — | E | E | 8 | + |
| AV-51 (2001) | broiler | 26 days | liver | E | E | — | 8 | + |
| AV-52a (2001) | broiler | NR | liver | — | E | — | ND (8) | + |
| AV-52b (2001) | broiler | NR | liver | — | E | — | ND (8) | + |
| AV-53 (2001) | broiler | 35 days | liver, kidney, spleen | — | E | E | 6 | + |
| Qu — 1 (2001) | broiler | NR | liver | D | D | — | ND (2) | + |
| Qu — 2 (2001) | broiler | NR | liver | E | E | — | ND (8) | + |
| Qu — 3 (2001) | broiler | NR | liver | D | D | — | ND (2) | + |
| Qu — 4 (2001) | broiler | 18 days | liver | E | E | — | ND (8) | + |
| Qu — 5 (2001) | broiler | 17 days | liver | E | E | — | ND (8) | + |
| Qu — 6 (2001) | broiler | 14 days | liver | D | D | D | ND (2) | + |
IBH — inclusion body hepatitis; NR — not recorded; Eco — EcoR1; Bam — BamH1; Hin — HindIII; ND — serotyping not done; ND (1), ND (2) or ND (8) — presumptive serotype based on DNA profiles.
Not done.
Only the species was recorded but not the operation type.
Figure 1.
A — uninfected cells. B — Cytopathic effect of chicken hepatoma cells (CH-SAH cell line) infected with a fowl adenovirus isolate AV-49 at 36 h post infection.
Virus neutralization assay
The plaque reduction assay, using a panel of Abs against FAdV serotypes was performed as described earlier (12,16). Briefly, the different isolates were first titrated in hepatoma cells and 100 plaque forming units (PFU) were mixed with an equal volume of 1:50 and 1:100 dilutions of the Abs followed by an incubation for 1 h. The samples were then added to hepatoma cells and after a 1-h adsorption, the cells were overlaid with 0.65% agarose in DMEM/F12 containing 5% fetal bovine serum. The plaques were visualized by the addition of 0.02% neutral red after 3 d of incubation. A sample that inhibited the formation of > 50% of the plaques compared to that of the negative control serum, was considered positive.
DNA analysis
Viruses were concentrated as described (17), and DNA was extracted by phenol/chloroform from samples digested in a 0.5% final concentration of sodium dodecyl sulphate (SDS) and 500 μg/mL of proteinase K for 2 h at 56°C, and overnight at room temperature. Restriction endonucleases (RE) were obtained (Invitrogen Life Technologies, Burlington, Ontario) and the digestions were carried out according to manufacturer’s recommendations. The DNA fragments were separated by electrophoresis in 0.8% agarose gels, stained with ethidium bromide, and viewed with a BioRad Gel doc system (17). Viruses were assigned to species A to E based on the similarities of RFLPs to reference isolates in each species (2).
Results
Description of FAdVs
A total of 52 FAdV isolates were included in this study (see Table I). Except for AV-10, which originated from a guinea fowl, all viruses were isolated from chicken samples; with most originating from broilers (44), 6 from broiler breeders, and 1 from layer pullets. Viruses Qu-1 to Qu-6 were isolated from IBH cases diagnosed in Quebec in 2001. The remaining 46 viruses were from Ontario, mainly from routine case submissions; 12 from 1998, 8 from 1999, 9 from the year 2000, and 17 from 2001. The age of the birds from which viruses were isolated ranged from 10-day-old to 33-week-old chickens, but most frequently samples were from 2- to 4-week-old birds. Most viruses were isolated from liver only (28 samples; 53.85%) and pooled samples of liver and other tissues (9 samples; 17.3%); 13 viruses (25%) were isolated from other tissues only. There were no records for 2 virus isolates (3.85%). When virus was isolated from other tissues, such as bursa of Fabricius, lung, cecal tonsil, and trachea, 11 of them were identified later as FAdV-1 and 2 as FAdV-4. All of the viruses in this study were isolated from flocks, which had increased mortality and/or clinical signs. As recovered from the case reports, based on postmortem and histological examinations and laboratory results, IBH was the final diagnosis for 34 of the 52 cases from which FAdV was isolated (Table I).
DNA analysis
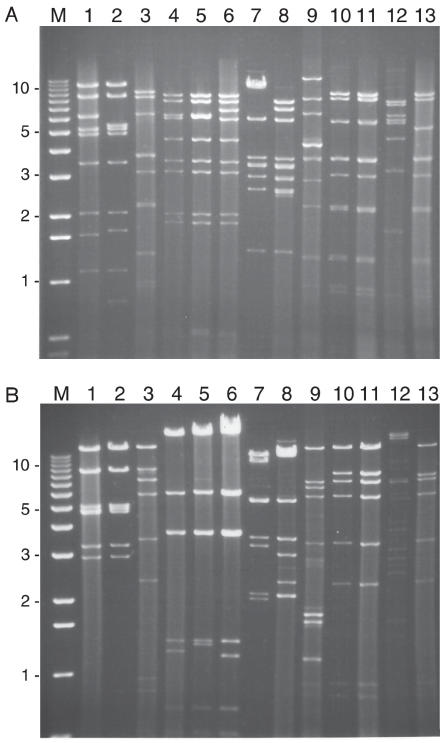
All of the extracted viral DNA was digested with BamHI and in addition, most samples were analyzed by either EcoRI and or HindIII. The DNA fragment profiles of isolates were compared to each other and published data (2). Representative gel profiles are shown in Figure 2. The DNA RE patterns allowed the differentiation of viruses into FAdV species as shown in Table I.
Figure 2.
A — Ethidium bromide stained gel following electrophoresis of BamHI. B — EcoRI digested viral DNA for representative fowl adenovirus isolates. The order of samples is the same for both A and B.
Lane M — 1 kb ladder. Lane 1 — FAdV-1; Lane 2 — AV-2; Lane 3 — FAdV-9; Lane 4 — FAdV-4; Lane 5 — AV-15; Lane 6 — AV-23; Lane 7 — AV-9; Lane 8 — AV-43; Lane 9 — AV-53; Lane 10 — AV-29; Lane 11 — Av-47; Lane 12 — AV-19; Lane 13 — AV-11.
The most frequent DNA profile, found for 30 out of the 52 isolates studied was compatible with FAdV species E (Figure 2, lanes 10 and 11) of which 28 were from IBH cases. Twenty-two of the 30 species E viruses had identical DNA patterns, while the others had only minor variations. In the year 2000, 8 out of 9 isolates were of the E pattern, and in 2001, there were 18 out of 23 isolates of this pattern. The DNA patterns of viruses (AV-52a and 52b) isolated from the different floors of the same barn were identical (data not shown).
Twelve isolates showed RE profiles identical to each other and to the reference serotype 1 virus in species A (Figure 2, lanes 1 and 2). While the BamHI profile of AV-2 indicated an additional cutting site on the DNA fragment in the 3rd band (Figure 2, panel A, lane 2), all the other 11 isolates (AV-3 to AV-8, AV-10, AV-12 and -13, AV-18, AV-24 and AV-40) were identical to the pattern of species A obtained for the Phelps strain including the virus isolated from a 57-week-old guinea fowl (AV-10).
Six isolates (AV-14, AV-27, AV-43, Qu-1, Qu-3, and Qu-6) had characteristic species D profiles of DNA (Figure 2, lane 8) and were almost identical. They were all identified as serotype 2 viruses. Three of the isolates (AV-15, AV-16, and AV-23) were grouped into species C (Figure 2, lanes 5 and 6). One virus (AV-19) had a unique RE profile, dissimilar to other viruses that were analyzed. None of the viral DNAs had an RE profile indicative of FAdV species B viruses.
Serotyping of FAdV field isolates
Plaque reduction assays with a panel of FAdV-serotype specific antibodies were performed to determine the serotypes of the isolates. The test was performed for 26 representative viruses, but not for isolates that displayed identical DNA patterns, since these isolates could be assigned to a serotype with a good degree of certainty. The determined and assigned serotypes (in brackets) are shown in Table I. Twelve viruses were identified as serotype 1, while 6, 3, 2, and 28 isolates were grouped into serotypes 2, 4, 6, and 8, respectively. The serotyping gave unambiguous results for all the isolates tested except 2 (AV-15 and AV-16), which were only partially neutralized by the serotype 4 specific Ab but not by Abs to any other serotype. Virus neutralization assays confirmed that 12 out of the 28 isolates grouped to species E by RFLP analysis were indeed serotype 8 viruses, while 2 (AV-41 and AV-53) were serotype 6.
One virus, AV-19, with unique RFLP patterns, was not neutralized with any of the available Abs, so the serotype for this could not be determined and may possibly represent a new serotype.
Discussion
Fowl adenoviruses isolated from samples that originated from Ontario and Quebec between 1998 and 2002 were studied. By 2001, the number of IBH cases had increased, and since then, this increasing trend has continued.
Of the 52 FAdV isolates, 23% (12) had RE profiles compatible with that of species A, FAdV-1 viruses. Except for AV-2, all the A type viruses were shown to be identical to the Phelps strain by RFLP. The BamHI profile of AV-2, which differed in 1 BamHI fragment from the other serotype A viruses, was identical to strain 112 isolated in Northern Ireland (2,18). Ninety-two percent (11 out of 12) of the FAdV-1 viruses was recovered from tissues such as trachea, lung, bursa of Fabricius, and cecal tonsil (but not from liver), and the source tissue was not recorded for one. These viruses were isolated from birds for which a variety of diseases, such as acute tracheitis, spiking mortality, atrophy of the gizzard, colibacillosis, were indicated as the final case diagnosis. In 1 case (AV-24), the virus was isolated from cecal tonsil, but the disease and diagnosis of hepatitis was recorded as associated with FAdV-1. Generally considered to be a low pathogenic, FAdV-1 is a ubiquitous virus. Okuda et al (19), however, recently showed the apparent pathogenicity of a serotype 1 virus (FAV-99ZH) isolated from older broiler chickens exhibiting gizzard erosion. Later, the same authors concluded that some serotype 1 strains induce gizzard erosion while others do not (20). One of the serotype 1 viruses (AV-10) analyzed in this study was isolated from a guinea fowl; a species rarely suggested in the literature as a host for fowl adenoviruses (18).
Until the year 2000, the diagnostic laboratory in Guelph had mainly used the chorioallantoic sac (CAS) inoculation of embryonated eggs for virus isolation. In subsequent years, both cell culture and eggs were utilized. Chorioallantoic sac inoculation is a common route for the isolation and propagation of serotype 1 FAdVs; however, it is not effective for the other serotypes (21,22). This might at least partly explain why 11 (55%) out of the 20 FAdVs isolated prior to the year 2000 were identified as serotype 1 virus.
The 3 isolates placed into species C were all serotype 4; however, none of these isolates were associated with hydropericardium syndrome, and all were isolated from broiler breeders with no clinical signs of IBH and no reported IBH associated problems in the progeny at the time. This finding is similar to that of Toro et al (23) regarding Chilean serotype 4 isolates, which did not produce hydropericardium/inclusion body hepatitis when administered orally. Although all 3 isolates were from the same year, they originated from distant premises and no association was found among them.
All 6 of the isolates identified in this study as species D, FAdV-2, were associated with IBH. While naturally occurring outbreaks associated with this serotype have been reported (24), they are considered as a less frequent cause of IBH. An outbreak in Canada, caused by a species D, FAdV-2 virus (AV-43) was described earlier (16). No epidemiological connection was found among premises from which the serotype 2 viruses were isolated.
Based on the DNA fragment profiles, more than half of the samples (58%) studied could be grouped into species E, and of these all but 2 were determined to be FAdV-8. These 2 viruses, AV-41 and AV-53, were serotype 6 FAdVs. The number of FAdV-8 virus isolations has been increasing over the years and by 2001 this serotype had become the dominant serotype in Ontario. All 34 cases of IBH were caused by either species E (28 cases) or by species D (6 cases) viruses. Except for the viruses AV-11 and AV-41, IBH was reported as the final diagnosis for all cases involving species E and species D viruses. Although the pathogenicity of the FAdV-8 viruses was not studied in a controlled environment and in experimentally infected chickens, the recorded case histories revealed that they were highly pathogenic in field settings. The presence and involvement of immunosuppressive viruses and/or other agents, however, were not investigated in this study. Although severe outbreaks and epidemics of IBH caused by fowl adenovirus 8 viruses were reported in New Zealand (25) and Australia (26), our study is the first to report on the distribution of FAdVs in Canada. Moreover, only a few sporadic cases of IBH associated with FAdV-8 have been reported in the United States, fowl adenovirus infections there are associated with other serotypes (27).
In summary, over a 4-year period, 52 FAdV isolates belonging to 5 different serotypes (1, 2, 4, 6, and 8) were isolated in 2 provinces that have the largest poultry industries in Canada. Fowl adenovirus FAdV-8, which was isolated from IBH cases in chickens between the ages of 2 to 5 wk mainly from southern Ontario, became the dominant virus by 2001, and continues as such. Since these viruses are very similar in their RE profiles, RFLP analysis alone would not be a suitable method for tracing the origin of serotype 8 viruses or following the spread of a given virus within and among poultry operations.
Acknowledgments
This work was supported by the Ontario Ministry of Agriculture, Food and Rural Affairs of Canada, and the Poultry Industry Council (Canada). The technical help of Samira Dahesh and Paul Huber is greatly appreciated.
References
- 1.Benkö M, et al. Family Adenoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy. 8. San Diego: Elsevier Acad Pr; 2005. pp. 213–228. [Google Scholar]
- 2.Zsák L, Kisary J. Grouping of fowl adenoviruses based upon the restriction patterns of DNA generated by BamHI and HindIII. Intervirology. 1984;22:110–114. doi: 10.1159/000149541. [DOI] [PubMed] [Google Scholar]
- 3.McFerran JB, Smyth JA. Avian adenoviruses. Rev Sci Tech. 2000;19:589–606. [PubMed] [Google Scholar]
- 4.McFerran JB, Gordon WA, Taylor SM, McParland PJ. Isolation of viruses from 94 flocks of fowl with respiratory disease. Res Vet Sci. 1971;12:565–569. doi: 10.1016/S0034-5288(18)34110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones R, Georgiou K. Experimental infection of chickens with adenoviruses isolated from tenosynovitis. Avian Pathol. 1984;13:13–23. doi: 10.1080/03079458408418504. [DOI] [PubMed] [Google Scholar]
- 6.Anjum AD, Sabri MA, Iqbal Z. Hydropericarditis syndrome in broiler chickens in Pakistan. Vet Record. 1989;124:247–248. doi: 10.1136/vr.124.10.247. [DOI] [PubMed] [Google Scholar]
- 7.McFerran JB, Adair BM. Group I adenovirus infections. In: Saif YM, editor. Diseases of Poultry. Ames, Iowa: Iowa State Pr; 2003. pp. 214–227. [Google Scholar]
- 8.Grgíc H, Philippe C, Ojkíc D, Nagy É. Study of vertical transmission of fowl adenoviruses. Can J Vet Res. 2006;70:230–233. [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang P, Ojkíc D, Tuboly T, Huber P, Nagy É. Application of the polymerase chain reaction to detect fowl adenoviruses. Can J Vet Res. 1999;63:124–128. [PMC free article] [PubMed] [Google Scholar]
- 10.Meulemans G, Boschmans M, van den Berg TP, Decaesstecker M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001;30:655–660. doi: 10.1080/03079450120092143. [DOI] [PubMed] [Google Scholar]
- 11.Meulemans G, Couvreur B, Decaesstecker M, Boschmans M, van den Berg TP. Phylogenetic analysis of fowl adenoviruses. Avian Pathol. 2004;33:164–170. doi: 10.1080/03079450310001652086. [DOI] [PubMed] [Google Scholar]
- 12.Ojkíc D, Nagy É. Antibody response and virus distribution in chickens inoculated with wild-type and recombinant fowl adenovirus. Vaccine. 2003;22:42–48. doi: 10.1016/s0264-410x(03)00544-9. [DOI] [PubMed] [Google Scholar]
- 13.Philippe C, Grgíc H, Ojkíc D, Nagy É. Serologic monitoring of a broiler breeder flock previously affected by inclusion body hepatitis and testing the progeny for vertical virus transmission. Can J Vet Res. 2007;71:98–102. [PMC free article] [PubMed] [Google Scholar]
- 14.Martin E, Binnington B, Youssef S, Shapiro J, Ojkíc D, McEwen B. Summary of AHL pathology diagnoses for Ontario poultry, 2003–2004. AHL Newsletter. 2005;9:4. [Google Scholar]
- 15.Alexander HS, Huber P, Cao J, Krell PJ, Nagy É. Growth characteristics of fowl adenovirus type 8 in a chicken hepatoma cell line. J Virol Methods. 1998;74:9–14. doi: 10.1016/s0166-0934(98)00062-7. [DOI] [PubMed] [Google Scholar]
- 16.Philippe C, Grgíc H, Nagy É. Inclusion body hepatitis in young broiler breeders associated with a serotype 2 adenovirus in Ontario, Canada. J Appl Poultry Res. 2005;14:588–593. [Google Scholar]
- 17.Ojkíc D, Nagy É. The long repeat region is dispensable of fowl adenovirus replication in vitro. Virology. 2001;283:197–206. doi: 10.1006/viro.2000.0890. [DOI] [PubMed] [Google Scholar]
- 18.McFerran JB, Clarke JK, Connor TJ. Serological classification of avian adenoviruses. Arch Gesamte Virusforsch. 1972;39:132–139. doi: 10.1007/BF01241536. [DOI] [PubMed] [Google Scholar]
- 19.Okuda Y, Ono M, Yazawa S, Imai Y, Shibata I, Sato S. Pathogenicity of serotype 1 fowl adenovirus in commercial broiler chickens. Avian Dis. 2001;45:819–827. [PubMed] [Google Scholar]
- 20.Okuda Y, Ono M, Shibata I, Sato S, Akashi H. Comparison of the polymerase chain reaction-restriction fragment length polymorphism pattern of the fiber gene and pathogenicity of serotype-1 fowl adenovirus isolates from gizzard erosions and from feces of clinically healthy chickens in Japan. J Vet Diagn Invest. 2006;18:162–167. doi: 10.1177/104063870601800204. [DOI] [PubMed] [Google Scholar]
- 21.Cook JKA. Fowl adenoviruses: Studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathol. 1983;12:35–43. doi: 10.1080/03079458308436147. [DOI] [PubMed] [Google Scholar]
- 22.Cowen BS. Chicken embryo propagation of type I adenoviruses. Avian Dis. 1988;32:347–352. [PubMed] [Google Scholar]
- 23.Toro H, Prusas C, Raue R, et al. Characterization of fowl adenoviruses from outbreaks of inclusion body hepatitis/hydropericardium syndrome in Chile. Avian Dis. 1999;43:262–270. [PubMed] [Google Scholar]
- 24.McFerran JB, McCracken RM, Connor TJ, Evans RT. Isolation of viruses from clinical outbreaks of inclusion body hepatitis. Avian Pathol. 1976;5:315–324. doi: 10.1080/03079457608418201. [DOI] [PubMed] [Google Scholar]
- 25.Saifuddin MD, Wilks CR. Pathogenesis of an acute viral hepatitis: Inclusion body hepatitis in the chicken. Arch Virol. 1991;116:33–43. doi: 10.1007/BF01319229. [DOI] [PubMed] [Google Scholar]
- 26.Erny KM, Barr DA, Fahey KJ. Molecular characterization of highly virulent fowl adenoviruses associated with outbreaks of inclusion body hepatitis. Avian Pathol. 1991;20:597–606. doi: 10.1080/03079459108418799. [DOI] [PubMed] [Google Scholar]
- 27.El-Attrache J, Villegas P. Genomic identification and characterization of avian adenoviruses associated with inclusion body hepatitis. Avian Dis. 2001;45:780–787. [PubMed] [Google Scholar]