Abstract
Cyclic AMP signaling plays a central role in regulating activity at a number of synapses in the brain. We showed previously that pairing activation of receptors that inhibit adenylate cyclase (AC) and reduce the concentration of cyclic AMP, with elevation of the concentration of cyclic GMP is sufficient to elicit a presynaptically expressed form of LTD at Schaffer collateral-CA1 synapses in the hippocampus. To directly test the role of AC inhibition and G-protein signaling in LTD at these synapses, we utilized transgenic mice that express a mutant, constitutively active inhibitory G protein, Gαi2, in principal neurons of the forebrain. Transgene expression of Gαi2 markedly enhanced LTD and impaired late-phase LTP at Schaffer collateral synapses, with no associated differences in input/output relations, paired-pulse facilitation, or NMDA receptor-gated conductances. When paired with application of a type V phosphodiesterase inhibitor to elevate the concentration of intracellular cyclic GMP, constitutively active Gαi2 expression converted the transient depression normally caused by this treatment to an LTD that persisted after the drug was washed out. Moreover, this effect could be mimicked in control slices by pairing type V phosphodiesterase inhibitor application with application of a PKA inhibitor. Electrophysiological recordings of spontaneous excitatory postsynaptic currents and two-photon visualization of vesicular release using FM1-43 revealed that constitutively active Gαi2 tonically reduced basal release probability from the rapidly recycling vesicle pool of Schaffer collateral terminals. Our findings support the hypothesis that inhibitory G-protein signaling acts presynaptically to regulate release, and, when paired with elevations in the concentration of cyclic GMP, converts a transient cyclic GMP-induced depression into a long-lasting decrease in release.
Long-term potentiation (LTP) and long-term depression (LTD) of synaptic strength are reciprocal, activity-dependent mechanisms that are thought to mediate synaptic competition during development and store information in mature networks. cAMP and its major effector kinase, cyclic AMP-dependent protein kinase (PKA), play key roles in the induction of LTP (Frey et al. 1993; Impey et al. 1996; Nguyen and Kandel 1997; Otmakhova et al. 2000; Matsushita et al. 2001). Evidence also suggests that inhibition of adenylate cyclase (AC) and reduced PKA activity promote the induction of LTD. Inhibiting PKA enhances the induction of LTD at Schaffer collateral-CA1 synapses (Santschi et al. 1999, 2006), and simultaneous elevation of the concentration of intracellular cGMP and inhibition of PKA is sufficient to elicit LTD at these synapses in the absence of afferent stimulation (Santschi et al. 1999; Stanton et al. 2001). This chemically induced form of LTD (CLTD) is presynaptically expressed and occludes stimulus-evoked LTD (SLTD), suggesting convergence of key mechanisms between CLTD and SLTD (Santschi et al. 1999; Stanton et al. 2001, 2003; Bailey et al. 2003).
A number of presynaptic receptors, including groups II/III metabotropic glutamate receptors (mGluR) and A1 adenosine receptors, are negatively coupled to AC via inhibitory heterotrimeric G-proteins. We recently showed that activating either of these receptors can promote the induction of LTD at Schaffer collateral-CA1 synapses, and that pairing activation of either of these receptors with elevations in the concentration of cyclic GMP is sufficient to elicit LTD (Santschi et al. 2006). At mossy fiber-CA3 synapses, we also showed that expression of a mutant, constitutively active form of an inhibitory G alpha subunit, Gαi2, can substitute for the actions of group II mGluRs in regulating synaptic plasticity at this synapse (Nicholls et al. 2006). However, unlike mossy fiber synapses, Schaffer collateral-CA1 synapses express a mixture of both pre- and postsynaptic alterations underlying differing forms of LTP and LTD (Reyes and Stanton 1996; Stanton and Gage 1996; Patterson et al. 2001; Duffy and Nguyen 2003; Huang et al. 2005).
To test whether inhibitory G-protein regulation of AC also contributes to the induction of LTD at Schaffer collateral-CA1 synapses in the hippocampus, and whether these actions may be, in part, presynaptic in nature, we examined synaptic plasticity at Schaffer collateral synapses in slices from transgenic mice that express an inducible, constitutively active form of Gαi2. We found that constitutively active Gαi2 expression caused a tonic inhibition of presynaptic release of FM1-43 from the rapidly recycling vesicle pool at Schaffer collateral terminals and enhanced stimulus-evoked LTD. Furthermore, Gαi2 converted the transient depression elicited by elevating the concentration of cyclic GMP to a persistent LTD, suggesting that inhibitory G-protein signaling participates in both short- and long-term regulation of presynaptic activity.
Results
Constitutively active Gαi2 does not alter synaptic input/output relations, paired-pulse facilitation, or the NMDA component of transmission at Schaffer collateral-CA1 synapses
To mimic G-protein-mediated inhibition of adenylate cyclase in vivo, we utilized transgenic mice that express a constitutively active form of the heterotrimeric G-protein alpha subunit, Gαi2 (Nicholls et al. 2006). By crossing animals bearing a tetO-Gαi2 transgene with animals bearing a second transgene in which the tTA synthetic trans-activator is under the control of the calcium/calmodulin kinase IIα promoter, we could drive constitutively active Gαi2 expression selectively in principal cells of the forebrain, including CA3 and CA1 pyramidal cells in the hippocampus (Nicholls et al. 2006). Since doxycycline blocks the binding of the tTA transactivator to the synthetic tetO promoter, we also used this system to prevent Gαi2 transgene expression during development. As we showed previously, raising pregnant mothers and their pups on doxycycline-containing food until 10 d of age effectively suppressed transgene expression (Nicholls et al. 2006). By shifting these animals off doxycycline 10 d after birth, we then induced transgene expression in animals by 15 d of age. We used this “shift off doxycycline” protocol in all of our experiments to temporally restrict the expression of the constitutively active Gαi2 transgene.
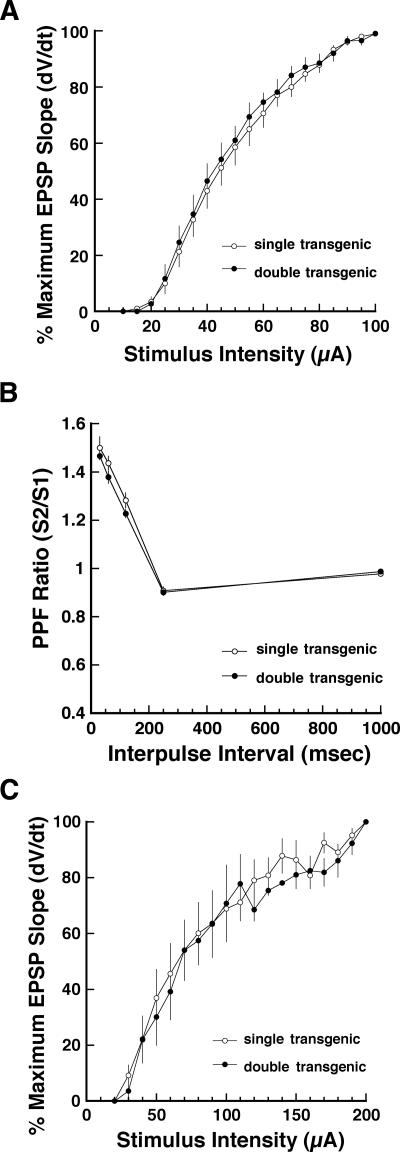
To evaluate the effects of constitutively active Gαi2 expression on basal synaptic transmission at Schaffer collateral-CA1 synapses, we compared field excitatory postsynaptic potential (fEPSP) input–output relations, paired-pulse facilitation, and NMDA receptor-mediated fEPSPs in slices from Gαi2-expressing double transgenic mice to single transgenic controls. Since synaptic responses in slices from tetO-Gαi2 and CaMKIIα-tTA single transgenic mice were similar in all experiments, data from these two groups were pooled throughout and are referred to simply as “controls”. As shown in Figure 1A, input/output relations (maximum fEPSP slope vs. stimulus intensity) at Schaffer collateral-CA1 synapses in hippocampal slices from double transgenic mice were similar to controls (P > 0.20; two-way ANOVA with repeated measures), suggesting that constitutively active Gαi2 did not affect low-frequency synaptic transmission. As a measure of presynaptic function, we compared paired-pulse facilitation at interstimulus intervals of 20, 50, 100, 200, and 1000 msec in slices from double-transgenic and control mice. As seen in Figure 1B, constitutively active Gαi2 also did not affect this property of Schaffer collateral synapses (P > 0.20; two-way ANOVA with repeated measures).
Figure 1.
Constitutively active Gαi2 does not alter input–output relations or paired-pulse facilitation at Schaffer collateral-CA1 synapses. (A) Input–output relation of Schaffer collateral-CA1 evoked EPSP slopes (percent maximum) as a function of stimulus intensity (μA) in Gαi2-expressing double-transgenic (●) versus pooled single-transgenic slices (◦). Each point is mean ± SEM of six slices. (B) Percent paired-pulse facilitation profiles (S2/S1) at Schaffer collateral-CA1 evoked EPSPs as a function of interstimulus interval in slices from Gαi2-expressing (●) and single-transgenic control (◦) mice. Each point is mean ± SEM of six slices. (C) Input–output relation of NMDA receptor-dependent evoked EPSP slopes (percent maximum) pharmacologically isolated by bath-applied CNQX (10 μM), in Gαi2-expressing double-transgenic (●) versus pooled single-transgenic slices (◦). Each point is mean ± SEM of six slices.
Since the induction of some forms of LTP and LTD depend on the activation of N-methyl-D-aspartate receptors (NMDARs), we tested the effect of constitutively active Gαi2 on NMDAR-mediated fEPSPs that were pharmacologically isolated by bath application of 10 μM CNQX to block AMPA receptor conductances and 10 μM picrotoxin to block GABAergic conductances. Figure 1C illustrates input/output functions of NMDA receptor-mediated responses at Schaffer collateral-CA1 synapses in hippocampal slices from constitutively active Gαi2-expressing double transgenic mice and controls. The responses in these two groups were similar for each of the stimulus intensities tested (P > 0.20; two-way ANOVA with repeated measures), suggesting that constitutively active Gαi2 does not alter NMDA receptor-mediated synaptic transmission at Schaffer collateral-CA1 synapses.
Constitutively active Gαi2 suppresses stimulus-evoked LTP at Schaffer collateral-CA1 synapses
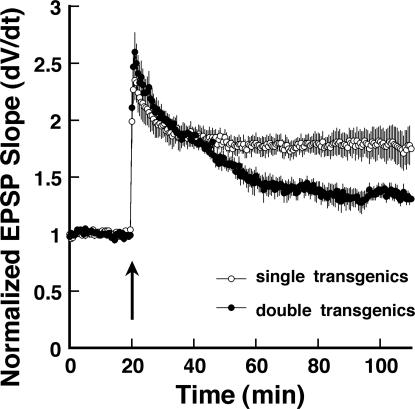
A large body of evidence has implicated a pathway involving adenylate cyclase, cAMP, and protein kinase A in the late phase of LTP at hippocampal Schaffer collateral synapses (Nguyen and Woo 2003). The activity of this pathway can be regulated in part by the actions of G-protein coupled receptors (GPCRs) whose activation has been variously shown to enhance or inhibit the induction of LTP (Winder et al. 1999; Santschi et al. 2006.). In the case of inhibitory GPCRs, this is thought to be mediated by inhibitory G alpha subunits that are capable of inhibiting the activity of certain adenylate cyclase isoforms, leading to a decrease in cAMP concentration and PKA activity (Simonds 1999; Chern 2000). Therefore, we decided to test the ability of the constitutively active Gαi2 transgene to affect the late phase of LTP. Figure 2 illustrates LTP induced at Schaffer collateral synapses by four theta burst trains (TBS; each train 10 × 100 Hz/five pulse bursts, 200 msec interburst interval) in slices from double transgenic and control mice. In control slices, this protocol elicited a stable LTP that persisted for over 2 h (180 ± 11% of baseline response 1 h post-TBS). Slices from double transgenic animals exhibited a similar magnitude of potentiation immediately after stimulation (short-term potentiation [STP]); however, this STP decayed rapidly within the first hour post-tetanus to significantly lower LTP levels than those observed in controls (134 ± 9% of baseline response 1 h post-TBS; P < 0.05, Student's t-test compared with controls). This observation is consistent with previous observations of the effects of protein kinase A and adenylate cyclase mutants (Abel et al. 1997; Wong et al. 1999) on the late phase of LTP at Schaffer collateral synapses, and suggests that constitutively active Gαi2-mediated inhibition of this pathway is sufficient to interfere with its role in late-phase LTP.
Figure 2.
Stimulus-evoked long-term potentiation (LTP) at Schaffer collateral-CA1 synapses is impaired in slices from mice expressing constitutively active Gαi2. Time course of experiments comparing theta burst-evoked LTP (arrow; 4× theta burst stimulation) at Schaffer collateral-CA1 synapses in slices from Gαi2-expressing double-transgenic (●; n = 12) versus pooled single-transgenic mice (◦; n = 15). Each point is mean ± SEM of n slices.
Constitutively active Gαi2 enhances stimulus-evoked LTD at Schaffer collateral-CA1 synapses
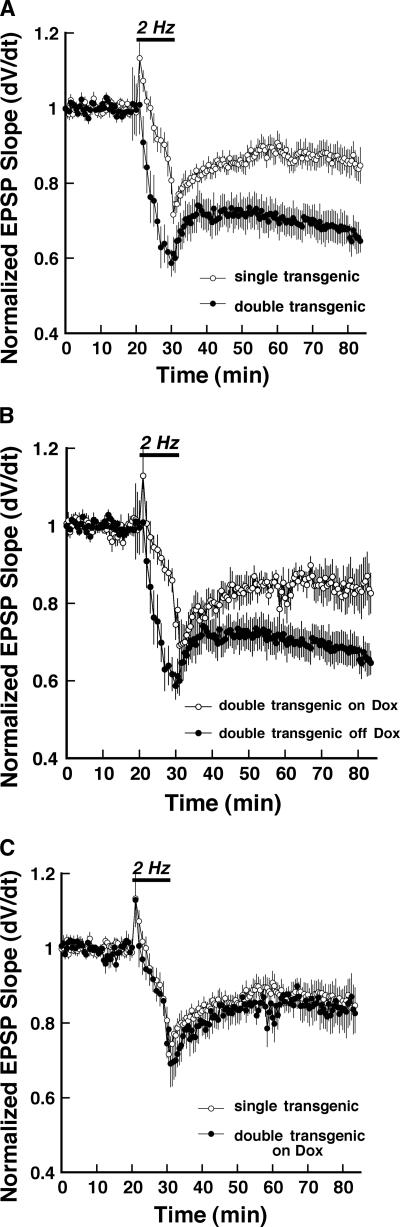
LTP and LTD are opposing processes, and manipulations that affect one of these forms of synaptic plasticity often have mirror-image effects on the other (Migaud et al. 1998; Zeng et al. 2001; Chen et al. 2003). Given this fact, and previous work showing that decreases in the activity of the cAMP pathway can enhance the induction of LTD at Schaffer collateral synapses (Kameyama et al. 1998; Santschi et al. 2006; but see Brandon et al. 1995; Qi et al. 1996), we tested whether constitutively active Gαi2 expression might also result in an enhancement of this form of synaptic plasticity. Figure 3A shows the time course of LTD in field CA1 induced by 2 Hz/10 min low-frequency stimulation (LFS) of Schaffer collateral axons in slices from double transgenic and control animals. We found that constitutively active Gαi2 expression more than doubled the amplitude of LTD elicited at Schaffer collateral-CA1 synapses as compared with controls (−35.4 ± 3.4% of pre-LFS baseline compared with –15.3 ± 4.8% in controls, 30 min after the end of LFS, P < 0.05, Student’s t-test). This enhancement was dependent on the expression of the constitutively active Gαi2 transgene, since it was not observed in slices from double-transgenic animals when expression was suppressed by continued administration of doxycycline (Fig. 3B, open circles, −17.4 ± 5.2%, 30 min post-LFS, P < 0.05; Student’s t-test double-transgenic animals on vs. off doxycycline). In fact, LTD in slices from double-transgenic mice maintained on doxycycline was indistinguishable from LTD in slices from control mice (Fig. 3C).
Figure 3.
Stimulus-evoked long-term depression (LTD) at Schaffer collateral-CA1 synapses is enhanced in slices from mice expressing constitutively active Gαi2. (A) Time course of experiments comparing stimulus-evoked LTD (solid bar; 2 Hz/10 min) at Schaffer collateral-CA1 synapses in slices from Gαi2-expressing double-transgenic (●; n = 11) versus pooled single-transgenic control mice (◦; n = 14). Each point is mean ± SEM of n slices. (B) Time course of stimulus-evoked LTD (solid bar; 2 Hz/10 min) at Schaffer collateral-CA1 synapses in Gαi2-expressing double-transgenics 5 d after being taken off doxycycline (off Dox; ●; n = 11), compared with double transgenics where expression was suppressed by doxycycline (on Dox; ◦; n = 5). Each point is mean ± SEM of n slices. (C) Time course of stimulus-evoked LTD (solid bar; 2 Hz/10 min) at Schaffer collateral-CA1 synapses in slices from single-transgenic control mice (◦; n = 14), compared with slices from Gαi2-expressing double transgenics where expression remained blocked by doxycycline (on Dox; ●; n = 5). Each point is mean ± SEM of n slices.
Constitutively active Gαi2 partially occludes A1 adenosine receptor-mediated depression of synaptic transmission
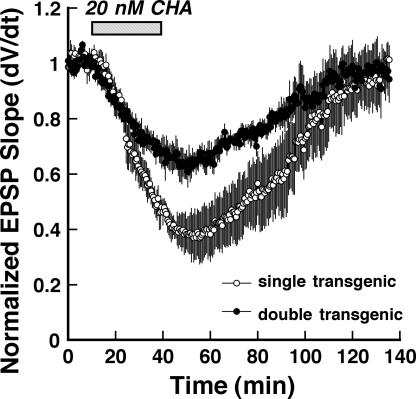
One of the ways in which the cAMP pathway contributes to LTD at Schaffer collateral synapses is by acting postsynaptically to control the PKA-dependent phosphorylation of GluR1 subunit-containing AMPA receptors (Kameyama et al. 1998). However, LTD at this synapse also involves changes in presynaptic activity that may be mediated, in part, by the presynaptic actions of the cAMP pathway. Since we previously observed that agonists of G protein-coupled receptors can promote Schaffer collateral LTD (Santschi et al. 2006), we tested the ability of the constitutively active Gαi2 transgene to substitute for inhibitory G protein-coupled receptor activation in other contexts. To do this, we compared the ability of the A1 adenosine receptor agonist, cyclohexyladenosine (CHA), to suppress responses at Schaffer collateral synapses in slices from constitutively active Gαi2-expressing mice to slices from control animals. A1 receptors are expressed presynaptically and are coupled to inhibitory heterotrimeric G proteins. The activation of these receptors leads to a well-characterized suppression of Schaffer collateral synaptic transmission that results from a decrease in presynaptic transmitter release (Mitchell et al. 1993; Dunwiddie and Diao 1994). We reasoned that if these receptors participate in this process via inhibitory G alpha subunits, then expression of the constitutively active Gαi2 transgene might mimic the effects of A1 adenosine receptor activation. As shown in Figure 4, 20 nM CHA evoked significantly less peak depression of Schaffer collateral-CA1 synaptic transmission in Gαi2-expressing slices (−35.8 ± 3.4%), compared with control slices (−63 ± 8.8%; P < 0.05, Student's t-test Gαi2 compared with control slices), suggesting that transgene-mediated inhibition of AC partially occluded the effects of A1 adenosine receptor activation.
Figure 4.
Adenosine receptor-mediated G protein-dependent depression of synaptic transmission at Schaffer collateral-CA1 synapses is impaired in Gαi2-expressing mice. Time course of experiments comparing the effects of the A1 adenosine receptor agonist cyclohexyladenosine (CHA; 20 nM; hatched bar) on Schaffer collateral-evoked EPSPs in slices from Gαi2 double transgenics (●) versus pooled single-transgenic slices (◦). Each point is mean ± SEM of four slices.
Two-photon FM1-43 imaging of Schaffer collateral terminals demonstrates reduced vesicular release in constitutively active Gαi2-expressing mice
The ability of the constitutively active Gαi2 transgene to partially occlude A1 adenosine receptor-mediated suppression of Schaffer collateral responses indicates that the transgene does, in fact, affect presynaptic activity at this synapse, even though we did not observe transgene-dependent changes in paired-pulse facilitation or input/output relations. In many instances, previous studies of genetically modified animals have failed to detect changes in these measures despite strong evidence that the transgene or knockout affected presynaptic activity (Castillo et al. 1997, 2002; Cabin et al. 2002; Hou et al. 2004). This discrepancy is thought to result from mechanisms that compensate for the effects of altered gene expression and renormalize mean synaptic efficacy. In these instances, PPF and input/output relations may be imperfect tools for detecting presynaptic alterations caused by chronic alterations in gene expression. We decided to directly test the effects of constitutively active Gαi2 transgene expression on presynaptic activity by measuring presynaptic release in brain slices from these animals using FM1-43 labeling of synaptic vesicles, and by analyzing miniature excitatory postsynaptic currents (mEPSCs).
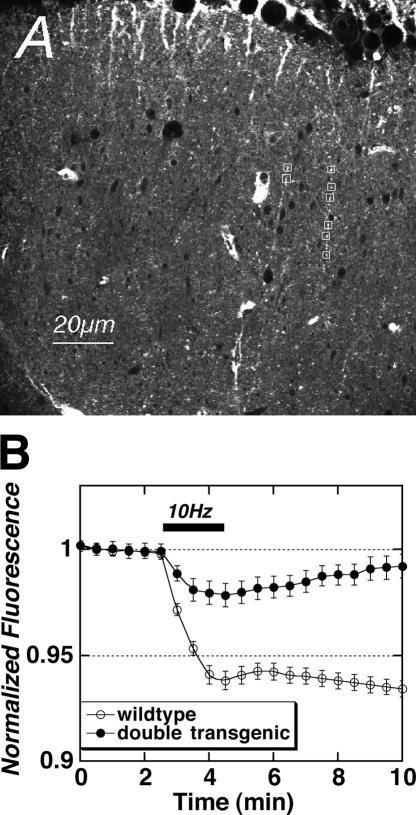
We began by examining the dynamic properties of vesicular release using two-photon imaging of stimulus-evoked release of the styryl dye FM1-43 from Schaffer collateral terminals in hippocampal slices (Stanton et al. 2001, 2003, 2005; Winterer et al. 2006). Figure 5A illustrates a typical two-photon image of FM1-43 labeled presynaptic boutons in stratum radiatum of field CA1 of a hippocampal slice. Figure 5B plots the mean destaining time courses of stimulus-evoked (10 Hz/2 min) FM1-43 release from Schaffer collateral presynaptic terminals in slices from wild-type control versus constitutively active Gαi2-expressing mice. While control slice terminals exhibited a 6.2 ± 0.44% reduction (closed circles) in FM1-43 fluorescence at the end of the 2-min LFS, constitutively active Gαi2-expressing double transgenic slices showed significantly less stimulus-evoked release (open circle, 2.2 ± 0.55%, P < 0.05, Student’s t-test compared with control slices), suggesting that tonic Gαi2-mediated inhibition of adenylate cyclase does reduce baseline-evoked transmitter release probability from Schaffer collateral boutons.
Figure 5.
Constitutively active Gαi2 reduces stimulus-evoked vesicular release from Schaffer collateral presynaptic terminals. (A) Typical two-photon image of Schaffer collateral presynaptic puncta, samples marked with white squares, labeled with FM1-43 in stratum radiatum of a hippocampal slice; 800 nm excitation, >600 nm emission filtering. (B) Time-course of stimulus-induced reductions in FM1-43 bouton fluorescence evoked by a 10 Hz/2 min train of stimulation (solid bar) applied to Schaffer collateral axons in slices from wild-type (◦) and Gαi2-expressing double-transgenic (●) mice. Each point is mean ± SEM of 20–24 puncta per slice from four wild-type and five double-transgenic mice.
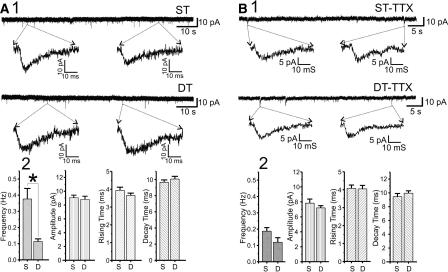
Constitutively active Gαi2 reduces frequency of spontaneous action potential-driven mEPSCs at Schaffer collateral synapses
To independently test the effects of constitutively active Gαi2 expression on presynaptic release probability at Schaffer collateral terminals, we examined TTX-sensitive and TTX-insensitive spontaneous mEPSCs in CA1 pyramidal neurons in slices from Gαi2-expressing and control mice (Fig. 6). Approximately half of mEPSC events were TTX sensitive, and half TTX resistant. TTX-sensitive mEPSCs driven by spontaneous action potentials were markedly reduced in frequency by constitutively active Gαi2 expression (Fig. 6A1, 2; *, P < 0.05, Student’s t-test compared with control slices), without any changes in peak amplitude, rise, or decay time kinetics (Fig. 6A2), consistent with the presynaptic reduction in probability of vesicular release we observed using FM1-43. In contrast, TTX-insensitive mEPSCs, representative of action potential-independent release events, showed no significant alterations in either frequency or waveform resulting from Gαi2 expression (Fig. 6B). While the selective effects of constitutively active Gαi2 on TTX-sensitive mEPSCs suggests that release from the rapidly-recycling pool of vesicles driven by single-action potentials is persistently altered by inhibition of adenylate cyclase, we cannot rule out the possibility that the frequency of spontaneous action potentials in CA3 pyramidal neurons might be altered as well.
Figure 6.
Constitutively active Gαi2 reduces frequency, but not amplitude or kinetics, of TTX-sensitive spontaneous mEPSCs, without altering TTX-insensitive mEPSCs. (A1) Sample mEPSCs recorded from CA1 pyramidal neurons voltage clamped at –55 mV in the absence of TTX. The top line is a representative segment of EPSC recordings from a pyramidal neuron in a slice from a single-transgenic control animal (ST), while the bottom is a similar representative segment from a slice from a Gαi2-expressing double-transgenic mouse (DT). (A2) Comparison of the mean ± SEM frequency (Hz), amplitude (pA), rise and decay times (ms) of mEPSCs in pyramidal neurons from single transgenic control (S) and Gαi2-expressing double transgenic (D) mice. Only frequency was significantly reduced by Gαi2 expression (*, P < 0.05, one-way ANOVA) (B1) Sample mEPSCs recorded from CA1 pyramidal neurons voltage clamped at –55 mV in the presence of 1 μM TTX. The top line is a representative segment of EPSC recordings from a pyramidal neuron in a slice from a single-transgenic control animal (ST-TTX), while the bottom is a similar representative segment from a slice from a Gαi2-expressing double-transgenic mouse (DT-TTX). (B2) Comparison of the mean ± SEM frequency (Hz), amplitude (pA), rise and decay times (ms) of mEPSCs in pyramidal neurons from single-transgenic (S) and Gαi2-expressing double-transgenic (D) mice.
However, the pairing of reduced basal transmitter release with the lack of alteration in synaptic input/output relations supplies evidence for postsynaptic compensatory up-regulation of transmitter sensitivity as a likely mechanism that normalizes overall synaptic strength during development in Gαi2-expressing mice.
Constitutively active Gαi2 converts cyclic-GMP mediated transient depression of Schaffer collateral synaptic transmission to LTD
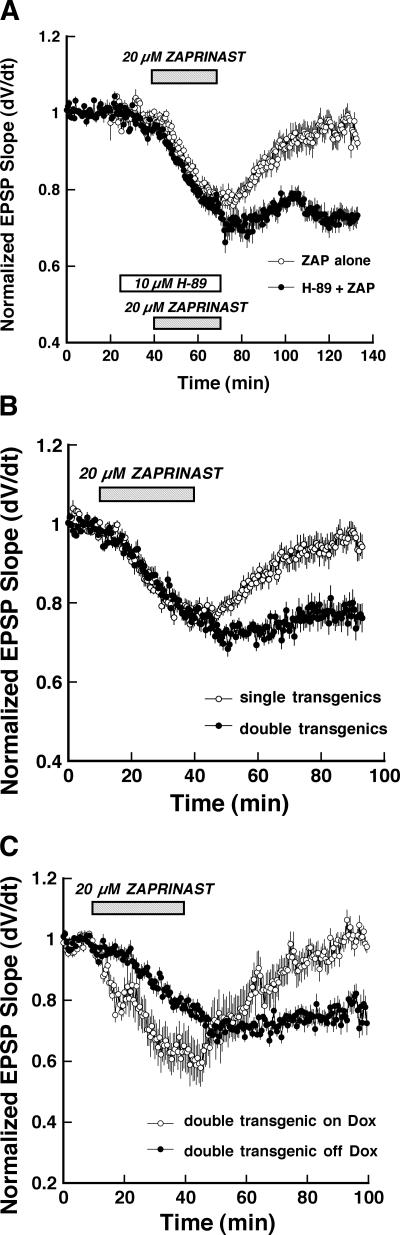
In previous studies in rat hippocampal slices, we showed that a chemical LTD of synaptic transmission was elicited by simultaneous elevation of the concentration of intracellular cyclic GMP and inhibition of PKA (Santschi et al. 1999). To confirm that this was also true in mice, we co-applied the PKA inhibitor, H-89, along with the type V phosphodiesterase inhibitor, zaprinast, to control slices. Figure 7A illustrates the effects of bath application of zaprinast (20 μM; solid bar) in the presence of the PKA inhibitor H-89 (10 μM; hatched bar), on Schaffer collateral-CA1 synaptic strength. The pairing of zaprinast and H-89 elicited significant LTD of synaptic transmission that persisted after drug washout (closed circles, −26.7 ± 2.7% 1 h post-washout), while zaprinast alone evoked only transient depression (open circles, −7.8 ± 1.7% 1 h post-washout).
Figure 7.
Elevating the concentration of cyclic GMP elicits transient depression of Schaffer collateral synaptic transmission in control mice, but LTD in mice expressing constitutively active Gαi2. (A) Time course comparing the effect of bath application of zaprinast alone (ZAP, 20 μM, hatched bar; n = 7) on Schaffer collateral-CA1 evoked EPSCs in slices from single-transgenic control mice (◦), to chemical LTD induced by combining zaprinast with the selective PKA inhibitor H-89 (H-89, 10 μM, open bar; ●; n = 6). Each point is mean ± SEM of n slices. (B) Time course of the effect of bath application of zaprinast (ZAP, 20 μM, hatched bar) on Schaffer collateral-CA1 evoked EPSPs in slices from Gαi2-expressing double-transgenic (●; n = 9) versus pooled single-transgenic slices (◦; n = 7). Each point is mean ± SEM of n slices. (C) Time course of the effect of bath application of zaprinast (ZAP, 20 μM, hatched bar) on Schaffer collateral-CA1 evoked EPSCs in slices from Gαi2 double-transgenic mice taken off doxycycline 5 d earlier (off Dox; ●; n = 9) versus slices from double-transgenic mice where expression was blocked by doxycycline (on Dox; ◦; n = 4) Each point is mean ± SEM of n slices.
We showed previously that this form of chemically induced LTD is the result of a persistent decrease in presynaptic activity, and moreover, its induction depends on the inhibition of presynaptic PKA activity (Santschi et al. 1999). Therefore, we tested the ability of the constitutively active Gαi2 transgene to participate in this form of LTD to determine whether the presynaptic actions of the transgene contribute to persistent decreases in synaptic strength. As above, bath application of zaprinast produced only a transient depression of Schaffer collateral responses in slices from control animals that returned to baseline following washout of the drug (Fig. 7B, open circles). In contrast, zaprinast application to slices prepared from constitutively active Gαi2-expressing double transgenic animals produced a long-term depression that persisted for over 1 h after drug washout (−23.3 ± 3.3% reduction in EPSP slope 50 min post-wash; P < 0.05 Student’s t-test comparison to single transgenic controls). As was the case for the enhancement of stimulus-induced LTD in these animals, this effect was dependent on the expression of constitutively active Gαi2, since it was not observed in slices from double-transgenic mice where expression was suppressed by continued administration of doxycycline (Fig. 7C; open circles, P < 0.05, Student’s t-test, double-transgenic animals on vs. off doxycycline).
Discussion
Activity-dependent changes in synaptic strength are essential for maintaining proper nervous system function, and storing information during learning acquisition and memory retention. Studies in a number of animals and synapses have found that signaling via the cAMP pathway plays an important role in these changes. To better understand how this pathway regulates synaptic activity, we examined the physiological consequences of expressing, in transgenic mice, a mutant Gαi2 transgene that constitutively inhibits susceptible forms of adenylate cyclase. We found that constitutively active Gαi2 expression enhanced stimulus-induced LTD at Schaffer collateral-CA1 synapses without altering input/output relations, paired-pulse facilitation, or the NDMAR-mediated component of postsynaptic potentials. Constitutively active Gαi2 also partially occluded the suppression of Schaffer collateral responses by the A1 adenosine receptor agonist, cyclohexyladenosine, suggesting that the transgene tonically suppressed presynaptic activity. Consistent with this interpretation, we found that constitutively active Gαi2 caused a decrease in vesicle release as measured by stimulus-evoked FM1-43 destaining, and a decrease in spontaneous action potential-evoked mEPSC frequency. In addition, we found that constitutively active Gαi2 expression, in combination with a pharmacologically induced increase in the concentration of cGMP, was sufficient to elicit a presynaptically expressed form of LTD. Together, our data suggest that inhibitory G-protein signaling regulates presynaptic activity, and participates in the induction of a presynaptic, cyclic GMP-dependent form of LTD.
Inhibition of the cAMP pathway has been found previously to enhance, and in some cases mimic, LTD at Schaffer collateral-CA1 synapses (Kameyama et al. 1998; Santschi et al. 1999, 2006). One of the ways in which this is thought to occur is through protein kinase A-dependent regulation of AMPA receptor activity in the postsynaptic neuron. Inhibiting postsynaptic PKA activity causes a rundown of AMPA currents that occludes some forms of stimulus-induced LTD, and this is thought to reflect both the role of basal GluR1 phosphorylation in maintaining AMPA receptor currents (Roche et al. 1996; Banke et al. 2000), and the role of AMPA receptor dephosphorylation in postsynaptic LTD (Kameyama et al. 1998). In addition to these postsynaptic functions of cAMP, we found that activation of presynaptic inhibitory G-protein coupled receptors enhanced stimulus-induced LTD, suggesting that decreases in the activity of the cAMP pathway in the presynaptic cell can also contribute to LTD at Schaffer collateral-CA1 synapses (Santschi et al. 1999, 2006). Therefore, the ability of the constitutively active Gαi2 transgene to partially occlude the suppression of Schaffer collateral responses by A1 adenosine receptor activation suggests that one of the ways in which this transgene may enhance stimulus-induced LTD is through inhibition of adenylate cyclase in presynaptic terminals.
A presynaptically expressed form of LTD can be chemically induced at Schaffer collateral-CA1 synapses by increasing presynaptic cGMP concentration while simultaneously decreasing presynaptic cAMP pathway activity (Santschi et al. 1999). We showed previously that either A1 adenosine or group II mGluR activation is sufficient to elicit the decrease in cAMP pathway activation necessary for this form of LTD (Santschi et al. 2006), and here we show that constitutively active Gαi2 transgene expression also converts the transient depression of synaptic transmission elicited by increased cGMP concentration into a persistent depression. The ability of this form of chemically induced depression to occlude LTD induced by LFS suggests that these two forms of LTD may share common induction and/or expression mechanisms. If this is the case, then the activation of inhibitory G alpha subunits by presynaptic G-protein coupled receptors during LFS may provide a necessary decrease in the concentration of cAMP. In support of this hypothesis, we found that antagonists of either A1 adenosine receptors or group II mGluRs inhibit stimulus-induced Schaffer collateral-CA1 LTD (Santschi et al. 2006).
If presynaptic inhibitory G-protein-coupled receptor activation in response to LFS is responsible for decreasing cAMP pathway activity, then what mechanism triggers the increase in the concentration of cGMP that is also necessary for presynaptic LTD? Our previous work suggests that the requisite increase in the concentration of cGMP is brought about by a signaling cascade activated by nitric oxide (NO) released from the postsynaptic neuron. This diffusible molecule could then act as a retrograde messenger, diffusing to the presynaptic terminal, where it activates guanylyl cyclase (Gage et al. 1997), leading to increased cGMP concentration and activation of PKG (Reyes-Harde et al. 1999). This model of LTD induction is attractive, because the existence of an intercellular second messenger provides a mechanism for coordinating the pre- and postsynaptic changes that occur during activity-dependent decreases in synaptic efficacy.
Just as evidence suggests that decreases in the concentration of cAMP act both pre- and postsynaptically to promote Schaffer collateral LTD, data also suggest that both pre- and postsynaptic increases in the concentration of cAMP can contribute to Schaffer collateral LTP. While postsynaptic infusion of PKA inhibitors has been found to block late-phase LTP (Duffy and Nguyen 2003), the application of the nonhydrolyzable cAMP analog, Sp-cAMPs, elicits a persistent increase in transmission that involves increased presynaptic activity (Bolshakov et al. 1997; Ma et al. 1999; Yu et al. 2001). Here too, NO has been proposed to act as a retrograde messenger that elicits presynaptic changes involved in Schaffer collateral LTP (Zhuo et al. 1994; Arancio et al. 1995; Lu et al. 1999). The apparently contradictory findings that retrograde signals mediated by NO may participate in both Schaffer collateral LTD and LTP raise the question of whether an additional signal determines whether NO leads to transient depression, LTD, or LTP in the presynaptic compartment. Our current finding that constitutively active Gαi2 expression both enhances LTD and inhibits LTP suggests that one factor controlling the outcome may be presynaptic cAMP concentration. This mechanism seems ideal to explain the contradictory data supporting roles for NO-stimulated cyclic GMP in the induction of both LTP and LTD, and suggests that evoked increases in both the concentration of glutamate, acting via group II/III mGluRs, and the concentration of adenosine, acting via A1 receptors, are transmitters that respond to elevations in local synaptic activity levels by shifting synapses in favor of greater LTD. Such a mechanism could represent a physiological basis for the dynamic “sliding threshold” for the induction of LTD versus LTP (Stanton 1996), an activity-dependent biasing of synapses toward induction of presynaptic LTD.
As attractive as this model is, it should be pointed out that there is much to be learned about the mechanisms underlying the involvement of NO in Schaffer collateral presynaptic plasticity. First, pharmacological and genetic evidence has suggested that it is still possible to elicit LTP when NO signaling is impaired (Cummings et al. 1994; Son et al. 1996), suggesting that NO may only be required under some conditions or for certain forms of LTP. Second, what appears to be normal LTP has been observed in PKG knockout mice (Kleppisch et al. 1999), suggesting that, if NO signaling is required for LTP, it may act via a PKG-independent pathway, perhaps involving ADP-ribosyltransferase (Schuman et al. 1994). Hopper and Garthwaite (2006) have recently supplied evidence that both tonic and phasic increases in the concentration of NO, mediated by different nitric oxide synthase isoforms, are involved in the induction of LTP, but their roles in LTD are currently unknown. Given the current uncertainty surrounding the mechanisms underlying the involvement of NO in synaptic plasticity, the cAMP-dependent conversion from potentiation to depression we propose remains one of a number of possible explanations for the involvement of NO in bidirectional synaptic plasticity at Schaffer collateral synapses.
It is also still unclear what are the downstream targets of inhibitory G alpha subunits to regulate presynaptic activity, and how these targets might interact with an NO/cGMP signaling cascade. Two likely immediate targets of inhibitory G alpha subunits are the adenylate cyclase isoforms AC1 and AC8. Both of these isoforms are sensitive to inhibition by Gi-coupled receptors (Nielsen et al. 1996) and mice lacking both of these genes exhibit deficient LTP at Schaffer collateral synapses (Wong et al. 1999). G alpha-mediated inhibition of adenylate cyclase would be expected to lead to a decrease in the concentration of cAMP, and a corresponding decrease in PKA activity. As mentioned above, PKA has been found to play an important postsynaptic role in Schaffer collateral LTP and LTD, but recent evidence also suggests that PKA-dependent phosphorylation of presynaptic substrates may be involved in these forms of plasticity. The synaptic vesicle protein Rab3A and its effector protein Rim1α are both believed to regulate presynaptic activity downstream of PKA, and an examination of the phenotypes of animals lacking these gene products reveals deficits in PKA-dependent forms of LTP at Schaffer collateral synapses (Huang et al. 2005). However, there are a number of additional presynaptic PKA substrates that might also regulate activity in response to changes in the concentration of cAMP. The ability of the cAMP pathway to interact with changes in the concentration of presynaptic cGMP means that a complete understanding of the mechanisms underlying the regulation of presynaptic activity will require not just understanding the function of these two pathways in isolation, but how they interact to bring about long-term changes in synaptic strength.
Materials and Methods
Transgenic mice and doxycycline-controlled transgene expression
The generation of constitutively active Gαi2 transgenic mice and the regulation of transgene expression by doxycycline was reported previously (Nicholls et al. 2006). Briefly, a mutant form of Gαi2 was generated that codes for a cysteine to arginine amino acid substitution at residue 179. This substitution interferes with the ability of the mutant protein to hydrolyze GTP, rendering it constitutively active (Wong et al. 1991; Pace et al. 1995). We used the tetO/tTA system to achieve region-restricted, drug-regulated expression of the constitutively active Gαi2 transgene by placing the Gαi2 transgene under the control of the synthetic tetO promoter (Mayford et al. 1996). This promoter is activated in a drug-dependent manner by the synthetic transactivator, tTA. We achieved cell-type specificity by placing the tTA transactivator under the control of the calcium-calmodulin kinase IIα (CaMKIIα) promoter in a second transgene. In animals that carried both the tetO-Gαi2 and the CaMKIIα-tTA transgenes, constitutively active Gαi2 transgene expression was observed only in principal cells in the forebrain (where the CaMKIIα promoter is active) (Nicholls et al. 2006). We achieved temporally restricted Gαi2 transgene expression by raising mothers and pups on food containing doxycycline until the pups were 10 d old, at which point mice were switched to food without doxycycline. This on doxycycline/off doxycycline regimen prevented the expression of constitutively active Gαi2 in pups prior to 15 d of age. In some control experiments, we suppressed Gαi2 transgene expression in the tetO-Gαi2; CaMKIIα-tTA double transgenic animals by continuing to feed animals doxycycline-containing food. We have previously shown that dopamine-stimulated adenylate cyclase activity is significantly inhibited in these mice when taken off doxycycline at 10 d of age, compared with both single transgenic controls and double transgenics maintained on doxycycline throughout (Nicholls et al. 2006).
Slice preparation
tetO-Gαi2; CamKIIα-tTA double-transgenic, single-transgenic, and wild-type control mice of mixed sex, 15–21 d of age, were decapitated under deep ether anesthesia, and the hippocampus plus entorhinal cortex dissected free from surrounding tissue and placed immediately in ice-cold artificial cerebrospinal fluid (ACSF) consisting of: 126 mM NaCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, and 10 mM D-glucose, gassed with 95% O2/ 5% CO2 (pH 7.2–7.4). For extracellular field potential recordings, 400 μm-thick transverse slices were cut using a vibrating tissue slicer (Vibroslice, Camden Instruments), and transferred to an interface recording chamber maintained at 33°C and continuously perfused with ACSF (3 mL/min), where they were incubated for a minimum of 90 min prior to recording. For whole-cell patch recordings from single CA1 pyramidal neurons, slices were incubated for 1–6 h in oxygenated ACSF at room temperature prior to transfer to a submerged recording chamber continuously perfused with room temperature ACSF (5 mL/min).
Electrophysiological recordings
Extracellular population fEPSP recordings were made using glass microelectrodes (2–3 MΩ when filled with ACSF) placed in stratum radiatum of the CA1 region under visual guidance, to a depth of 100–150 μm. Bipolar stainless-steel stimulation electrodes (Frederick Haer) were placed in stratum radiatum to activate Schaffer collateral/commissural afferents. For baseline recordings, synaptic inputs were stimulated once per min (150 μsec square DC pulse). Baseline stimulus strength (10–100 μA) was adjusted in order to elicit a response ≈50% of the maximum fEPSP amplitude prior to the generation of a population action potential, and monitored for at least 30 min prior to induction of LTD/LTP. Slices in which there was a drift in baseline of >5% during this period were excluded from further analysis. Synaptic strength was quantified by measuring the maximum slope of the initial falling phase of the fEPSP, using a six-point interpolation least-squares linear regression analysis, which marched along the response until the maximum value was retrieved. Electrical signals were collected with an Axoclamp-2A amplifier (Axon Instruments) filtered at 1 kHz, sampled at 10 kHz, and digitized on an IBM clone computer using DataWave Technologies software to acquire and analyze data on-line.
Whole-cell patch-clamp recordings (Axoclamp 700B, Axon Instruments) were obtained from CA1 pyramidal neurons visualized using infrared differential interference contrast optics on a Zeiss Axioskop FS upright microscope. The intracellular patch pipette filling solution contained: 130 mM CsMeSO4, 4 mM NaCl, 10 mM HEPES, 0.5 mM EGTA, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 2 mM QX-314 (pH 7.25; 280–290 mOsm; 4–6 MΩ). EPSCs were acquired at 5 kHz sample frequency and filtered at 1 kHz with an eight-pole low-pass Bessel filter. After whole-cell configuration was established, membrane potential was held at −55 mV. Recordings with leak currents of <−100 pA or series resistances >25 MΩ were discarded. Spontaneous miniature EPSCs (mEPSCs) were recorded in 10 μM bicuculline, and automatically detected using minianalysis software (Synaptosoft). RMS noise level for each recording was calculated from a segment of data without obvious EPSCs, and the event detection threshold set to four times the RMS noise level. A period-to-search-maximum length of 10 msec was used, and if a local maximum was detected, the previous –12 to −8 msec was used to set the baseline. If peak amplitude of the local maximum minus the average baseline was greater than or equal to detection threshold, peak amplitude, rise, and decay times were calculated. Time to peak was calculated as the time from the first data point 0.5% of peak amplitude to peak amplitude. Decay time was calculated as the time from the first point after peak to the point of 37% of peak amplitude. All program-identified EPSCs were confirmed by visual inspection before inclusion in results.
LTD/LTP stimulation induction protocols
LTD was induced using a prolonged train of low-frequency stimuli (LFS), which consisted of 1200 × 150 μsec duration DC square pulses at a frequency of 2 Hz, for a total stimulation period of 10 min. In some experiments where LTD was electrically evoked, two separate inputs were isolated, verified as such by a lack of paired-pulse cross-interactions (50 msec interstimulus interval), and one input served as a control to verify input specificity of LTD. At the end of some experiments, reversibility of LTD was verified by inducing LTP. LTP was induced in naïve and LTD slices by a theta burst stimulation protocol (TBS) consisting of 10 bursts (five pulse/100 Hz each), at a frequency of 5 Hz, repeated four times, 15-sec apart. Changes in synaptic strength after LFS, TBS, or drug addition, were normalized to the pretreatment baseline in the same slice before averaging across slices.
Two-photon imaging of FM1-43 release
After confirming the presence of Schaffer collateral-evoked fEPSPs >1 mV in amplitude in CA1 stratum radiatum, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was bath applied to prevent synaptically driven action potentials in CA3 pyramidal neurons from accelerating dye release. Presynaptic boutons were loaded by bath applying 5 μM FM1-43 (Molecular Probes) in 45 mM [K+] ACSF for 10 min, then returning to normal ACSF. Stimulus-induced destaining was measured after 30 min perfusion with dye-free ACSF, by a 2-min train of 10 Hz bipolar stimuli (150 μsec DC pulses).
FM1-43 fluorescence of single release sites was evoked by two-photon excitation, and visualized using a Leica DM LFS E upright microscope with water immersion ultraviolet objective APO L 63x/0.90 W and a Leica multispectral confocal laser scan unit. The light source was a Millenia 5 W diode laser source pumping a Tsunami Ti:sapphire laser (Spectra-Physics) that provided ≈130-fsec pulses at 82 MHz, tuned to 840-nm center wavelength. Epifluorescence was detected with photomultiplier tubes of the confocal laser scan head with pinhole maximally opened and emission spectral window optimized for signal over background (560–660 nm). Kinetic data were recorded with bandpass-filtered nondescanned photomultiplier tubes behind the objective and a 1.3 numerical aperture oil condenser, optimized for signal over background (540–600 nm) based on spectral analyses (Winterer et al. 2006). Laser intensity was controlled with a variable beam splitter exploiting polarization of the laser light and neutral density filters. Though there were no signs of photodamage, we always used the lowest intensity necessary for adequate signal-to-noise ratio.
Using Leica TCS MP software, 512 × 512 pixel images were acquired, 0.15 μm/pixel in the x-y axes. In offline analyses, rectangular regions of interest (ROI) (∼2–4 μm2) were defined around the center of bright, punctate fluorescence spots, and 12–16 boutons and three to four background fields measured in each slice. If lateral displacement of a bouton beyond the ROI occurred, that bouton data set was discarded. Only puncta that showed stimulus-dependent unloading were included in the analysis (∼90% of puncta fulfilled this criteria). All fields imaged were within the first 100 μm depth in the slice, typically between 25 and 60 μm deep, and were from 40–60 μm away from the stimulating electrode poles. A fluorescence time course was generated by normalizing each ROI time course by dividing by starting intensity, averaging the background fields to produce a dye bleaching time course (2 h bleaching 12.1 ± 1.0%), and then dividing each bouton ROI by bleaching at corresponding time points throughout the experiment. Vertical bars denote SEM for the average of all normalized and corrected boutons across experiments.
Statistical analyses
Values of LTD and LTP were calculated as the change in fEPSP slope measured at least 1 h post-treatment. Summary data are presented as mean ± SEM at each 1-min time point throughout the experiment, with the vertical bars representing the SEM. The significance of differences between group means was calculated using a Student's t-test for unpaired data, significance before and after induction of LTD within slices with a paired t-test, with significance level preset to P < 0.05. Reported n is number of slices throughout, and typically one, but no more than two slices per mouse were used.
Chemicals
All drugs were stored frozen as stock solutions 100–1000 times the desired final concentration, and thawed and diluted immediately before addition to the perfusion ACSF. Drugs were used at the indicated final concentrations: cyclohexyladenosine (CHA; Sigma), 20 nM; 6-cyano-7-nitroquinoxalone-2,3-dione (CNQX; Tocris), 10 μM; 1 mM; H-89, (Biomol) 10 μM; Zaprinast, (Sigma) 20 μM; Picrotoxin, (Sigma) 10 μM. CNQX and H-89 were dissolved in DMSO stock solutions, with the final concentration of DMSO applied to slices always ≤0.1%. When these drugs were used, control experiments used equal concentrations of DMSO alone, which had no effect on the induction of LTD or LTP.
Acknowledgments
This work is dedicated to the memory of Lewis N. Stanton Sr., Gary L. Stanton, and John M. Sarvey. Supported by the Alexander von Humboldt Foundation and National Institutes of Health Grant R01-NS44421 (P.K.S.), National Institutes of Mental Health Conte Center Grant MN50733 (E.R.K.), and Deutsche Forschungsgemeinschaft Grant Mu 809/7-2 (W.M.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.810208.
References
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Arancio O., Kandel E.R., Hawkins R.D. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Bailey C.P., Trejos J.A., Schanne F.A.X., Stanton P.K. Pairing elevation of [cyclicGMP] with inhibition of PKA produces long-term depression of glutamate release from isolated rat hippocampal presynaptic terminals. Eur. J. Neurosci. 2003;17:903–908. doi: 10.1046/j.1460-9568.2003.02507.x. [DOI] [PubMed] [Google Scholar]
- Banke T.G., Bowie D., Lee H., Huganir R.L., Schousboe A., Traynelis S.F. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov V.Y., Golan H., Kandel E.R., Siegelbaum S.A. Recruitment of new sites of synaptic transmission during the cAMP-dependent late phase of LTP at CA3-CA1 synapses in the hippocampus. Neuron. 1997;19:635–651. doi: 10.1016/s0896-6273(00)80377-3. [DOI] [PubMed] [Google Scholar]
- Brandon E.P., Zhuo M., Huang Y.Y., Qi M., Gerhold K.A., Burton K.A., Kandel E.R., McKnight G.S., Idzerda R.L. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin D.E., Shimazu K., Murphy D., Cole N.B., Gottschalk W., McIlwain K.L., Orrison B., Chen A., Ellis C.E., Paylor R., et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J. Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P.E., Janz R., Sudhof T.C., Tzounopoulos T., Malenka R.C., Nicoll R.A. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo P.E., Schoch S., Schmitz F., Sudhof T.C., Malenka R.C. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chen A., Muzzio I.A., Malleret G., Bartsch D., Verbitsky M., Pavlidis P., Yonan A.L., Vronskaya S., Grody M.B., Cepeda I., et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Chern Y. Regulation of adenylyl cyclase in the central nervous system. Cell. Signal. 2000;12:195–204. doi: 10.1016/s0898-6568(99)00084-4. [DOI] [PubMed] [Google Scholar]
- Cummings J.A., Nicola S.M., Malenka R.C. Induction in the rat hippocampus of long-term potentiation (LTP) and long-term depression (LTD) in the presence of a nitric oxide synthase inhibitor. Neurosci. Lett. 1994;176:110–114. doi: 10.1016/0304-3940(94)90883-4. [DOI] [PubMed] [Google Scholar]
- Duffy S.N., Nguyen P.V. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J. Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T.V., Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J. Pharmacol. Exp. Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- Frey U., Huang Y.Y., Kandel E.R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gage A.T., Reyes M., Stanton P.K. Nitric oxide-guanylyl-cyclase-dependent and independent components of multiple forms of long-term synaptic depression. Hippocampus. 1997;7:286–295. doi: 10.1002/(SICI)1098-1063(1997)7:3<286::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hopper R.A., Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J. Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Gao X., Zhang X., Kong L., Wang X., Bian W., Tu Y., Jin M., Zhao G., Li B., et al. SNAP-25 in hippocampal CA1 region is involved in memory consolidation. Eur. J. Neurosci. 2004;20:1593–1603. doi: 10.1111/j.1460-9568.2004.03600.x. [DOI] [PubMed] [Google Scholar]
- Huang Y.Y., Zakharenko S.S., Schoch S., Kaeser P.S., Janz R., Sudhof T.C., Siegelbaum S.A., Kandel E.R. Genetic evidence for a protein kinase-A mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc. Natl. Acad. Sci. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S., Mark M., Villacres E.C., Poser S., Chavkin C., Storm D.R. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Lee H.K., Bear M.F., Huganir R.L. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kleppisch T., Pfeifer A., Klatt P., Ruth P., Montkowski A., Fassler R., Hofmann F. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP-dependent protein kinases is normal and susceptible to inhibition of nitric oxide synthase. J. Neurosci. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.F., Kandel E.R., Hawkins R.D. Nitric oxide signaling contributes to late-phase LTP and CREB phosphrylation in the hippocampus. J. Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Zablow L., Kandel E.R., Siegelbaum S.A. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nat. Neurosci. 1999;2:24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Tomizawa K., Morikawa A., Li S.T., Terada H., Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J. Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M., Bach M.E., Huang Y.Y., Wang L., Hawkins R.D., Kandel E.R. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Migaud M., Charlesworth P., Dempster M., Webster L.C., Watabe A.M., Makhinson M., He Y., Ramsay M.F., Morris R.G., Morrison J.H., et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B., Lupica C.R., Dunwiddie T.V. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J. Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R.E., Zhang X.L., Bailey C.P., Conklin B., Kandel E.R., Stanton P.K. mGluR2 acts through inhibitory Gα subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc. Natl. Acad. Sci. 2006;103:6380–6385. doi: 10.1073/pnas.0601267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.D., Chan G.C., Poser S.W., Storm D.R. Differential regulation of type I and type VIII Ca2+-stimjulated adenylyl cyclases by Gi-coupled receptors in vivo. J. Biol. Chem. 1996;271:33308–33316. doi: 10.1074/jbc.271.52.33308. [DOI] [PubMed] [Google Scholar]
- Nguyen P.V., Kandel E.R. Brief, theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn. Mem. 1997;4:230–243. doi: 10.1101/lm.4.2.230. [DOI] [PubMed] [Google Scholar]
- Nguyen P.V., Woo N.H. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Otmakhova N.A., Otmakhov N., Mortenson L.H., Lisman J.E. Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 2000;20:4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace A.M., Faure M., Bourne H.R. Gi2-mediated activation of the MAP kinase cascade. Mol. Biol. Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S.L., Pittenger C., Morozov A., Martin K.C., Scanlin H., Drake C., Kandel E.R. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Qi M., Zhuo M., Skalhegg B.S., Brandon E.P., Kandel E.R., McKnight G.S., Izerda R.L. Impaired hippocampal plasticity in mice lacking the Cβ catalytic subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M., Stanton P.K. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J. Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Harde M., Potter B.V.L., Galione A., Stanton P.K. Induction of hippocampal LTD requires nitric oxide-stimulated PKG activity and Ca2+ release from cyclic ADP ribose-sensitive stores. J. Neurophysiol. 1999;82:1569–1576. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- Roche K.W., O’Brien R.J., Mammen A.L., Bernhardt J., Huganir R.L. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Santschi L.A., Reyes-Harde M., Stanton P.K. Chemically-induced, activity-independent LTD elicited by simultaneous activation of PKG and inhibition of PKA. J. Neurophysiol. 1999;82:1577–1589. doi: 10.1152/jn.1999.82.3.1577. [DOI] [PubMed] [Google Scholar]
- Santschi L., Zhang X.L., Stanton P.K. Activation of receptors negatively coupled to adenylate cyclase is required for induction of long-term synaptic depression. J. Neurobiol. 2006;66:205–219. doi: 10.1002/neu.20213. [DOI] [PubMed] [Google Scholar]
- Schuman E.M., Meffert M.K., Schulman H., Madison D.V. An ADP-ribosyltransferase as a potential target for nitric oxide action in hippocampal long-term potentiation. Proc. Natl. Acad. Sci. 1994;91:11958–11962. doi: 10.1073/pnas.91.25.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W.F. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 1999;20:66–73. doi: 10.1016/s0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- Son H., Hawkins R.D., Martin K., Kiebler M., Huang P.L., Fishman M.C., Kandel E.R. Long-term potentiation is reduced in mice that are double mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- Stanton P.K. LTP, LTD and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stanton P.K., Gage A.T. Distinct synaptic loci of Ca2+/calmodulin-dependent protein kinase II necessary for long-term potentiation and depression. J. Neurophysiol. 1996;76:2097–2101. doi: 10.1152/jn.1996.76.3.2097. [DOI] [PubMed] [Google Scholar]
- Stanton P.K., Heinemann U., Müller W.2001FM1-43 imaging reveals cGMP-dependent long-term depression of presynaptic transmitter release J. Neurosci. 21 . http://www.jneurosci.org/cgi/content/full/21/19/RC167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P.K., Winterer J., Bailey C.P., Kyrozis A., Raginov I., Laube G., Veh R.W., Nguyen C.Q., Müller W. Long-term depression of presynaptic release from the readily-releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J. Neurosci. 2003;23:5936–5944. doi: 10.1523/JNEUROSCI.23-13-05936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P.K., Winterer J., Zhang X.L., Müller W. Imaging LTP of presynaptic release of FM1-43 from the rapidly-recycling vesicle pool at Schaffer collateral-CA1 synapses in hippocampal slices. J. Neurosci. 2005;22:2451–2461. doi: 10.1111/j.1460-9568.2005.04437.x. [DOI] [PubMed] [Google Scholar]
- Winder D.G., Martin K.C., Muzzio I.A., Rohrer D., Chruscinski A., Kobilka B., Kandel E.R. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- Winterer J., Stanton P.K., Müller W. Direct monitoring of vesicular release and uptake in brain slices by multiphoton excitation of FM1-43. Biotechniques. 2006;40:343–351. doi: 10.2144/000112120. [DOI] [PubMed] [Google Scholar]
- Wong Y.H., Federman A., Pace A.M., Zachary I., Evans T., Pouyssegur J., Bourne H.R. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- Wong S.T., Athos J., Figueroa X.A., Pineda V.V., Schaefer M.L., Chavkin C.C., Muglia L.J., Storm D.R. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Yu T.P., McKinney S., Lester H.A., Davidson N. γ-Aminobutyric acid type A receptors modulate cAMP-mediated long-term potentiation and long-term depression at monosynaptic CA3-CA1 synapses. Proc. Natl. Acad. Sci. 2001;98:5264–5269. doi: 10.1073/pnas.091093998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Chattarji S., Barbarosie M., Rondi-Reig L., Philpot B.D., Miyakawa T., Bear M.F., Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Hu Y., Schultz C., Kandel E.R., Hawkins R.D. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368:635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]