Abstract
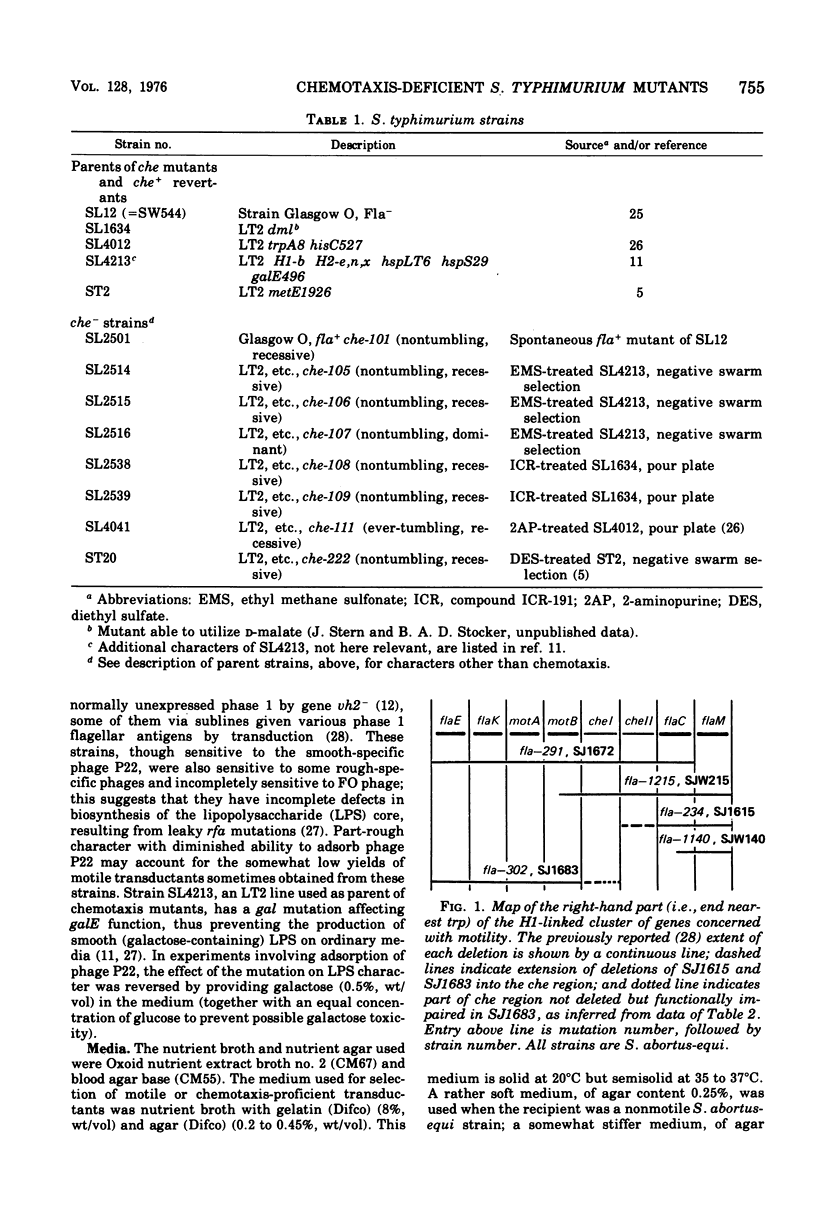
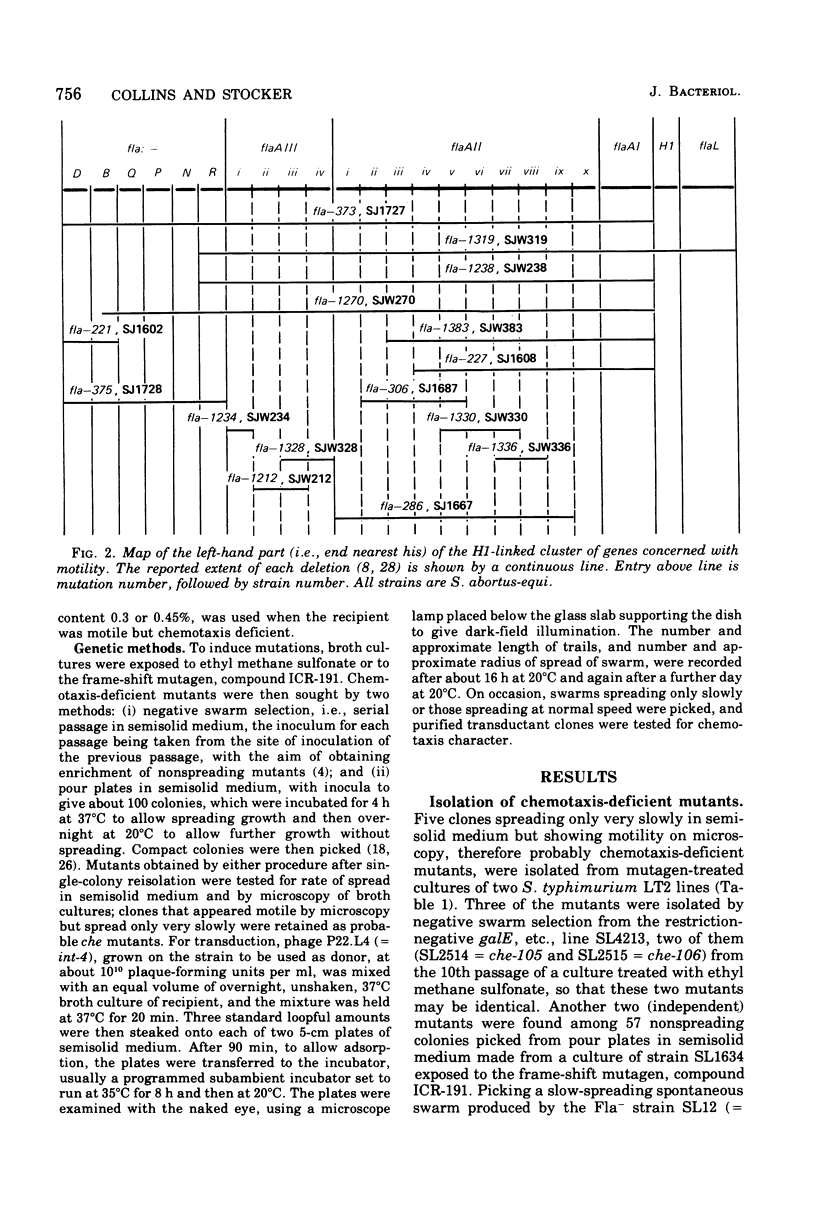
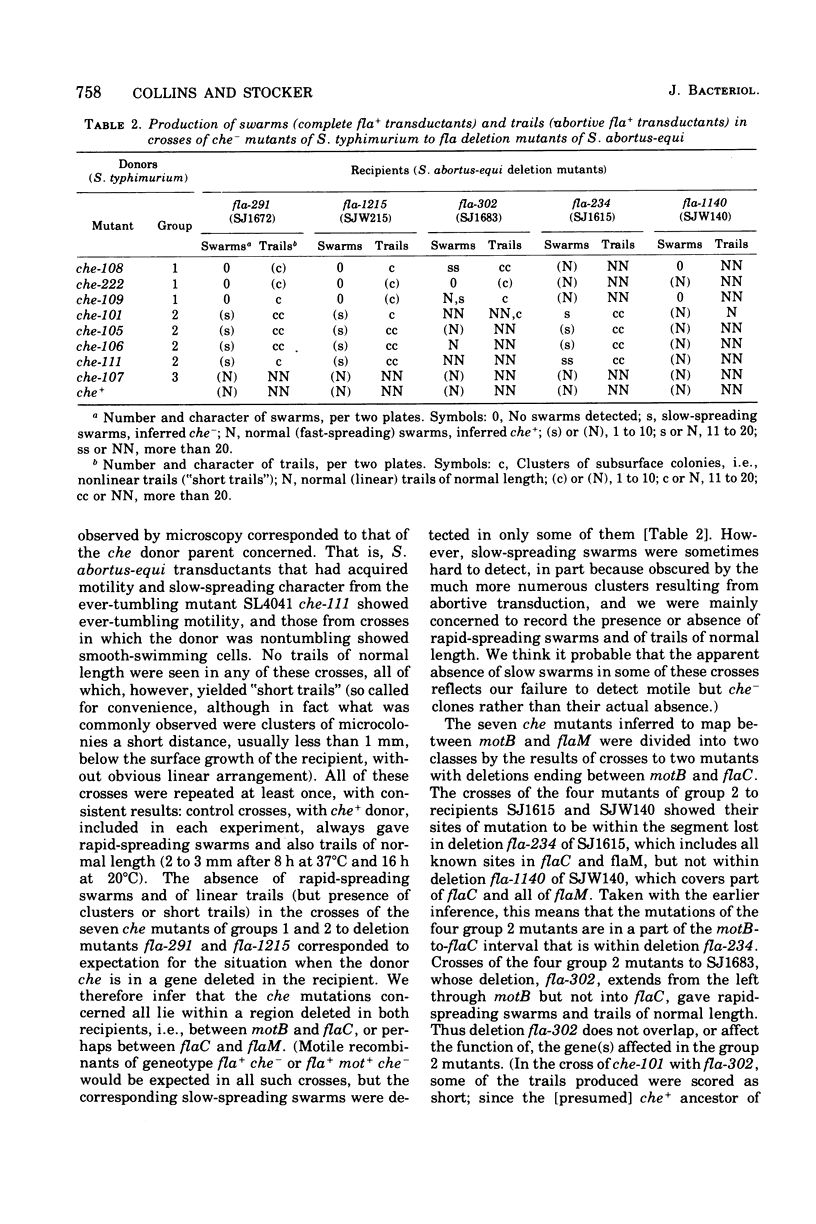
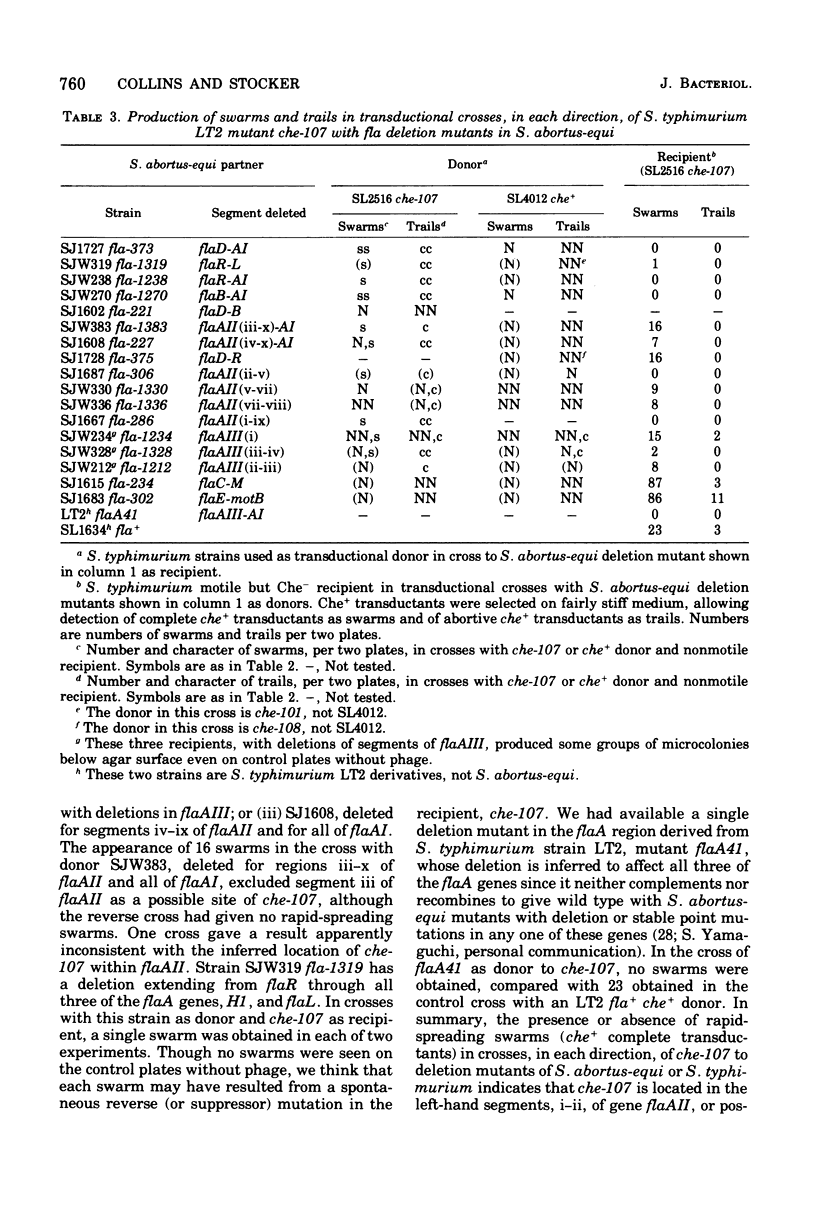
The mutations of eight chemotaxis-deficient strains of Salmonella typhimurium, including five new mutants in strain LT2, were mapped by P22 transduction in relation to various fla mot deletions in S. abortus-equi. Seven recessive che mutations mapped between motB and flaC: three, all nontumbling, the che region I, adjacent to motB, and four, including one ever-tumbling, in che region II, adjacent to flaC. Mutant che-107, never-tumbling and dominant to wild type, mapped at flaAII, other mutations of which cause either absence of flagella or lack of locomotor function. We surmise that gene flaAII specifies a protein that polymerizes to form an essential component of the basal apparatus (so that absence of gene product prevents formation of flagela); that a component built up from certain mutationally altered proteins cannot transmit (or generate) active rotation of the hook and flagellum, and so causes the Mot (paralysis) phenyotype; and that a component built up from protein with the che-107 alteration permits only counterclockwise rotation, so that the tumble, normally produced by transient clockwise rotation, cannot be effected.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. B., Adler J. Complementation of nonchemotactic mutants of Escherichia coli. Genetics. 1969 Jan;61(1):61–66. doi: 10.1093/genetics/61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Genetics of motility in Escherichia coli: complementation of paralysed mutants. Genetics. 1967 Jul;56(3):363–373. doi: 10.1093/genetics/56.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Boro H., Brenchley J. E. A new generalized transducing phage for Salmonella typhimurium LT2. Virology. 1971 Sep;45(3):835–836. doi: 10.1016/0042-6822(71)90208-x. [DOI] [PubMed] [Google Scholar]
- Colson C., Colson A. M. A new Salmonella typhimurium DNA host specificity. J Gen Microbiol. 1971 Dec;69(3):345–351. doi: 10.1099/00221287-69-3-345. [DOI] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Slow motile mutant in Salmonella typhimurium. J Bacteriol. 1965 Dec;90(6):1696–1702. doi: 10.1128/jb.90.6.1696-1702.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Iino T. A Stabilizer of Antigenic Phases in Salmonella Abortus-Equi. Genetics. 1961 Nov;46(11):1465–1469. doi: 10.1093/genetics/46.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Complementation of non-flagellate Salmonella mutants. J Gen Microbiol. 1965 Oct;41(1):47–55. doi: 10.1099/00221287-41-1-47. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Data processing by the chemotaxis machinery of Escherichia coli. Nature. 1974 Nov 22;252(5481):317–319. doi: 10.1038/252317a0. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J Bacteriol. 1976 May;126(2):758–770. doi: 10.1128/jb.126.2.758-770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Stocker B. A., Yamaguchi S. A new fla gene in Salmonella typhimurium--flaR--and its mutant phenotype-superhooks. Arch Mikrobiol. 1973 Mar 26;90(2):107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- Pearce U., Stocker B. A. Variation in composition of chromosome fragments transduced by phage P22. Virology. 1965 Nov;27(3):290–296. doi: 10.1016/0042-6822(65)90108-x. [DOI] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. The occurrence of rare motile bacteria in some non-motile Salmonella strains. J Gen Microbiol. 1957 Oct;17(2):424–436. doi: 10.1099/00221287-17-2-424. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Abortive transduction of motility in Salmonella; a nonreplicated gene transmitted through many generations to a single descendant. J Gen Microbiol. 1956 Dec;15(3):575–598. doi: 10.1099/00221287-15-3-575. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Transduction of flagellar characters in Salmonella. J Gen Microbiol. 1953 Dec;9(3):410–433. doi: 10.1099/00221287-9-3-410. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of bacteriophage Mu-induced flagellar mutants in Escherichia coli. J Bacteriol. 1973 Oct;116(1):114–122. doi: 10.1128/jb.116.1.114-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. S., Stocker B. A. Nonsense motility mutants in Salmonella typhimurium. Genetics. 1973 Feb;73(2):229–245. doi: 10.1093/genetics/73.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Horiguchi T., Ota K. Genetic analysis of fla and mot cistrons closely linked to H1 in Salmonella abortusequi and its derivatives. J Gen Microbiol. 1972 Apr;70(1):59–75. doi: 10.1099/00221287-70-1-59. [DOI] [PubMed] [Google Scholar]


