Abstract
Adenovirus (Ad) gene transfer vectors are rapidly cleared from infected hepatocytes in mice. To determine which effector mechanisms are responsible for elimination of the Ad vectors, we infected mice that were genetically compromised in immune effector pathways [perforin, Fas, or tumor necrosis factor α (TNF-α)] with the Ad vector, Ad5-chloramphenicol acetyl transferase (CAT). Mice were sacrificed at 7–60 days postinfection, and the levels of CAT expression in the liver determined by a quantitative enzymatic assay. When the livers of infected mice were harvested 28 days postinfection, the levels of CAT expression revealed that the effectors most important for the elimination of the Ad vector were TNF-α > Fas > perforin. TNF-α did not have a curative effect on infected hepatocytes, as the administration of TNF-α to infected severe combined immunodeficient mice or to infected cultures in vitro had no specific effect on virus persistence. However, TNF-α-deficient mice demonstrated a striking reduction in the leukocytic infiltration early on in the infection, suggesting that TNF-α deficiency resulted in impaired recruitment of inflammatory cells to the site of inflammation. In addition, the TNF-deficient mice had a significantly reduced humoral immune response to virus infection. These results demonstrate a dominant role of TNF-α in elimination of Ad gene transfer vectors. This result is particularly important because viral proteins that disable TNF-α function have been removed from most Ad vectors, rendering them highly susceptible to TNF-α-mediated elimination.
Adenovirus (Ad) gene transfer vectors are currently the most efficient technique available for in vivo gene transduction. Accompanying transduction of target cells is an equally efficient host immune response to the defective virus, which has proven to be a major obstacle to the successful use of Ad vectors in a variety of gene therapy applications (reviewed in ref. 1). Although some of the complications revealed by the use of Ad vectors undoubtedly will be Ad specific, many of the problems are generic to in vivo gene therapy strategies. The replication defective Ad vectors present an opportunity to characterize both shared and Ad-specific aspects of the host immune response to gene therapy vectors.
The response of the host to the introduction of Ad vector can vary depending on the dose of virus, the site of delivery, and the genetic makeup of the vector as well as the transgene expressed from the viral backbone. When administered intravenously into mice, the vast majority of Ad is localized to the liver (2). During the first 24–48 hr of infection, 90% of vector DNA is eliminated, presumably through innate pathways of virus clearance mediated by the Kupffer cells within the liver (2). Despite the clearance of the majority of virus within days, over 95% of hepatocytes can be transduced by Ad vectors (3) with maximum transgene expression occurring during the first week of postinfection. In immune-competent animals, transgene expression rapidly declines to baseline levels by 2 to 3 weeks postinfection.
The rapid clearance of the virus is attributed to the cellular arm of the immune system. This observation is supported by experiments showing that Ad-mediated transgene expression extends beyond 4 months in immunodeficient mice that lack mature T and B lymphocytes (4–9); that adoptive transfer of CD8+ and CD4+ cytotoxic T cells (CTL) from Ad vector-infected mice clear the vector and the transgene from infected RAG-2 mice (5, 9); and that immune depletion of CD8+ or CD4+ cells in immunocompetent mice results in persistence of transgene expression (5, 8–11).
Recent studies have reported that the perforin and Fas pathways are the major effector pathways responsible for T cell cytotoxicity (12–15). Clearance of Ad gene transfer vectors by antigen-specific CTL has been reported to be mediated by the perforin/granzyme pathway (16). Because, as discussed in relation to hepatitis infection (17, 18), cytolytic destruction of 90% of hepatocytes over a short period of time would most likely be fatal, we considered the possibility that Ad vector clearance could occur by other clearance pathways. Here, we report that TNF-α plays a major role in the elimination of the Ad vector.
MATERIALS AND METHODS
Mice.
C57BL/6 and C57BL/6-Faslpr mice (B6/lpr) were obtained from The Jackson Laboratory. The mutant strain, CBA/lprcg (CBA/K1Jms/lprcg/lprcg) and the wild-type, CBA/++ (CBA/K1Jms) have been described in detail previously (19). Homozygous C.B-17 severe combined immunodeficient (SCID) mice (kindly provided by George Carlson, McLaughlin Research Institute, Great Falls, MT) were bred and maintained in a specific pathogen-free environment at the animal facilities of the Hospital for Special Surgery (New York, NY). Mice deficient in TNF-α expression (TNF-α −/−, TNF-α −/− C57BL/6×129) were created by replacement-type homologous recombination and are described elsewhere (20). Perforin knockout mice (perf−/−) have been described elsewhere (21).
Ad.
Ad5-chloramphenicol acetyl transferase (CAT) expressing the CAT gene from a first-generation Ad vector was used in all studies and has been previously described (22).
In Vivo Gene Transfer of Ad5-CAT.
Mice were anesthetized with methoxyflurane and the skin incised to expose the jugular vein. The mice then were injected intrajugularly with 5 × 109 viral particles of Ad5-CAT diluted to 100 μl with PBS (137 mM NaCl/2.7 mM KCl/10 mM Na2HPO4, pH 7.4 using a 28 g 1/2 (0.36 mm × 13 mm) needle affixed to a 0.5-cc insulin syringe. After the injection, the incision was sutured. Mice were sacrificed on the specified day, and liver removed, homogenized in 2 ml of PBS per gram (wet weight) of tissue, and the homogenates processed for CAT assays. CAT assays were performed as previously described (22). Initially, 1% to 5% of the total tissue homogenate was assayed. When values were out of the range of assay (generally >60% acetylated chloramphenicol) lysates were diluted in PBS containing 1 mg of BSA per ml. Each assay point included five animals from a single experiment.
Cells.
Murine embryonic fibroblasts were prepared from embryos of C57BL/6 (H2b) strain at fetal age of 16 days, following a standard procedure (23) with minor modifications. Briefly, embryos were aseptically removed, rinsed with PBS, cut into small pieces, and incubated at 4°C in PBS containing 0.05% trypsin and 1 mM EDTA (PBS/trypsin/EDTA) overnight. After the removal of particulate debris, embryonic cells released into the medium were plated onto 100-mm plates and cultured in α-modified minimum essential medium supplemented with 5% fetal bovine serum (HyClone), penicillin/streptomycin (5,000 units/50 μg per ml), and 5 × 10−5 M 2-mercaptoethanol at 37°C. Embryonic fibroblasts were harvested by trypsinization, washed, and either split for continuing culture or frozen as stocks. Fibroblasts of the third passage were used in most experiments.
In Vitro Stimulation of Splenic Lymphocytes and Lymphocyte-Mediated Cytotoxicity Assays.
The spleens from Ad5-CAT-infected mice were aseptically removed and single cell suspensions prepared. Spleen cells were cultured at a density of 4 × 106 cells/ml (1.5 ml per well of 24-well plates) in α-modified minimum essential medium with supplements as above in the presence of Ad5-CAT at a multiplicity of infection of 50. After a 5-day culture, reactivated CTLs were harvested, washed, and resuspended at the desired concentrations in culture medium. Embryonic fibroblasts were infected with Ad5-CAT at a multiplicity of infection of 500 for 24 hr before use as target cells. Infected, as well as uninfected control, fibroblasts were detached from the culture plates and labeled with 51Cr (≈ 100 μCi/106 cells) for 1.5 hr in suspension. CTLs and labeled target cells (104 cells/well), at different effector/target ratios, were added to individual wells of round-bottomed 96-well microtiter plates and incubated at 37°C for 5 hr. The percentage of target cell lysis induced by CTLs was determined by quantifying 51Cr released from target cells, based on the equation: % specific release (cytolysis) = (experimental release − spontaneous release)/(total release − spontaneous release) × 100%.
TNF Cytotoxicity.
H-2b fibroblasts were infected with Ad5-CAT at 1,000 particles/cell. Twenty-four hours postinfection, cells were labeled with 51Cr and exposed to increasing concentrations of TNF. Cell lysis was examined over the next 18 hr by 51Cr release, and CAT transgene expression was quantified as described above.
Histology.
Liver was fixed in formalin, embedded in paraffin, and sections stained with hematoxylin and eosin.
Neutralizing Antibody Assay and ELISA.
The determination of Ad-neutralizing antibody titers was performed on 28 days postinfection serum as described in Gall et al. (24). Anti-Ad antibodies were measured by ELISA in 96-well plates coated with 1010 particles (approximately 50 ng) per well of Ad in PBS at 4°C overnight. The wells were washed and then blocked with 1% BSA at room temperature for 1 hr. The plates were then serially incubated with mouse sera (1/1,000 dilution in 3% BSA/10% normal goat serum/0.05% Tween-20/PBS), a 1/1000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG and developed with substrate (Sigma 104 Phosphatase Substrate). Plates were read at 405 nm in an ELISA reader.
RESULTS
Fas-Deficient Mice Have a Modest Impairment of Ad5-CAT Transgene Clearance.
Recognition of the peptide/major histocompatibility complex by the T cell receptor is the first of several receptor ligand interactions that take place between the effector and target cell. To examine the role of CTL effector pathways and cell surface interactions considered important for lytic function, mice that were defective in either the Fas (lpr) (25) or perforin (21) pathways were evaluated for their efficiency of clearance of Ad5-CAT from the liver.
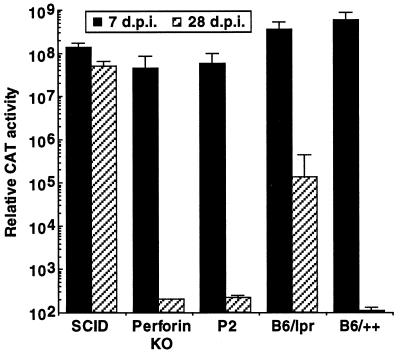
Administration of 5 × 109 particles of Ad5-CAT by intrajugular injection produced uniformly high levels of gene expression in all strains examined 7 days postinfection (Fig. 1). As expected from previous studies (4, 22, 26), SCID mice had persistently high elevations of CAT expression, whereas normal immunocompetent mice (P2, B6/++), had baseline levels of CAT expression at 28 days postinfection. Surprisingly, the levels of CAT gene expression also were reduced to baseline in perforin-deficient mice (perf−/−) at day 28, indicating that perforin was not required for elimination of transgene expression.
Figure 1.
Persistence of reporter gene expression in immunodeficient mice after i.v. administration of Ad5-CAT. Particles of Ad5-CAT (5 × 109) were administered to mutant strains (perf−/− or B6/lpr) and their wild-type counterparts (P2 and B6/++) by i.v. injection to the jugular vein (as described in Materials and Methods). SCID mice were used as a control for animals that do not clear Ad vectors due to a lack of B and T cells. Animals were sacrificed 7 or 28 days postinfection, and the livers harvested for CAT expression assays as indicated in the text. Each group includes a minimum of five animals. Relative CAT activity corresponds to the calculated CAT activity present in the total liver.
In contrast to the results obtained from Ad5-CAT-infected perf−/− mice, Fas-deficient lpr mice showed a low level of persistence of CAT gene expression when compared with the appropriate wild-type control strain at 28 days. This result indicates that the Fas pathway contributes modestly to host clearance of Ad5-CAT. Because similar results were observed in CBA lprcg mice (not shown) that have a point mutation in the signal transducing region of the Fas receptor (27), these findings are not explained by strain differences.
TNF-α Deficiency Markedly Attenuates Clearance of Ad5-CAT from the Liver.
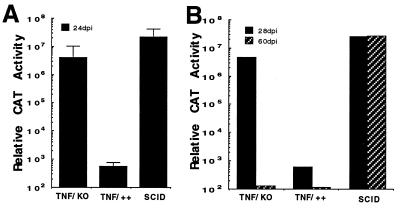
As shown in Fig. 1, neither Fas nor perforin alone could account for the efficient clearance of the transgene from the liver of infected animals. Using an identical strategy to that described in Fig. 1, we next determined whether TNF-α played a role in immune clearance of the virus. Particles of Ad5-CAT (5 × 109) were administered i.v. to TNF-α −/−, TNF-α ++, and SCID mice. Twenty-four days postinfection, animals were sacrificed and CAT expression levels determined. In comparison to TNF-α +/+ animals, high levels of CAT expression were observed in TNF-α −/− mice (Fig. 2A). To determine if CAT expression levels in the TNF-α −/− mice had stabilized at the 24-day time point, the experiment was repeated and CAT expression levels determined at 28 and 60 days postinfection. As shown in Fig. 2B, TNF-α-deficient mice were able to completely inhibit transgene expression by day 60 postinfection, indicating that other host defense mechanisms were available to eliminate transgene expression over a prolonged period of time.
Figure 2.
Persistence of CAT gene expression in TNF-α −/− mice transduced by Ad5-CAT. (A) Ad5-CAT administered to TNF-deficient mice (TNF-α −/−), the parental strain (TNF-α+/+), or SCID mice and sacrificed at day 24 postinfection. Liver CAT enzyme measurements were performed as described previously. (B) Extended time course of CAT expression in TNF-α −/− mice after administration of 5 × 109 particles of Ad5-CAT. Animals were sacrificed at 28 and 60 days postinfection.
TNF-α Administration Does Not Alter Ad5-CAT Expression in Vivo or in Vitro.
The experimental findings indicate that TNF-α plays an important role in facilitating elimination of virus-encoded transgene expression. Chisari and colleagues (28, 29) have shown in the transgenic hepatitis model that a single dose of TNF-α injected into the transgenic animals was sufficient to clear the hepatitis B genomic transcript. To determine whether TNF-α exerted its protective effect directly on hepatocytes after Ad5-CAT infection, 12 SCID mice were infected with Ad5-CAT. On days 7 and 14 postinfection, half the animals received either 80,000 units of recombinant mouse TNF-α or saline by i.p. injection. Animals were sacrificed 21 days postinfection, and CAT gene expression in the liver was measured. All animals receiving i.p. injections of TNF-α had levels of CAT expression identical to those found in the control group (data not shown). These findings indicate that systemic TNF-α did not seem likely to mediate a direct curative effect on Ad5-CAT-infected hepatocytes in vivo.
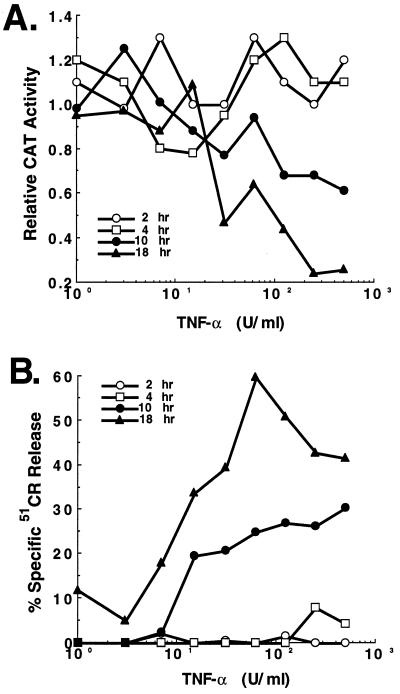
To examine whether TNF-α was directly capable of mediating noncytolytic clearance of Ad in vitro, a fibroblast assay was established. Primary mouse fibroblasts were infected with Ad5-CAT (103 particles/cell), exposed to varying concentrations of TNF-α, and harvested after 2, 4, 10 or 18 hr. As shown in Fig. 3, the decline in CAT activity assays (Fig. 3A) directly correlated with the level of cell lysis (Fig. 3B), indicating that the cytokine did not appear to have a curative effect separable from the ability to induce cell lysis.
Figure 3.
In vitro clearance of CAT transgene expression from primary mouse fibroblasts directly correlates with TNF-α cytolytic activity. Primary mouse fibroblasts infected for 24 hr with 1,000 particles/cell of Ad5-CAT were exposed to increasing concentrations of TNF-α as shown. At the indicated time intervals (2, 4, 10, 18 hr) samples were examined for (A) CAT enzymatic activity and (B) cytolysis as described in the text.
TNF-α-Deficient Lymphocytes Lysed Virus-Infected Targets in Vitro But Showed Reduced Homing to the Liver.
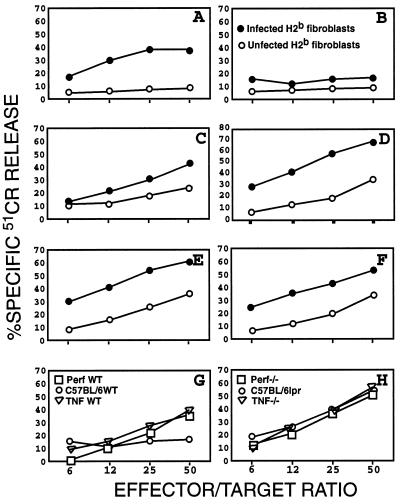
To further explore how TNF-α deficiency allowed persistence of Ad5-CAT infection, we examined CTL activity from wild-type and mutant strains at 28 days postinfection. Significant lysis of virus-infected, and to a lesser extent of uninfected, fibroblasts was detected in the assays using CTLs derived from wild-type (Fig. 4 A, C, and E), lpr (Fig. 4D), and TNF-α −/− (Fig. 4F) mice. In contrast, CTLs derived from perf−/−mice exhibited only a marginal cytolytic activity against infected fibroblasts (Fig. 4B). Interestingly, the reactivated CTLs prepared from all strains of mice (wild-type, perf−/−, TNF-α −/−, and lpr) mice were capable of inducing substantial lysis of yeast artificial chromosome-1 cells, the classical natural killer (NK) target cells (Fig. 4 G and H), suggesting the presence of NK cells and lymphokine-activated killer cells in the bulk population of reactivated cells. The presence of NK/lymphokine-activated killer activities also provides an explanation for the low degree of killing of uninfected fibroblasts observed in the CTL cytotoxicity assays. Because the subclone of YAC-1 cells used expressed a substantial level of Fas antigen (data not shown), the lysis of these cells by perf−/− lymphocytes most likely was mediated via the Fas-dependent pathway.
Figure 4.
Cytolytic functions of splenic lymphocytes obtained from Ad-infected mice. (A-F) CTL response. Splenic lymphocytes harvested from perf−/− (B), lpr (D), TNF-α −/− (F), and their respective wild-type (WT) controls (A, C, and E) were stimulated in vitro for 5 days (see text) and tested for specific lysis of Ad-infected fibroblasts (•) or uninfected fibroblasts (○). (G and H) NK/lymphokine-activated killer activity. Stimulated splenic lymphocytes derived from all six strains of mice were tested for lysis of yeast artificial chromosome-1 cells, a classical target for NK cells.
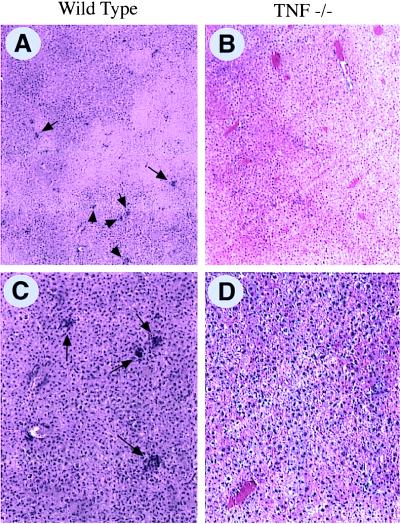
Because CTL were generated from TNF-α-deficient mice in vitro, we next asked whether lymphocytes adequately homed to the site of infection. Studies previously have demonstrated that Ad-vector infection leads to CD4+ and CD8+ lymphocyte infiltration of the liver, which peaks 7–10 days postinfection (5). TNF-α is known to be a potent stimulator of adhesion molecules, which, in turn, facilitate leukocyte adhesion to vessels and transendothelial migration (30). TNF-α −/− and TNF-α +/+ mice were infected with Ad5-CAT and sacrificed at day 7 postinfection. As shown in Fig. 5, wild-type mice showed extensive lymphocytic infiltration of the liver (average number of inflammatory foci per low power field was 8.6 ± 2.3). In marked contrast, TNF-α-deficient mice had a significant reduction in lymphocytic infiltrates (1.6 ± 1.5 foci per field, P < 0.0005, n = 5, Student’s t test) (Fig. 5).
Figure 5.
Impaired intrahepatic recruitment of leukocytes in TNF-α-deficient mice. Wild-type (A and C) and TNF-α-deficient (B and D) mice were infected with Ad5-CAT as in Fig. 1 and sacrificed 7 days postinfection. The livers were fixed in formalin and stained with hematoxylin and eosin. A significant reduction in the number of clusters of mononuclear cells (arrows) was observed in the TNF-deficient compared with the wild-type mice (A and B ×40; C and D ×10).
TNF-α-Deficient Mice Have an Impaired Humoral Immune Response to Ad.
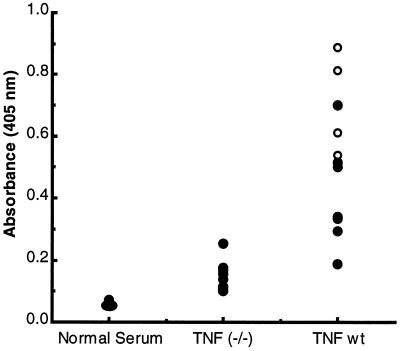
It recently has been observed that TNF-α −/− mice have an impaired antibody production (20, 31). To determine whether the humoral immune response to Ad was compromised in these mice, we quantified IgG anti-Ad antibody titers by ELISA. Analysis of serum from 28-day Ad5-CAT-infected wild-type or TNF-α −/− mice showed significantly reduced anti-Ad5 antibody compared with wild-type mice (Fig. 6). The low titer of anti-Ad5 antibody in the TNF-α −/− mice also was reflected by the lack of serum neutralization activity by in vitro anti-Ad5 serum neutralization assays.
Figure 6.
The humoral immune response to Ad5-CAT infection is impaired in TNF-α-deficient mice. TNF-α-deficient and wild-type control mice were infected with Ad5-CAT as described in Fig. 1. Sera were analyzed at 28 days postinfection for anti-Ad IgG antibody by ELISA. Each dot represents the ELISA titer of serum from an individual animal at a 1:1,000 dilution. In vitro neutralization of Ad infections were determined for each sample. ○ represent sera that have Ad-neutralizing titers >20. • represent undetectable neutralization at a 1:20 serum dilution.
DISCUSSION
Despite genetic reengineering of the Ad gene delivery vector, the efficiency with which the host eliminates replication defective Ad vectors is striking. After i.v. vector administration, the vast majority of Ad vector is localized to the liver. Greater than 90% of the vector is eliminated within 24 hr of administration, well before maximal levels of transgene are expressed (2). A small percentage of the input Ad vector therefore is responsible for subsequent transgene expression found in most of the hepatocyte population. During the second and third weeks postinfection, immune activation results in clearance of viral-mediated transgene expression, and it generally has been assumed that cells harboring the Ad vector are subject to immune-mediated lysis.
Although perforin-dependent CD8+ CTLs have long been thought to be the dominant cytolytic effector of virus-infected cells, recent studies indicate that perforin-mediated cytotoxicity against lymphocytic choriomeningitis virus is only one of multiple host antiviral strategies (15). Perf−/− mice are able to mount efficient immune responses against viruses, such as vesicular stomatitis virus, influenza, Semliki forest virus, and rotavirus (15, 32). In the present study, perf−/− mice had little or no impairment of Ad vector clearance in vivo as determined by a quantitative CAT transgene expression assay. These results were unexpected based on a previous report demonstrating a major role for the perforin pathway in Ad clearance (16). The differences between the results obtained in the present study and those of Yang et al. (16) may be explained by genetic makeup of the viral backbone, the transgene being expressed, the quantitative CAT assay used in the present study, or strain differences in the recipient mice. Despite efficient in vivo clearance, spleen cells obtained from Ad-infected perf−/− mice demonstrated profoundly impaired in vitro CTL lysis of fibroblasts. This may be attributed to the absence of Fas and perhaps other relevant surface receptors on target primary fibroblasts (C.-C. L., unpublished observations).
Fas recently has been shown to be a second major pathway of T cell-mediated cytotoxicity (12, 13). Fas-deficient (lpr) mice were modestly compromised in their ability to clear the Ad vector, suggesting that Fas-mediated apoptosis of either hepatocytes or macrophages (33) helped to facilitate virus clearance. Hepatocytes express high levels of Fas and are not protected by Bcl-2 (34), rendering them highly susceptible to Fas-mediated apoptosis (35). CD4+ T cells can transfer lethal lymphocytic choriomeningitis virus disease to β2-microglobulin-deficient mice by a Fas-dependent pathway (36), and evidence for FasL-mediated tissue injury has been observed both in the hepatitis transgene model and in fas null mice (37) as well as human livers infected with hepatitis B virus (38, 39). Of interest, humans with Fas mutations (Canale Smith syndrome), who received contaminated blood transfusions, failed to clear hepatitis B and hepatitis C infections compatible with a role for Fas in immune-mediated clearance of hepatotropic viruses (40).
Because neither perforin nor Fas deficiency alone substantially prevented clearance of the Ad vector, either both pathways need to be disabled to impair clearance or another effector pathway(s) is quantitatively more important for Ad-vector elimination. Cytokines such as TNF-α (41) and the interferons (42, 43) play prominent roles in the host defense against viruses. TNF-α is particularly important in host Ad interactions as 4 of 25 Ad-encoded early proteins inhibit TNF-α function (42). Two early transcription units, E3 and E1B, code for polypeptides [E3–14.7 K (44), 10.4/14.5 (45); E1B-19K (45–47) that function to directly block TNF-α activation as well as apoptotic pathways shared by TNF-α and Fas (42)]. Of note, these transcription units have been deleted or rendered nonfunctional in all of the generic Ad gene transfer vectors currently in use. Viral vectors deleted only in E1A also are lacking anti-TNF function because the E1A gene product is required to transcriptionally transactivate both E1B and E3. Of considerable interest, a vector that overexpresses a transcriptionally active E3 transcription unit suppressed the production of neutralizing antibody and resulted in transgene persistence (48).
Administration of Ad5-CAT to TNF-α −/− animals resulted in persistence of transgene expression 28 days postinfection. Compared with the experimental results from the Fas-deficient or perf−/− animals, the contribution of TNF-α to immune-mediated clearance of Ad5-CAT was considerable and demonstrates a key role for this cytokine in host elimination of the replication defective Ad5-CAT gene transfer vector. Because CAT activity approximated background levels 60 days postinfection in the TNF-α-deficient animals, other pathways are able to compensate for the effects of TNF-α deficiency over time.
To understand how TNF-α functions to eliminate the Ad5 vector, we first considered the possibility that, as in the hepatitis B transgene model (28, 29), TNF-α could directly eliminate virus expression in hepatocytes. Chisari and colleagues (29) reported that a single administration of a noncytopathic dose of TNF-α cured hepatocytes of hepatitis B virus expression whereas interferon-γ, interleukin 1, interleukin 3, interleukin 6, and other cytokines were ineffective (29). TNF-α most likely acts by inducing degradation of the viral transcript in the cytoplasm (49). However, the administration of 80,000 units of TNF-α on two separate occasions failed to clear Ad5-CAT-mediated transgene expression in SCID mice. Preliminary experiments administering exogenous TNF-α to TNF−/− animals have failed to reverse the prolongation of CAT expression. It previously has been shown that the TNF−/− animals are resistant to exogenous TNF (20), indicating some secondary defect in TNF receptor signaling arises in these animals. Furthermore, we could find no evidence for inhibition of transgene expression independent of the cytolytic effect of TNF-α in vitro. These findings suggested that TNF-α was important either for promoting T cell cytolytic activity or that TNF-α facilitated immune clearance of the transgene by some other route. The former possibility was considered less likely because T cells obtained from TNF-α-deficient mice were cytolytic in vitro as also shown previously (20).
TNF-α is a multifunctional cytokine with many proinflammatory properties (50). Among its actions are the up-regulation of adhesion molecules, such as intercellular adhesion molecule 1, which recruit leukocytes to the site of inflammation (51). In the present study, we observed a marked difference in the numbers and size of mononuclear cell infiltrates in TNF-α-deficient compared with wild-type mice. Because 90% of the Ad vector is rapidly taken up by hepatic Kupffer cells (2) and TNF-α is the major proinflammatory cytokine produced by these cells, failure to induce adhesion molecule expression could explain the limited mononuclear cell infiltrate and enhanced transgene expression in TNF-α-deficient mice. This interpretation is consistent with the enhanced susceptibility of TNF-α-deficient mice to Listeria monocytogenes (31) as well as a variety of other infectious agents (20). Similarly, it has been found that the major anti-inflammatory effect of anti-TNF-α antibody administration in autoimmune diseases is to decrease adhesion molecule expression, which secondarily reduces inflammation (52, 53).
The results in the present study indicate a prominent role for TNF-α in the clearance of the Ad5 gene vector. We propose that lack of leukocyte recruitment is the major factor responsible for impaired clearance of transgene expression in TNF-α-deficient mice. However, TNF-α also is involved in lymphoid activation and in the humoral immune response to infectious agents (20, 31). The reduced humoral immune response against Ad infection observed in TNF-α −/− mice is also of considerable interest as the antibody responses prevent reinfection with Ad vectors. Studies are underway to determine whether TNF-deficient mice can be reinfected with Ad5-CAT. The data also complements the observations made by Ilan et al. (48) where overexpression of the anti-TNF E3 genes effected both cellular and humoral components of the immune response to Ad infection. Understanding how the Ad-infected cell triggers immune effector pathways should facilitate strategies designed to selectively block immune-mediated clearance of Ad-infected cells without global immunosuppression.
Acknowledgments
This work was supported in part by National Institutes of Health Grants Specialized Center of Research in SLE P50-AR42588 (to K.E.) and PO1 HL51746 (to E.F.P. and R.G.C.) and Cystic Fibrosis Foundation Grant Z990 (to E.F.P.). E.F.P. and R.G.C. receive sponsored research support from GenVec.
ABBREVIATIONS
- Ad
adenovirus
- CAT
chloramphenicol acetyl transferase
- TNF
tumor necrosis factor
- perf−/−
perforin-deficient
- CTL
cytotoxic T cells
- NK
natural killer
- SCID
severe combined immunodeficient
References
- 1.Wilson C, Kay M A. Nat Med. 1995;1:887–889. doi: 10.1038/nm0995-887. [DOI] [PubMed] [Google Scholar]
- 2.Worgall S, Wolff G, Falck-Pedersen E, Crystal R G. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Kay M A, Finegold M, Stratford-Perricaudet L D, Woo S L. Hum Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 4.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. Gene Therapy. 1994;1:395–402. [PubMed] [Google Scholar]
- 5.Yang Y, Ertl H C, Wilson J M. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay M A, Holterman A X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wilson J M. J Immunol. 1995;155:2564–2570. [PubMed] [Google Scholar]
- 10.Kolls J K, Lei D, Odom G, Nelson S, Summer W R, Gerber M A, Shellito J E. Hum Gene Ther. 1996;7:489–97. doi: 10.1089/hum.1996.7.4-489. [DOI] [PubMed] [Google Scholar]
- 11.Guerette B, Gingras M, Wood K, Roy R, Tremblay J P. Transplantation. 1996;62:962–967. doi: 10.1097/00007890-199610150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, Shinkai Y, Alt F W, Matsuzawa A, Yonehara S, Takayama H. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 13.Henkart P A. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 14.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 15.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Xiang Z, Ertl H C, Wilson J M. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti L G, Borrow P, Hobbs M V, Matzke B, Gresser I, Oldstone M B, Chisari F V. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, Katagiri T. J Exp Med. 1990;171:519–531. doi: 10.1084/jem.171.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh C M, Matloubian M, Liu C-C, Ueda R, Kurahara C G, Christensen J L, Huang M T F, Young J D-E, Ahmed R, Clark W R. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick P M, Wittenberg B A, Cipriani L, Leinwand L. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doetschman T C, Eistetter J, Katz M, Schmidt W, Kemler R. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 24.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 26.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Gene Therapy. 1996;3:154–162. [PubMed] [Google Scholar]
- 27.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 28.Gilles P N, Fey G, Chisari F V. J Virol. 1992;66:3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidotti L G, Guilhot S, Chisari F V. J Virol. 1994;68:1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Springer T A. Nature (London) 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 31.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco M A, Tin C, Rott L S, VanCott J L, McGhee J R, Greenberg H B. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashany D, Song X, Lacy E, Nikolic-Zugic J, Friedman S M, Elkon K B. Proc Natl Acad Sci USA. 1995;92:11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 35.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Nature (London) 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 36.Zajac A J, Quinn D G, Cohen P L, Frelinger J A. Proc Natl Acad Sci USA. 1996;93:14730–14735. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 38.Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- 39.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drappa J, Vaishnaw A K, Sullivan K E, Chu J L, Elkon K B. N Engl J Med. 1996;335:1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 41.Wong G H W, Goeddel D V. Nature (London) 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 42.Wold W S, Hermiston T W, Tollefson A E. Trends Microbiol. 1994;2:437–443. doi: 10.1016/0966-842x(94)90801-x. [DOI] [PubMed] [Google Scholar]
- 43.Biron C A. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 44.Krajcsi P, Dimitrov T, Hermiston T W, Tollefson A E, Ranheim T S, Vande Pol S B, Stephenson A H, Wold W S. J Virol. 1996;70:4904–4913. doi: 10.1128/jvi.70.8.4904-4913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart A R, Tollefson A E, Krajcsi P, Yei S P, Wold W S. J Virol. 1995;69:172–181. doi: 10.1128/jvi.69.1.172-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gooding L R, Aquino L, Duerksen-Hughes P J, Day D, Horton T M, Yei S P, Wold W S. J Virol. 1991;65:3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White E, Sabbatini P, Debbas M, Wold W S, Kusher D I, Gooding L R. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ilan Y, Droguett G, Chowdhury N R, Li Y, Sengupta K, Thummala N, R, Davidson A, Chowdhury J R, Horowitz M S. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilhot S, Guidotti L G, Chisari F V. J Virol. 1993;67:7444–7449. doi: 10.1128/jvi.67.12.7444-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazzoni F, Beutler B. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 51.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak T W, Holzmann B, Kronke M. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 52.Paleolog E, Hunt M, Elliot M J, Feldmann M, Maini R N, Woody J N. Arthritis Rheum. 1996;39:1082–1091. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- 53.Tak P P, Taylor P C, Breedveld F C, Smeets T J M, Daha M R, Kluin P M, Meinders A E, Maini R N. Arthritis Rheum. 1996;39:1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]