Abstract
Recent studies demonstrate that context-specific memory retrieval after extinction requires the hippocampus. However, the contribution of hippocampal subfields to the context-dependent expression of extinction is not known. In the present experiments, we examined the roles of areas CA1 and CA3 of the dorsal hippocampus in the context specificity of extinction. After pairing an auditory conditional stimulus (CS) with an aversive footshock (unconditional stimulus or US), rats received extinction sessions in which the CS was presented without the US. In Experiment 1, pretraining neurotoxic lesions in either CA1 or CA3 eliminated the context dependence of extinguished fear. In Experiment 2, lesions of CA1 or CA3 were made after extinction training. In this case, only CA1 lesions impaired the context dependence of extinction. Collectively, these results reveal that both hippocampal areas CA1 and CA3 contribute to the acquisition of context-dependent extinction, but that only area CA1 is required for contextual memory retrieval.
In recent years, there has been considerable interest in understanding how the brain encodes memories for emotional experiences (Davis 1992; LeDoux 2000; Maren 2001). Far less is known concerning the neural mechanisms involved in suppressing fear memories after they are formed. After Pavlovian fear conditioning, for example, presentations of the conditional stimulus (CS) in the absence of the unconditional stimulus (US) decrease the fear response engendered by the CS (Pavlov 1927). This phenomenon, known as extinction, is an important form of inhibitory learning that not only allows animals to adapt their behavior to a changing environment but also provides a behavioral model for clinical treatment for fear disorders (Myers and Davis 2002; Maren 2005). Importantly, extinction is not an erasure of the original memory but instead involves new learning.
There are many important differences between conditioning and extinction memories, one being that extinction memories are preferentially expressed in the context in which they are learned. For example, fear to an extinguished CS will show a robust return or “renewal” if the CS is presented outside of the extinction context (Bouton 1993; Bouton 2002, 2004). This context-dependent expression of extinction is observed in both animals (Bouton 1993; Maren and Holt 2000; Rauhut et al. 2001) and humans (Maguire et al. 1996; Mineka et al. 1999; Mystkowski et al. 2006). Given the critical role of the hippocampus in memory for context (Good and Honey 1991; Kim and Fanselow 1992; Phillips and LeDoux 1992; Honey and Good 1993; Maren et al. 1997; Frankland et al. 1998; Good et al. 1998; Rudy and O’Reilly 2001; Kennedy and Shapiro 2004), it is not surprising that recent studies have implicated the hippocampus in the context-specific encoding (Corcoran et al. 2005; Ji and Maren 2005) and retrieval (Corcoran and Maren 2001, 2004; Ji and Maren 2005) of fear extinction (Wilson et al. 1995; Frohardt et al. 2000). These studies indicate that the hippocampus plays a critical role in using contextual information to modulate behavior to stimuli with ambiguous meanings, such as an extinguished CS.
Within the hippocampus, recent studies have suggested important differences in the function of areas CA1 and CA3 (the principal pyramidal cell fields in the hippocampus) in spatial and contextual memory (Lee et al. 2004; Leutgeb et al. 2005). For example, neurotoxic lesions or pharmacological inactivation of hippocampal area CA3 or CA1 have been reported to produce differential effects on the encoding and retrieval of contextual memories (Lee and Kesner 2004; Daumas et al. 2005). Because hippocampal areas CA1 and CA3 may have different functional roles in encoding and retrieving memories for context, we examined whether these areas make a unique contribution to the context dependence of extinguished fear responses. To this end, we made neurotoxic lesions in area CA1 or CA3 prior to fear conditioning (Experiment 1) or after extinction (Experiment 2) in rats. The context dependence of extinction was assessed by examining fear (freezing behavior, in this case) to the CS in either the extinction context or another context.
Results
Experiment 1: Pretraining lesions of hippocampal areas CA1 or CA3 and contextual encoding of extinction
The context dependence of extinction relies on two inter-related processes: contextual encoding and contextual retrieval. Contextual encoding indexes the extinction memory to the context in which it is acquired, and this enables contextual retrieval of that memory to occur in the extinction context. In Experiment 1, we examined whether pre-training neurotoxic lesions of hippocampal areas CA1 or CA3 would interfere with the encoding of context-dependent extinction memories.
Histology
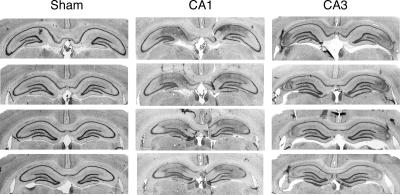
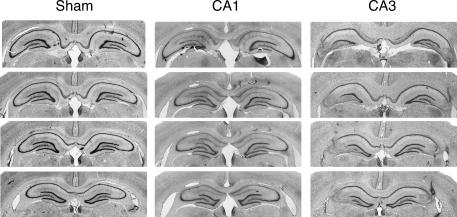
Photomicrographs of coronal brain sections from representative rats in the sham and lesion group are shown in Figure 1. Schematic drawing of the minimum and maximum extent of CA1 or CA3 lesions is illustrated in Figure 2. As shown in Figure 1, NMDA infusions into area CA1 produced an almost complete removal of pyramidal cells in this area, but spared the majority of neurons in area CA3 and the dentate gyrus (DG). In some sections, however, damage induced by CA1 infusions encroached into the DG (Fig. 2). Similarly, CA3 lesions were typically complete and selective (Fig. 1), although in some sections, damage in rats with CA3 lesions involved CA1 and DG (Fig. 2).
Figure 1.
Representative photomicrographs showing four thionin-stained coronal sections along the septotemporal axis for CA1 and CA3 lesions in the dorsal hippocampus (Experiment 1).
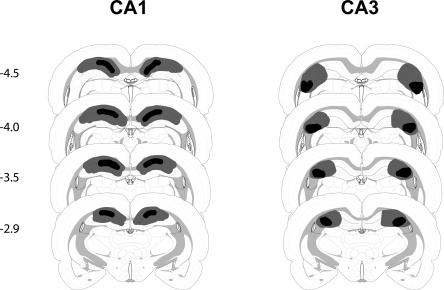
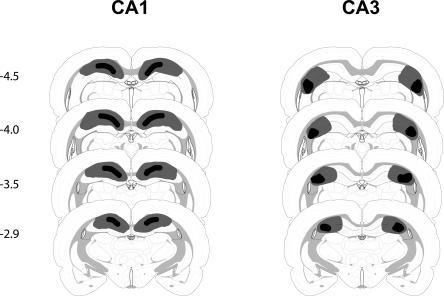
Figure 2.
Schematic representation of brain lesions mapped onto coronal rat brain sections showing the minimum (black areas) and maximum (black> + gray areas) extent of CA1 or CA3 lesions (Experiment 1). Coordinates are given in millimeters from bregma.
Quantification of the lesions revealed that NMDA infusions into CA1 resulted in substantial damage of the pyramidal cells in CA1 (mean = 83%; range = 76%–95%). These lesions were also associated with minimal damage in area CA3 (mean = 12%; range = 3.2%–23%) and DG (mean = 12%; range = 3%–37%). NMDA infusions into area CA3 produced a substantial loss of pyramidal cells in this (mean = 88%; range = 84%–93%). The other hippocampal cell fields exhibited some cell loss as well (CA1: mean = 10%, range = 5%–22%; DG, mean = 12%, range = 3.1%–24.8%).
Behavior
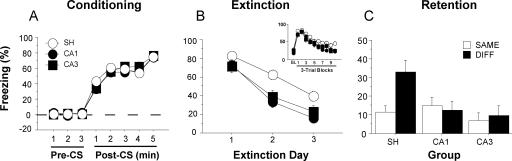
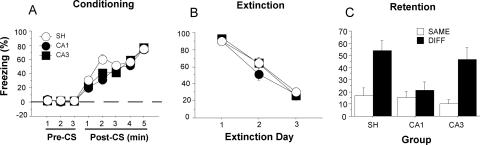
Conditional freezing during the conditioning, extinction, and testing sessions is shown in Figure 3. On the conditioning day (Fig. 3A), immediate post-shock freezing increased significantly across the five conditioning trials (F(7,658) = 301.47, p < 0.001). Pre-training CA1 or CA3 lesions did not affect post-shock freezing; these rats had a similar level of post-shock freezing compared to sham rats. ANOVA demonstrated that there was neither a significant main effect of lesion (F(2,94) = 0.37, p = 0.69) nor an interaction of lesion and minute on freezing (F(14,658) = 1.11, p = 0.35). To assess extinction across days, we averaged freezing after the first four tone-alone trials on each day (Fig. 3B). During the extinction sessions, freezing to the tone CS declined significantly across the three extinction days (F(2,188) = 152.51, p < 0.001). There was a significant main effect of lesion (F(2,94) = 6.61, p < 0.01) and a significant interaction of lesion and extinction day (F(4,188) = 2.69, p < 0.05). Post hoc comparisons showed that the CA1 and CA3 groups exhibited significantly lower levels of freezing than rats in the sham group on the second (CA1 vs. sham: p < 0.001; CA3 vs. sham: p < 0.05) and third (CA1 vs. sham: p < 0.01; CA3 vs. sham: p < 0.05) extinction days, but not on the first extinction day.
Figure 3.
Effects of pre-training CA1 or CA3 lesions on fear conditioning, extinction, and testing (Experiment 1). (A) Percentage of freezing during the fear conditioning session for the three groups of rats. Freezing is shown for the 3-min period prior to the first trial (baseline) and during 1-min periods after each conditioning trial. (B) Percentage of freezing during the extinction sessions for the three groups of rats. Freezing was averaged across the 1-min periods after the first four CS presentations across the 3 d of extinction. (Inset) Freezing in three-trial blocks during the first extinction session. (C) Normalized conditional freezing across the retention test (CS freezing – baseline). Rats were tested either in the extinction context (SAME; open bars) or outside of the extinction context (DIFF; filled bars). Neurotoxic CA1, CA3, or sham (SH) lesions were made prior to conditioning. All data are presented as mean ± SEM.
The significant interaction of extinction day and lesion reveals that extinction was faster in animals with CA1 or CA3 lesions. To determine whether this was also true for within-session extinction, we analyzed the freezing across the first extinction session. As illustrated in Figure 3B (inset), freezing declined significantly across the 30 CS-alone trials (indicated by three-trial blocks) (F(9,846) = 65.6, p < 0.001). Although there was a significant main effect of lesion (F(2,94) = 3.59, p < 0.05), there was not a significant interaction of lesion by trial block (F(18,846) = 1.27, p = 0.20). Given that lesions had no effect on pre-tone freezing levels, these data suggest that CA1 or CA3 lesions did not affect the within-session extinction rate.
One week after extinction, rats were placed in either the extinction context (SAME; ABB, where each letter denotes the context of conditioning, extinction, and testing) or in a different (but familiar) context (DIFF; AAB) to assess the context dependence of extinction. Figure 3C shows conditional freezing averaged across the eight post-CS minutes of the testing session; the data were normalized by subtracting pre-CS freezing levels for each rat. The ANOVA revealed a significant main effect of lesion (F(2,91) = 3.43, p < 0.05), and significant interaction of lesion and test context (F(2,91) = 3.33, p < 0.05). Planned comparisons showed that SH/DIFF rats (n = 23) had much higher freezing levels than SH/SAME rats (n = 21, p < 0.01), whereas there was no difference between the CA1/SAME (n = 21) and CA1/DIFF (n = 12) groups (p = 0.67) or between the CA3/SAME (n = 10) and CA3/DIFF (n = 10) groups (p = 0.78). Thus, renewal of fear to the auditory CS occurred when intact rats were tested outside the extinction context, and CA1 or CA3 lesions disrupted the renewal of extinguished fear.
It is worth noting that the deficits in fear renewal in rats with CA1 or CA3 lesions were not due to a performance impairment, insofar as freezing to the CS in the rats with lesion was the similar to that of shams in first extinction session (see Fig. 3B). Another possibility is that hippocampal lesions may have produced impairments in contextual processing. Such a deficit may have rendered rats with hippocampal lesions unable to discriminate the test contexts. To assess this possibility, we examined whether rats with hippocampal lesions discriminated the conditioning context from a novel context before extinction. In the present experiment, exposure to each context was equated and extinction was preceded by context exposure. Therefore, half of the rats were returned to the conditioning context (COND) and the other half were placed in a novel context (NOVEL) to equate exposure to the other (extinction) context on the first day of extinction. This allowed us to assess the effects of pre-training CA1 or CA3 lesions on contextual discrimination (conditioning context vs. novel context) before extinction by analyzing the data from the exposure sessions on the first day of extinction.
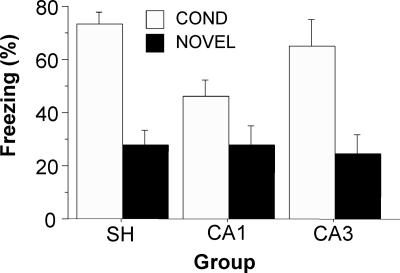
The average freezing for the first 10 min of the context exposure session on the first extinction day is shown in Figure 4. The main effect of context (F(2,91) = 2.54, p < 0.001) was highly significant. Planned comparisons (p < 0.001) showed that SH/COND and CA3/COND rats had much higher freezing levels than SH/NOVEL and CA3/NOVEL rats. Although only approaching statistical significance (p = 0.053), CA1/COND rats also had higher levels of freezing than CA1/NOVEL rats (p = 0.053). This indicates that hippocampal lesions had minimal effects on context discrimination before extinction.
Figure 4.
Effects of pre-training CA1 or CA3 lesions on context discrimination (Experiment 1). The figure shows the percentage of freezing for the first 10 min of context exposure during the first equilibration session. Exposure was either in the same context as the conditioning context (COND; open bars) or in a novel context (NOVEL; filled bars). Neurotoxic CA1, CA3, or sham (SH) lesions were made before conditioning. All data are presented as mean ± SEM.
Previous studies have reported a strong correlation between the magnitude of contextual freezing and locomotor activity produced by electrolytic dorsal hippocampal lesions (Maren and Fanselow 1997; Maren et al. 1997), and increased locomotor activity has been observed after hippocampal lesions (Bardgett et al. 1997, 2003, 2006). We examined locomotor activity, cage crossovers, rearing, and grooming during the 3-min period prior to the onset of conditioning on the first day of training. We did not observe any difference in rats with hippocampal lesions on any of these parameters (data not shown). This suggests that nonspecific effects of the lesion on locomotor activity or competing behaviors did not account for the impairments in conditional freezing observed on the retention test.
Experiment 2: Post-extinction lesions of hippocampal areas CA1 or CA3 and contextual retrieval of extinction
In Experiment 1, we found that pre-training lesions of either CA1 or CA3 disrupted the context dependence of extinction to an auditory CS. These lesions did not affect context discrimination or locomotor activity. Therefore, the data from Experiment 1 suggest that both CA1 and CA3 are required for the acquisition of context dependence of extinction.
However, it is also possible that CA1 and CA3 are involved in the retrieval or expression of extinction memories insofar as each brain area was also dysfunctional at the time of retention testing. To address this issue, we made CA1 or CA3 lesions in the dorsal hippocampus after extinction and examined the context specificity of extinction in a subsequent retention test. In this case, the rats were intact during extinction training, and any deficits during retention testing would therefore be attributable to an influence of hippocampal damage on contextual retrieval.
Histology
Figure 5 shows photomicrographs of coronal brain sections from representative rats in the sham and CA1 or CA3 lesion groups, and Figure 6 shows the schematic drawing of the minimum and maximum extent of CA1 or CA3 lesions. The histological results were similar to those in Experiment 1: NMDA infusions into either area CA1 or CA3 produced relatively selective damage to those areas, respectively. Quantification of the lesions revealed that NMDA infusions into CA1 eliminated a substantial number of the pyramidal cells in this region (mean = 85%, range = 73%–96%). These lesions also involved some cell loss in area CA3 (mean = 11%, range = 6.1%–23%) and DG (mean = 12%, range = 6%–33%). NMDA infusions into area CA3 resulted in substantial cell loss in this region (mean = 87%, range = 71%–95%). Cell loss in the other hippocampal regions was generally limited (CA1: mean = 11%, range = 0.6%–39%; DG: mean = 12%, range = 3%–28%).
Figure 5.
Representative photomicrographs showing four thionin-stained coronal sections along the septotemporal axis for each of subfield lesions in the dorsal hippocampus (Experiment 2).
Figure 6.
Schematic representation of brain lesions mapped onto coronal rat brain sections showing the minimum (black areas) and maximum (black + gray areas) extent of CA1 or CA3 lesions (Experiment 2). Coordinates are given in millimeters from bregma.
Behavior
Conditional freezing during the conditioning, extinction, and testing sessions is shown in Figure 7. Although the lesion group designations are shown in this figure, the rats did not have lesions during the conditioning or extinction sessions (the lesions were made after extinction). On the conditioning day (Fig. 7A), rats in each of the designated lesion groups exhibited similar levels of freezing and showed comparable increases in freezing as the conditioning session progressed. This was confirmed by a nonsignificant main effect lesion (F(2,63) = 3.02, p = 0.06) and a significant main effect of minute (F(7,441) = 220.55, p < 0.001). During extinction, the average freezing during the first four CS-alone presentations on each extinction day is shown in Figure 7B. All groups demonstrated similar levels of freezing, and freezing to the tone-CS declined significantly across the three extinction days. These observations were verified by a significant effect of day (F(2,126) = 127.36, p < 0.001); there was no main effect of group (F(2,63) = 0.79, p = 0.46). In addition, there was no interaction of extinction day and lesion group (F(4,126) = 0.78, p = 0.54), suggesting that the decrease in freezing across the extinction training session was equivalent for all the groups.
Figure 7.
Effects of post-extinction CA1 or CA3 lesions on fear conditioning, extinction, and testing (Experiment 2). (A) Percentage of freezing during the fear conditioning session for the three groups of rats. Freezing is shown for the 3-min period prior to the first trial (baseline) and during 1-min periods after each conditioning trial. (B) Percentage of freezing during the extinction sessions for the three groups of rats. Freezing was averaged across the 1-min periods after the first four CS presentations across the 3 d of extinction. Although lesion group designations are indicated in A and B, the rats had not undergone surgery at this point in training. (C) Normalized conditional freezing across the retention test (CS freezing − baseline). Rats were tested either in the extinction context (SAME; open bars) or outside of the extinction context (DIFF; filled bars). Neurotoxic CA1, CA3, or sham (SH) lesions were made after extinction. All data are presented as mean ± SEM.
After recovery from surgery, retrieval testing occurred either in the extinction context (SAME; ABB) or in a different context (DIFF; AAB) to assess the context dependence of extinction. Figure 7C shows conditional freezing averaged across the eight post-CS minutes of the testing session; the data were normalized by subtracting pre-CS freezing levels for each rat. The main effect of test context was highly significant (F(1,60) = 21.54, p < 0.001). The main effect of lesion (F(2,60) = 2.85, p = 0.066) and interaction of lesion and test context (F(2,60) = 3.00, p = 0.057) were just short of significance. Nonetheless, planned comparisons demonstrated that rats in the SH/DIFF group (n = 12) exhibited more freezing than those in the SH/SAME group (n = 12, p < 0.001); CA3/DIFF rats (n = 11) also exhibited more freezing than CA3/SAME rats (n = 12, p < 0.001). In contrast, there was no difference between the CA1/DIFF (n = 11) and CA1/SAME (n = 8) groups (p = 0.58). Thus, rats with CA3 lesions, like sham rats, exhibited renewal of fear when the extinguished CS was presented outside of extinguished context, whereas neurotoxic CA1 lesions made after extinction abolished the context-dependent expression of extinction.
Discussion
In the present experiments, neurotoxic CA1 or CA3 lesions in the dorsal hippocampus disrupted the context dependence of extinction when lesions were made prior to conditioning, but only CA1 lesions eliminated renewal when the lesions were made after extinction. Contextual discrimination (an index of context processing) was relatively unaffected by either CA1 or CA3 lesions, suggesting that hippocampal subfields are selectively involved in the context dependence of extinction, as opposed to the representation or discrimination of contexts per se. In addition, the renewal deficits did not appear to be secondary to a sensorimotor deficit, as rats with lesions made prior to conditioning exhibited similar levels of locomotor activity, rearing, and grooming. Lastly, hippocampal damage did not affect fear to the CS or the ability to perform the freezing response. Collectively, these results reveal a role for both CA3 and CA1 in the dorsal hippocampus in encoding the context dependence of extinction and demonstrate a selective role for CA1 in the retrieval of this form of memory. These data confirm previous reports of a role for the hippocampus in the encoding and retrieval of context dependency (Corcoran and Maren 2001, 2004; Corcoran et al. 2005; Ji and Maren 2005; Hobin et al. 2006), and extend these reports to delineate distinct roles for hippocampal areas CA1 and CA3 in these processes.
The impaired context-dependent encoding of extinction memory in rats with CA1 or CA3 lesions is in accordance with previous work showing the requirement of these two hippocampal subfields in higher-order spatial and contextual learning tasks. For instance, rats with CA3 lesions exhibit deficits in encoding of object–place and odor–place paired associations (Gilbert and Kesner 2003). Animals receiving CA3 lesions are impaired in encoding, not retrieval, on the modified Hebb–Williams maze (Jerman et al. 2006). After CA3 removal, rats exhibit deficiency in encoding of spatial memory (Brun et al. 2002; Florian and Roullet 2004). Transgenic mice lacking CA3 NMDA receptors also exhibit deficits in spatial and contextual learning (Nakazawa et al. 2003; Cravens et al. 2006). Similar to rats with CA3 lesions, rats with selective CA1 lesions display deficits in encoding of sequential odor associations (Kesner et al. 2005). Mice lacking functional NMDA receptors in CA1 also exhibit impaired spatial and contextual learning (Tsien et al. 1996). Hence, both CA1 and CA3 appear to be involved in encoding higher-order representations of stimuli or objects embedded in contexts and places.
Although CA3 or CA1 lesions made prior to conditioning impaired the contextual encoding of extinction, it is important to note that the capacity for processing contextual information was minimally affected by the hippocampal lesions. In Experiment 1, rats with pre-training lesions were able to discriminate between the context in which they were conditioned and a novel context. This is consistent with previous reports that lesions or inactivation of the dorsal hippocampus selectively impair contextual retrieval without affecting the expression of a context discrimination (Holt and Maren 1999; Ji and Maren 2005). The failure of pre-training hippocampal lesions to interfere with the retention of contextual fear after a multiple-trial conditioning procedure has also been demonstrated in other studies (Maren et al. 1997; Wiltgen et al. 2006). This suggests that encoding the memory of a context in which a CS is extinguished is dissociable from encoding (or retrieving) a context memory per se.
Nonetheless, in the present experiment, we examined contextual discrimination 1 d after conditioning, and prior to the substantial context exposure that is concomitant with extinction training. It is possible that additional context exposure and the passage of time render the contextual discrimination more dependent on the hippocampus at the time of renewal testing (Cravens et al. 2006), a possibility we are currently examining. However, the present results are inconsistent with another report showing deficits in contextual discrimination following hippocampal lesions in mice under some circumstances (Frankland et al. 1998). This discrepancy may be due to the different conditioning protocols used between the two studies. Here we conducted five conditioning trials followed by exposure trials in highly discriminable contexts prior to extinction training, whereas Frankland et al. (1998) used alternating single-trial shock sessions and shock-free sessions with less discriminable contexts. Therefore, selective hippocampal CA1/CA3 lesions are required in processing context information only when contexts are less discriminable.
In addition to influencing the context dependency of fear to an extinguished CS, hippocampal lesions accelerated extinction across days. Although conditional freezing to the auditory CS was similar in control rats and rats with hippocampal lesions during the first extinction session, rats with hippocampal lesions showed progressively less conditional freezing to the CS in subsequent extinction sessions. Accelerated extinction was therefore not due to a performance impairment, insofar as rats with hippocampal damage exhibited high levels of freezing to the CS during the first extinction session (see Fig. 3B). Similar results have been observed after complete electrolytic lesions of the dorsal hippocampus (Ji and Maren 2005). These results contrast with a recent inactivation study showing that muscimol infusions into the dorsal hippocampus retard extinction of fear to an auditory CS, at least during a single massed-extinction session (Corcoran et al. 2005). The reason for this discrepancy is not clear, although differences between permanent lesions and temporary inactivation may be responsible.
Another possible account for the impairments in fear renewal is a reduction in contextual fear in rats with CA1 or CA3 lesions. This account seems unlikely because rats with CA3 lesions, for example, exhibited relatively normal contextual fear (prior to extinction) and yet showed substantial renewal deficits during the post-extinction renewal test. Moreover, the test context in which rats were expected to renew their fear (context B) is not one in which they had ever received footshock (context A). So it seems unlikely that different levels of fear to the conditioning context or differential extinction of contextual fear in rats with hippocampal lesions would influence later renewal. Indeed, Bouton (1993) has argued that contextual fear is not necessary for fear renewal after extinction. Hippocampal manipulations also produce deficits in three-context designs in which animals are never exposed to the original conditioning context after conditioning occurs (e.g., Corcoran and Maren 2001; Corcoran et al. 2005). Hence, it would appear unlikely that deficits in fear renewal in rats with hippocampal lesions are due to a loss or accelerated extinction of context fear.
Interestingly, when CA1 or CA3 lesions were made after extinction, there was a selective deficit in fear renewal with CA1 lesions alone; contextual retrieval of fear extinction was normal in rats with CA3 lesions. These data suggest that hippocampal areas CA1 and CA3 have different roles in encoding and retrieving context-dependent fear memories. Whereas CA1 is required for both of these processes (Bevilaqua et al. 2003; Marti Barros et al. 2004; Akbari et al. 2006, 2007), CA3 appears to have a more selective role in encoding the context dependency of extinction and is not required for the expression of this memory. The differential effect of CA3 lesions on the contextual encoding versus contextual retrieval of fear to an extinguished CS is surprising insofar as CA1 receives a major excitatory input from CA3. However, CA3 lesions have been reported to have greater effects on encoding of fear to context (Lee and Kesner 2004), and animals with CA3 lesions are more deficient in encoding spatial information in a Hebb-Williams maze when within-day versus between-day indices of performance were used to infer encoding and retrieval processes, respectively (Jerman et al. 2006). Moreover, others have reported that mice lacking NMDA receptors in hippocampal area CA3 exhibit normal retention of contextual fear over a 24-h interval, despite showing transient impairments in contextual discrimination shortly after conditioning (Cravens et al. 2006). Although these studies did not explicitly examine pre- versus post-training lesions of hippocampal subfields to isolate the role of these areas in memory encoding and retrieval, respectively, our experiments also appear to support a differential role for CA3 in the encoding versus retrieval of context-dependent memory. Nonetheless, other work shows that CA3 is involved in memory retrieval under some conditions (Brun et al. 2002; Nakazawa et al. 2002; Day et al. 2003).
It is important to note that the CA1 or CA3 lesions in the present study are restricted in the dorsal hippocampus. A recent study shows that ventral hippocampal inactivation also impairs the contextual modulation of fear memory retrieval (Hobin et al. 2006), suggesting that the ventral hippocampus, like the dorsal hippocampus, may enable contextual cues to retrieve the appropriate CS association. Maren and Holt (2004) have argued that the ventral hippocampus may serve as a conduit for the transfer of contextual information between the dorsal hippocampus and the amygdala. Therefore, it is possible that spared hippocampal subfields in each lesion group still communicate with the rest of the brain via preserved ventral hippocampal tissue to support the context dependency of fear extinction. This might account for normal expression of renewal in rats with post-extinction lesion of CA3.
Many theoretical models of hippocampal function have proposed different roles for the hippocampal subfields in memory encoding and retrieval. According to most computational models (Treves and Rolls 1992, 1994; Rolls and Treves 1994; McClelland and Goddard 1996; Wiebe et al. 1997), CA3 is posited to participate in the rapid encoding of new information insofar as its recurrent excitatory projections from an auto-associative memory network are optimized for this function. It has been hypothesized that CA3 interacts with CA1 to recode and stabilize information in neocortex, therefore serving the retrieval of information from long-term memory (Rolls and Kesner 2006; Treves and Rolls 1992, 1994). Direct connections between the entorhinal cortex and hippocampal area CA1 may then support memory retrieval in the absence of hippocampal area CA3. Hence, this model accounts for the fact that both hippocampal areas CA1 and CA3 are involved in encoding the context in which a CS is extinguished, but that the retrieval of this information ultimately becomes independent of CA3. Moreover, preliminary work from our laboratory indicates a role for entorhinal cortex in both the encoding and retrieval of context-dependent extinction memories (Ji and Maren 2006). Thus, it appears likely that connections between CA1 and the entorhinal cortex are sufficient for memory retrieval in the absence of CA3.
In conclusion, the present experiments demonstrate that both hippocampal areas CA1 and CA3 contribute to the context dependence of extinguished fear. CA1 and CA3 are both required for contextual encoding of extinction, whereas area CA1 is essential for context-dependent retrieval. Interestingly, CS-elicited spike firing in the lateral nucleus of the amygdala, which is a neural correlate of fear memory (Quirk et al. 1995; Collins and Pare 2000; Maren 2000; Repa et al. 2001; Goosens et al. 2003; Maren and Quirk 2004), shows contextual modulation after extinction (Hobin et al. 2003), and this modulation requires the functional hippocampus (Maren and Hobin 2007). Further work is required to determine how hippocampal CA1 and CA3 modulate lateral amygdala neuronal activity during fear conditioning and extinction to contextualize memories of traumatic events.
Materials and Methods
We conducted two experiments examining the consequences of hippocampal damage on the encoding and retrieval of context-dependent fear to an extinguished CS in rats. These experiments differed only with respect to when hippocampal lesions were made relative to training. In Experiment 1, hippocampal lesions were made prior to fear conditioning, and in Experiment 2, the lesions were made after extinction.
Subjects
The subjects were 232 (Experiment 1, n = 128; Experiment 2, n = 104) adult male Long-Evans rats (200–224 g) obtained from a commercial supplier (Harlan Sprague Dawley). After arrival, the rats were housed individually in stainless-steel hanging cages on a 14:10 h light/dark cycle (lights on at 7:00 a.m.) and were allowed unlimited access to food and water. After being housed, the rats were handled (10–20 sec per rat per day) for 5 d to habituate them to the experimenter.
Surgery
In Experiment 1, rats underwent surgical procedures before any behavioral testing, whereas in Experiment 2, these surgical procedures were performed after extinction training (see below). Rats were treated with atropine methyl nitrate (0.3 mg/kg, i.p.), anesthetized with sodium pentobarbital (Nembutal; 65 mg/kg, i.p.), and mounted in a stereotaxic apparatus (Kopf Instruments). The scalp was incised and retracted, and the head was positioned to place bregma and lambda in the same horizontal plane. Small burr holes (2-mm diameter) were drilled bilaterally in the skull for the placement of lesions in either area CA1 (Experiment 1, n = 48; Experiment 2, n = 40) or area CA3 (Experiment 1, n = 32; Experiment 2, n = 40) of the dorsal hippocampus. Other rats received sham surgery (Experiment 1, n = 48; Experiment 2, n = 24) for which the scalp was incised and burr holes drilled, but no injections were made in the brain. The coordinates for lesions of area CA1 were (a) 3.6 mm posterior to bregma; 1.0, 2.0, 3.0 lateral to midline; and 1.9 ventral to dura. Area CA3 coordinates were (a) 2.5 mm posterior to bregma, 2.6 mm lateral to midline, and 3.2 mm ventral from dura; (b) 3.3 mm posterior to bregma, 3.3 mm lateral to midline, and 3.2 mm ventral from dura; (c) 4.1 mm posterior to bregma, 4.2 mm to midline, and 3.1 mm from dura. Neurotoxic lesions in CA1 and CA3 were made with NMDA (N-methyl-D-aspartic acid; 20 μg/μL; Sigma) in 100 mM phosphate-buffered saline (PBS, pH = 7.4). Pairs of injection cannulae (28-gauge), which were attached with polyethylene tubing to 10-μL Hamilton syringes mounted in an infusion pump (Harvard Apparatus), were lowered into each targeted region. NMDA was then slowly infused (0.07 μL/min; 0.1–0.15 μL/site for CA1; 0.1–0.2 μL/site for CA3) into the targeted pyramidal cell layer.
Behavioral apparatus
Eight identical observation chambers (30cm × 24cm × 21cm; MED-Associates) were used in all experiments. The chambers were constructed from aluminum (side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and were situated in sound-attenuating cabinets located in a brightly lit and isolated room. The floor of each chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center to center). Rods were wired to a shock source and solid-state grid scrambler (MED-Associates) for the delivery of footshock USs. A speaker mounted outside a grating in one wall of the chamber was used for the delivery of acoustic CSs. Closed-circuit videocameras mounted above each chamber were used to videotape the behavior of each rat. Sensory stimuli were adjusted within these chambers to generate two distinct contexts. For the first context, a 15 W house light mounted opposite the speaker was turned on, and room lights remained on. The chambers were cleaned with a 1% acetic acid solution, and stainless steel pans containing a thin film of the same solution were placed underneath the grid floors to provide a distinct odor before the rats were placed inside. Ventilation fans in each cabinet supplied background noise (65 dB, A scale). Rats were transported to this context in white plastic boxes. For the second context, all room and chamber houselights were turned off; a pair of 40 W red lights provided illumination. Additionally, the doors on the sound-attenuating cabinets were closed, the ventilation fans were turned off, and the chambers were cleaned with a 1% ammonium hydroxide solution. To provide a distinct odor, stainless steel pans containing a thin film of this solution were placed underneath the grid floors before the rats were placed inside. Rats were transported to this context in black plastic boxes.
Procedure
Rats received neurotoxic lesions in either CA1 or CA3 of the dorsal hippocampus, or sham surgery. This resulted in three lesion groups: CA1 lesion (CA1), CA3 lesion (CA3), and sham (SH) groups. All rats were submitted to three experimental phases: fear conditioning, extinction, and testing. In Experiment 1, surgery was conducted 7 d prior to fear conditioning, and in Experiment 2, surgery was conducted 24 h after extinction. For these behavioral procedures, “context A” refers to the context in which fear conditioning occurred, while “context B” refers to a neutral context. The actual contexts in which the experimental phases occurred were counterbalanced across groups. For fear conditioning, rats were transported in squads of eight and placed in the conditioning chambers; chamber position was counterbalanced for each squad. The rats received five tone (10 sec; 80 dB; 2 kHz)-footshock (1 sec; 1 mA) trials (70 sec intertrial interval) beginning 3 min after being placed in context A. Sixty seconds after the final shock, the rats were returned to their home cages.
Twenty-four hours after the conditioning session, rats were assigned to two groups: one group was extinguished in the original conditioning context (context A) and another group extinguished in a novel context (context B). The extinction phase lasted for 3 d. To equate exposure to the two contexts in each group of animals, the rats were first placed in the context that was different from their designated extinction context, but were not presented with any CSs. The extinction training and exposure sessions were each 38 min in duration. During extinction training, rats were presented with 30 tone presentations (10 sec; 80 dB; 2 kHz; 60 sec ISI) without the footshock US, beginning 3 min after placement in the context.
Seven days after extinction (to allow for surgical recovery in Experiment 2), all the rats were returned to a neutral context (context B) for renewal testing. Testing consisted of a continuous CS presentation (80 dB; 2 kHz; 8 min) beginning 2 min after rats were placed in the context. The squads were counterbalanced for both lesion and test context. For each experiment, this yielded a total of six groups in a 3 × 2 (lesion × test context) design: SH/SAME, SH/DIFF, CA1/SAME, CA1/DIFF, CA3/SAME, and CA3/DIFF. The labels SAME and DIFF refer to whether the CS was tested in the same context as extinction (SAME) or in a different context (DIFF).
We assessed fear to the tone CS by measuring freezing behavior as described previously (Maren 1998). Freezing was quantified by computing the number of observations for each rat that had a value less than the freezing threshold (a load cell value below which animals are observed to be freezing) (Maren 1998). To avoid counting momentary inactivity as freezing, we only scored an observation as freezing if it fell within a continuous group of at least five observations that were all less than the freezing threshold. Thus, freezing was only scored if the rat was immobile for at least 1 sec. For each session, the freezing observations were transformed to a percentage of total observations.
Histology
Histological verification of the lesions was performed after behavioral testing. Rats were perfused across the heart with physiological saline followed by a 10% formalin solution. After extraction from the skull, brains were post-fixed in 10% formalin solution for 2 d, at which time the solution was replaced with a 10% formalin/30% sucrose solution until sectioning. Sections (50 μm thick) were cut on a cryostat (−19°C), wet-mounted on microscope slides, and stained with 0.25% thionin for visualization of the lesions.
Scan area quantification of lesions
The damage to cell layers in hippocampal subregions was measured by computed-based scan area MCID (Multiple-line Caller Identification) analysis. Due to the large number of animals in the study, we randomly selected a subset of each lesion group (n = 6) for detailed analysis. Briefly, the boundaries of each cell layer in dorsal hippocampal sections (bregma 2.0 mm–4.8 mm) were digitized and calculated. The CA2 region was regarded as a part of CA3 for convenience, because, in many respects, CA2 resembles a terminal portion of the CA3 region (Amaral and Witter 1989). Next, the boundaries of the lesion in each cell layer were calculated. The percentage of damage to each subregion of the hippocampus was calculated by comparing the average size of the lesion with the average size of each subregion.
Data analysis
After histological examination, 97 rats were included in the final data analysis in Experiment 1 [sham (n = 44), CA1 (n = 33) and CA3 (n = 20)], and 66 rats were included in the final data analysis in Experiment 2 [sham (n = 24), CA1 (n = 19), and CA3 (n = 23)]. For each behavioral session, the freezing data were transformed to a percentage of the total observations, a probability estimate that is amenable to analysis with parametric statistics. These probability estimates of freezing were analyzed using ANOVA. Post hoc comparisons in the form of Fisher’s PLSD tests were performed after a significant omnibus F-ratio. All data are represented as mean ± SEM.
Acknowledgments
This research was supported by a grant from the NIH (R01MH065961) to S.M. The authors thank Stephanie Jimenez for commenting on an earlier draft of the manuscript and Stephanie Jimenez and Richard Barnes for assisting with video scoring.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.794808.
References
- Akbari E., Naghdi N., Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Akbari E., Naghdi N., Motamedi F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 2007;28:650–656. doi: 10.1016/j.peptides.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Witter M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Bardgett M.E., Jacobs P.S., Jackson J.L., Csernansky J.G. Kainic acid lesions enhance locomotor responses to novelty, saline, amphetamine, and MK-801. Behav. Brain Res. 1997;84:47–55. doi: 10.1016/s0166-4328(96)00132-5. [DOI] [PubMed] [Google Scholar]
- Bardgett M.E., Boeckman R., Krochmal D., Fernando H., Ahrens R., Csernansky J.G. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Res. Bull. 2003;60:131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bardgett M.E., Griffith M.S., Foltz R.F., Hopkins J.A., Massie C.M., O’Connell S.M. The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiol. Learn. Mem. 2006;85:86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bevilaqua L.R., Kerr D.S., Medina J.H., Izquierdo I., Cammarota M. Inhibition of hippocampal Jun N-terminal kinase enhances short-term memory but blocks long-term memory formation and retrieval of an inhibitory avoidance task. Eur. J. Neurosci. 2003;17:897–902. doi: 10.1046/j.1460-9568.2003.02524.x. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context and behavioral processes in extinction. Learn. Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Brun V.H., Otnass M.K., Molden S., Steffenach H.A., Witter M.P., Moser M.B., Moser E.I. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Collins D.R., Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS+ and CS−. Learn. Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn. Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K.A., Desmond T.J., Frey K.A., Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J. Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens C.J., Vargas-Pinto N., Christian K.M., Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur. J. Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- Daumas S., Halley H., Frances B., Lassalle J.M. Encoding, consolidation, and retrieval of contextual memory: Differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn. Mem. 2005;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: Implications for animal models of anxiety. Trends Pharmacol. Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Day M., Langston R., Morris R.G. Glutamate-receptormediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- Florian C., Roullet P. Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav. Brain Res. 2004;154:365–374. doi: 10.1016/j.bbr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Frankland P.W., Cestari V., Filipkowski R.K., McDonald R.J., Silva A.J. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Frohardt R.J., Guarraci F.A., Bouton M.E. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav. Neurosci. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gilbert P.E., Kesner R.P. Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behav. Neurosci. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Good M., Honey R.C. Conditioning and contextual retrieval in hippocampal rats. Behav. Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Good M., de Hoz L., Morris R.G. Contingent versus incidental context processing during conditioning: Dissociation after excitotoxic hippocampal plus dentate gyrus lesions. Hippocampus. 1998;8:147–159. doi: 10.1002/(SICI)1098-1063(1998)8:2<147::AID-HIPO7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Goosens K.A., Hobin J.A., Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: Mnemonic code or fear bias? Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Hobin J.A., Goosens K.A., Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin J.A., Ji J., Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holt W., Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J. Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey R.C., Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav. Neurosci. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- Jerman T., Kesner R.P., Hunsaker M.R. Disconnection analysis of CA3 and DG in mediating encoding but not retrieval in a spatial maze learning task. Learn. Mem. 2006;13:458–464. doi: 10.1101/lm.246906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn. Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Maren S. In Abstract of the Neuroscience Society Meeting. Atlanta, GA: 2006. Fornix and entorhinal cortex are essential for renewal of fear memory after extinction; pp. 46–49. [Google Scholar]
- Kennedy P.J., Shapiro M.L. Retrieving memories via internal context requires the hippocampus. J. Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner R.P., Hunsaker M.R., Gilbert P.E. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav. Neurosci. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee I., Kesner R.P. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Lee I., Yoganarasimha D., Rao G., Knierim J.J. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Leutgeb S., Leutgeb J.K., Barnes C.A., Moser E.I., McNaughton B.L., Moser M.B. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Frackowiak R.S., Frith C.D. Learning to find your way: A role for the human hippocampal formation. Proc. Biol. Sci. 1996;26:1745–1750. doi: 10.1098/rspb.1996.0255. [DOI] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S., Fanselow M.S. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol. Learn. Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S., Hobin J.A. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn. Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav. Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S., Holt W. Hippocampus and Pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav. Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S., Aharonov G., Fanselow M.S. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Marti Barros D., Ramirez M.R., Dos Reis E.A., Izquierdo I. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. doi: 10.1016/j.neuroscience.2004.03.010. [DOI] [PubMed] [Google Scholar]
- McClelland J.L., Goddard N.H. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mineka S., Mystkowski J.L., Hladek D., Rodriguez B.I. The effects of changing contexts on return of fear following exposure therapy for spider fear. J. Consult. Clin. Psychol. 1999;67:599–604. doi: 10.1037//0022-006x.67.4.599. [DOI] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Mystkowski J.L., Craske M.G., Echiverri A.M., Labus J.S. Mental reinstatement of context and return of fear in spider-fearful participants. Behav. Ther. 2006;37:49–60. doi: 10.1016/j.beth.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Quirk M.C., Chitwood R.A., Watanabe M., Yeckel M.F., Sun L.D., Kato A., Carr C.A., Johnston D., Wilson M.A., et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Sun L.D., Quirk M.C., Rondi-Reig L., Wilson M.A., Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Pavlov I.P. Conditioned reflexes. G.V. Anrep. Oxford University Press; London: 1927. [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Repa C., LeDoux J.E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rauhut A.S., Thomas B.L., Ayres J.J. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: Implications for treating human phobias. J. Exp. Psychol. Anim. Behav. Process. 2001;27:99–114. [PubMed] [Google Scholar]
- Repa J.C., Muller J., Apergis J., Desrochers T.M., Zhou Y., LeDoux J.E. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat. Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Kesner R.P. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Treves A. Neural networks in the brain involved in memory and recall. Prog. Brain Res. 1994;102:335–341. doi: 10.1016/S0079-6123(08)60550-6. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., O’Reilly R.C. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn. Affect. Behav. Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Treves A., Rolls E.T. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tsien J.Z., Huerta P.T., Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Wiebe S.P., Staubli U.V., Ambros-Ingerson J. Short-term reverberant memory model of hippocampal field CA3. Hippocampus. 1997;7:656–665. doi: 10.1002/(SICI)1098-1063(1997)7:6<656::AID-HIPO7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wilson A., Brooks D.C., Bouton M.E. The role of the rat hippocampal system in several effects of context in extinction. Behav. Neurosci. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wiltgen B.J., Sanders M.J., Anagnostaras S.G., Sage J.R., Fanselowand M.S. Context fear learning in the absence of the hippocampus. J. Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]