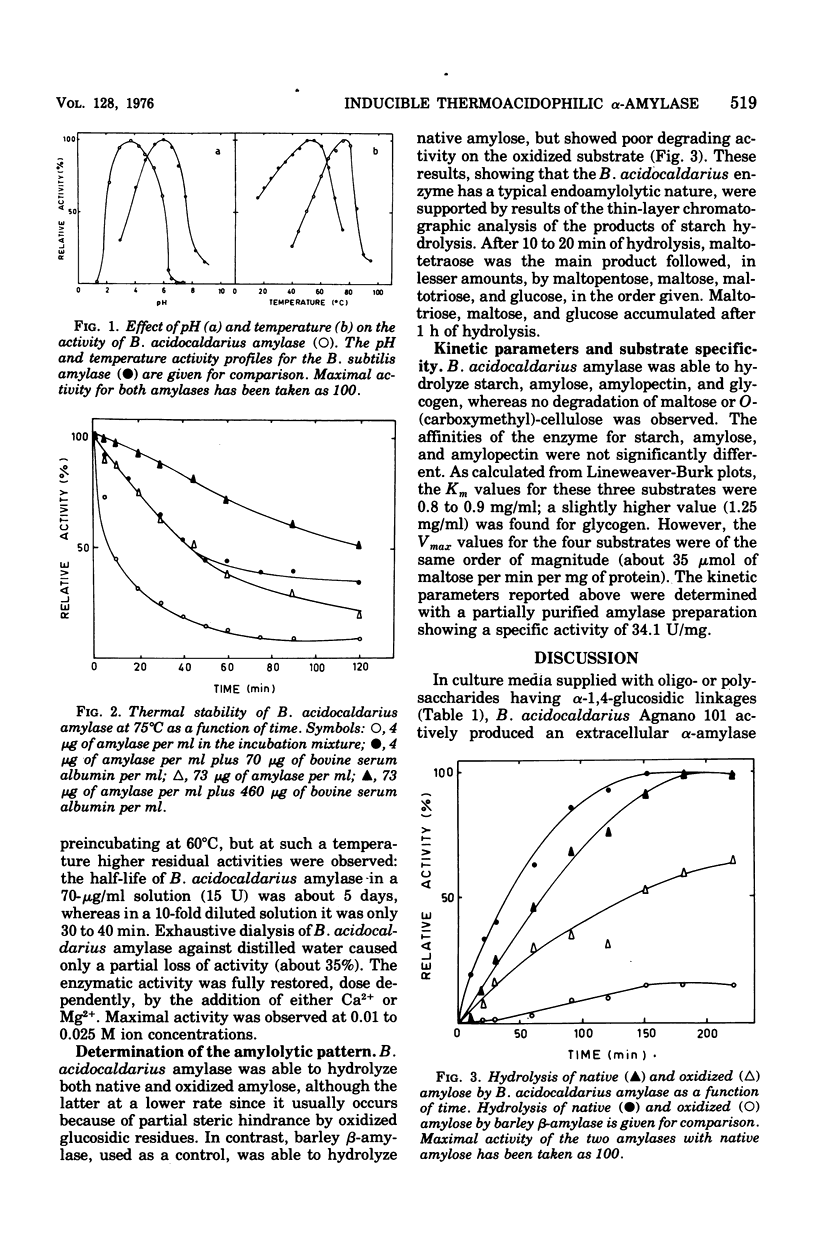
Abstract
Bacillus acidocaldarius Agnano 101 produces an inducible thermoacidophilic alpha-amylase. The enzyme production occurs during the stationary phase of growth in the presence of compounds with alpha-1,4-glucosidic linkages. The enzymatic activity is both present in the culture medium and associated with the cells; the enzymes purified from both sources show identical molecular and catalytic properties. The purified amylase has a single polypeptide chain of molecular weight 68,000 and behaves like an alpha-amylase with affinity constants for starch and related substances of 0.8 to 0.9 mg/ml. The pH and temperature optima for activity are 3.5 and 75degreesC, respectively. The amylase is stable at acidic pH (below 4.5). Its thermal stability is strictly dependent upon protein concentration; the half-life at 60degreesC of the amylase in a 70-mug/ml solution is about 5 days.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buonocore V., Poerio E., Gramenzi F., Silano V. Affinity column purification of amylases on protein inhibitors from wheat kernel. J Chromatogr. 1975 Nov 12;114(1):109–114. doi: 10.1016/s0021-9673(00)85247-4. [DOI] [PubMed] [Google Scholar]
- Buonocore V., Poerio E., Silano V., Tomasi M. Physical and catalytic properties of alpha-amylase from Tenebrio molitor L. larvae. Biochem J. 1976 Mar 1;153(3):621–625. doi: 10.1042/bj1530621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Smith E. E., Whelan W. J. A general method for distinguishing between endo and exo actions of carbohydrases. FEBS Lett. 1971 Jul 1;15(4):302–304. doi: 10.1016/0014-5793(71)80643-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Rivera Río L., Arroyo-Begovich A. Evidence for the presence of alpha amylase in the cell membrane of Bacillus amyloliquefaciens. Biochem Biophys Res Commun. 1975 Jul 8;65(1):161–169. doi: 10.1016/s0006-291x(75)80074-x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L., FOSTER R. J. Thermostable alpha-amylase of Bacillus stearothermophilus. II. Physical properties and molecular weight. J Biol Chem. 1961 Nov;236:2958–2961. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Ogasahara K., Imanishi A., Isemura T. Studies on thermophilic alpha-amylase from Bacillus stearothermophilus. I. Some general and physico-chemical properties of thermophilic alpha-amylase. J Biochem. 1970 Jan;67(1):65–75. doi: 10.1093/oxfordjournals.jbchem.a129235. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Elliott W. H. The extracellular alpha-amylase of bacillus stearothermophilus. J Biol Chem. 1969 Jan 10;244(1):48–54. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCED BIOSYNTHESIS OF ALPHA-AMYLASE BY GROWING CULTURES OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Dec;86:1196–1201. doi: 10.1128/jb.86.6.1196-1201.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yutani K. Molecular weight of thermostable alpha-amylase from B. stearothermophilus. J Biochem. 1973 Sep;74(3):581–586. doi: 10.1093/oxfordjournals.jbchem.a130279. [DOI] [PubMed] [Google Scholar]
- Yutani K., Sasaki I., Ogasahara K. Comparison of thermostable alpha-amylases from B. stearothermophilus grown at different temperatures. J Biochem. 1973 Sep;74(3):573–579. doi: 10.1093/oxfordjournals.jbchem.a130278. [DOI] [PubMed] [Google Scholar]


