Abstract
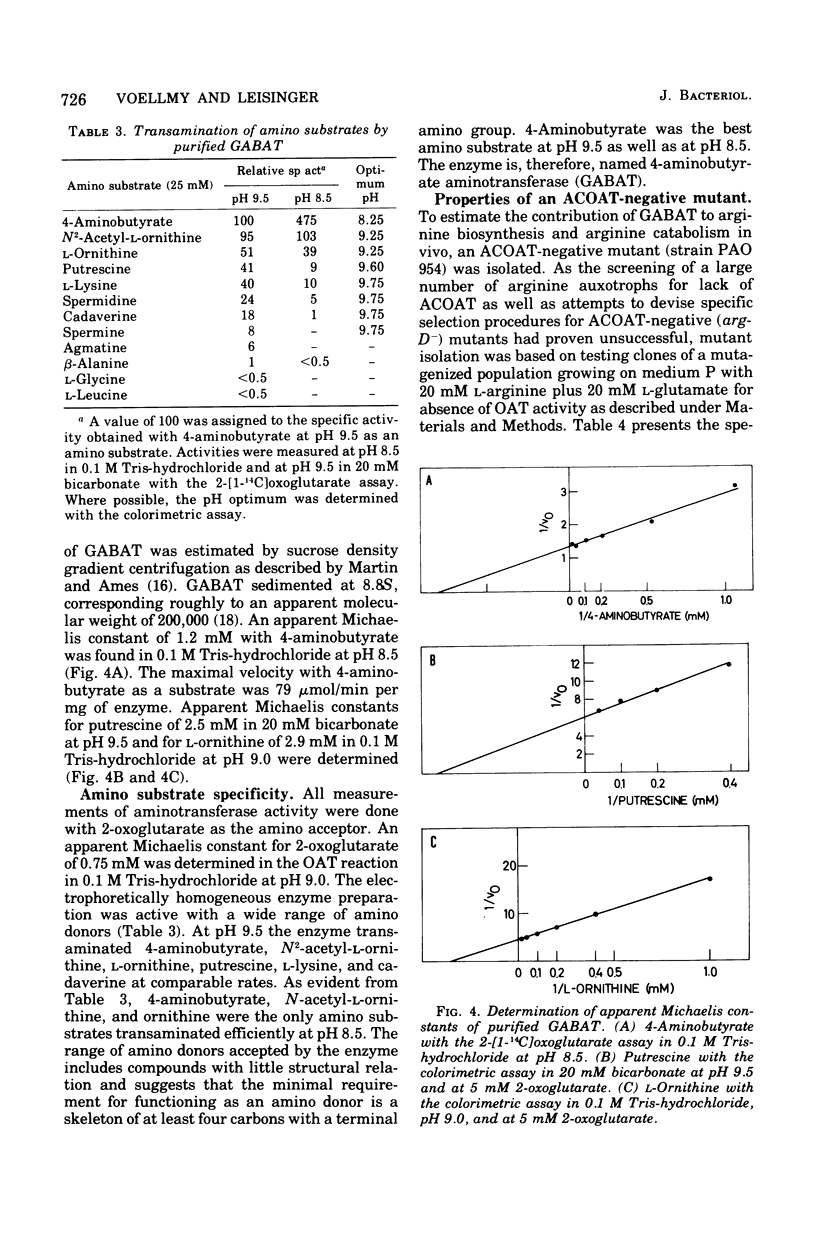
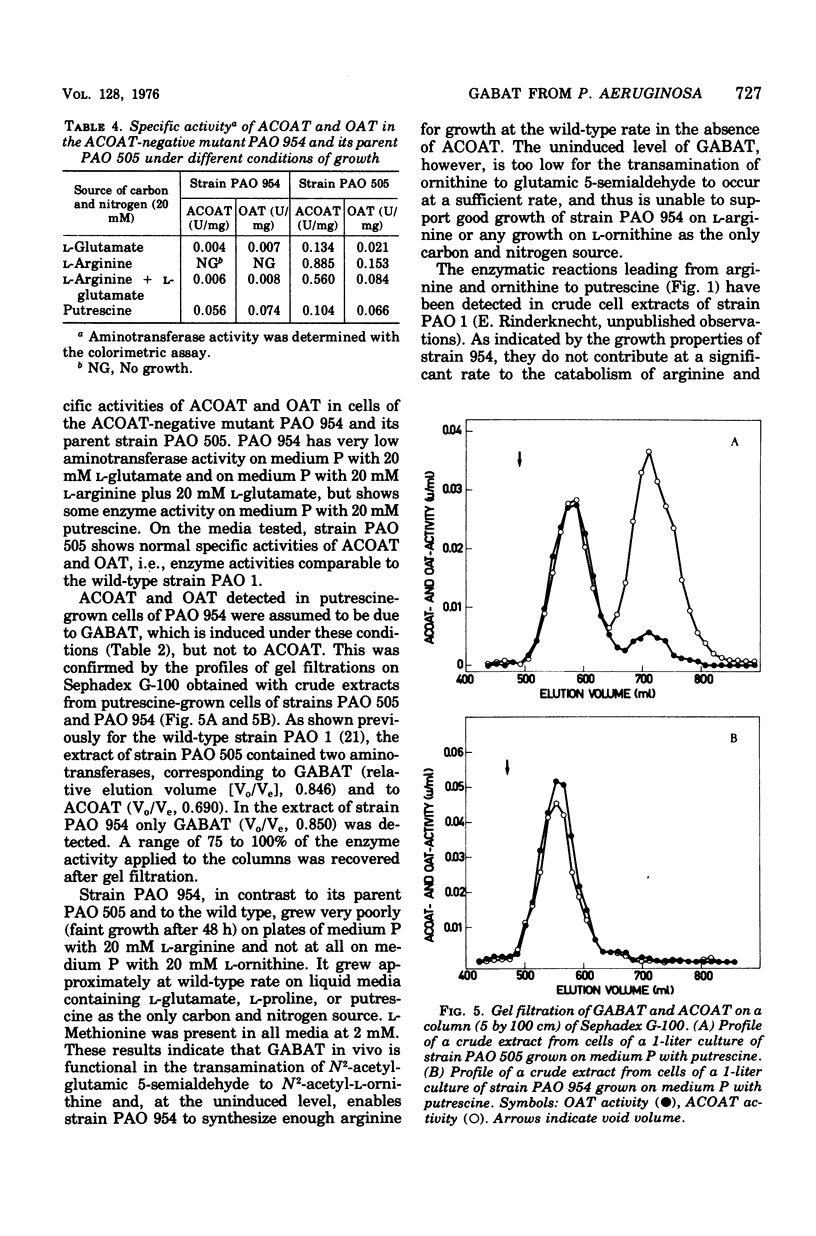
4-Aminobutyrate aminotransferase (GABAT) from Pseudomonas aeruginosa was purified 64-fold to apparent electrophoretic homogeneity from cells grown with 4-aminobutyrate as the only source of carbon and nitrogen. Purified GABAT catalyzed the transamination of 4-aminobutyrate, N2-acetyl-L-ornithine, L-ornithine, putrescine, L-lysine, and cadaverine with 2-oxoglutarate (listed in order of decreasing activity). The enzyme is induced in cells grown on 4-guanidinobutyrate, 4-aminobutyrate, or putrescine as the only carbon and nitrogen source. Cells grown on arginine or on glutamate contained low levels of the enzyme. The regulation of the synthesis of GABAT as well as the properties of the mutant with an inactive N2-acetyl-L-ornithin 5-aminotransferase suggest that GABAT functions in the biosynthesis of arginine by convertine N2-acetyl-L-glutamate 5-semialdehyde to N2-acetyl-Lornithine as well as in catabolic reactions during growth on putrescine or 4-guanidinobutyrate but not during growth on arginine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Bloch-Tardy M., Rolland B., Gonnard P. Pig brain 4-aminobutyrate 2-ketoglutarate transaminase. Purification, kinetics and physical properties. Biochimie. 1974;56(6-7):823–832. doi: 10.1016/s0300-9084(74)80503-1. [DOI] [PubMed] [Google Scholar]
- Brohn F., Tchen T. T. A single transaminase for 1,4-diaminobutane and 4-aminobutyrate in a Pseudomonas species. Biochem Biophys Res Commun. 1971 Nov 5;45(3):578–582. doi: 10.1016/0006-291x(71)90456-6. [DOI] [PubMed] [Google Scholar]
- Callewaert D. M., Rosemblatt M. S., Tchen T. T. Purification and properties of 4-aminobutanal dehydrogenase from a Pseudomonas species. J Biol Chem. 1974 Mar 25;249(6):1737–1741. [PubMed] [Google Scholar]
- Cash C., Maitre M., Ciesielski L., Mandel P. Purification and partial characterisation of 4-aminobutyrate 2-ketoglutarate transaminase from human brain. FEBS Lett. 1974 Oct 15;47(2):199–203. doi: 10.1016/0014-5793(74)81011-2. [DOI] [PubMed] [Google Scholar]
- Chou C. S., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. -guanidinobutyrate amidinohydrolase. J Biol Chem. 1972 Jul 25;247(14):4486–4490. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GROSS D. Two-dimensional high-voltage paper electrophoresis of amino--and other organic acids. Nature. 1959 Oct 24;184:1298–1301. doi: 10.1038/1841298b0. [DOI] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., FREDERICKS J. Pyrrolidine and putrescine metabolism: gamma-aminobutyraldehyde dehydrogenase. J Biol Chem. 1959 Aug;234(8):2145–2150. [PubMed] [Google Scholar]
- KIM K. H. PURIFICATION AND PROPERTIES OF A DIAMINE ALPHA-KETOGLUTARATE TRANSAMINASE FROM ESCHERICHIA COLI. J Biol Chem. 1964 Mar;239:783–786. [PubMed] [Google Scholar]
- Kakimoto T., Shibatani T., Chibata I. Crystallization of L-arginine deiminase from Pseudomonas Putida. FEBS Lett. 1971 Dec 1;19(2):166–168. doi: 10.1016/0014-5793(71)80505-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesinger T., Haas D., Hegarty M. P. Indospicine as an arginine antagonist in Escherichia coli and Pseudomonas aeruginosa. Biochim Biophys Acta. 1972 Mar 14;262(2):214–219. doi: 10.1016/0005-2787(72)90235-3. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nikolaeva Z. K., Vasil'ev V. Iu. O mekhanizme deistviia gamma-aminobutirat-glutamat-transaminazy iz pochek svin'i. Biokhimiia. 1972 May-Jun;37(3):572–578. [PubMed] [Google Scholar]
- Paetkau V., Lardy H. A. Phosphofructokinase. Correlation of physical and enzymatic properties. J Biol Chem. 1967 May 10;242(9):2035–2042. [PubMed] [Google Scholar]
- Ramos F., Stalon V., Piérard A., Wiame J. M. The specialization of the two ornithine carbamoyltransferases of Pseudomonas. Biochim Biophys Acta. 1967 May 16;139(1):98–106. doi: 10.1016/0005-2744(67)90116-7. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., JAKOBY W. B. Soluble gamma-aminobutyric-glutamic transaminase from Pseudomonas fluorescens. J Biol Chem. 1959 Apr;234(4):932–936. [PubMed] [Google Scholar]
- Voellmy R., Leisinger T. Dual role for N-2-acetylornithine 5-aminotransferase from Pseudomonas aeruginosa in arginine biosynthesis and arginine catabolism. J Bacteriol. 1975 Jun;122(3):799–809. doi: 10.1128/jb.122.3.799-809.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]


