Abstract
The activation of the AMP-activated protein kinase (AMPK) and inhibition of the mammalian target of rapamycin complex 1 (mTORC1) is hypothesized to underlie the fact that muscle growth following resistance exercise is decreased by concurrent endurance exercise. To directly test this hypothesis, the capacity for muscle growth was determined in mice lacking the primary upstream kinase for AMPK in skeletal muscle, LKB1. Following either 1 or 4 weeks of overload, there was no difference in muscle growth between the wild type (wt) and LKB1−/− mice (1 week: wt, 38.8 ± 7.75%; LKB1−/−, 27.8 ± 12.98%; 4 week: wt, 75.8 ± 15.2%; LKB1−/−, 85.0 ± 22.6%). In spite of the fact that the LKB1 had been knocked out in skeletal muscle, the phosphorylation and activity of the α1 isoform of AMPK were markedly increased in both the wt and the LKB1−/− mice. To identify the upstream kinase(s) responsible, we studied potential upstream kinases other than LKB1. The activity of both Ca2+–calmodulin-dependent protein kinase kinase α(CaMKKα) (5.05 ± 0.86-fold) and CaMKKβ (10.1 ± 2.59-fold) increased in the overloaded muscles, and this correlated with their increased expression. Phosphorylation of TAK-1 also increased 10-fold following overload in both the wt and LKB1 mice. Even though the α1 isoform of AMPK was activated by overload, there were no increases in expression of mitochondrial proteins or GLUT4, indicating that the α1 isoform is not involved in these metabolic adaptations. The phosphorylation of TSC2, an upstream regulator of the TORC1 pathway, at the AMPK site (Ser1345) was increased in response to overload, and this was not affected by LKB1 deficiency. Taken together, these data suggest that the α1 isoform of AMPK is preferentially activated in skeletal muscle following overload in the absence of metabolic adaptations, suggesting that this isoform might be important in the regulation of growth but not metabolism.
Concurrent training for strength and endurance decreases the gain in muscle mass seen when training for strength alone (Hickson 1980). One potential mechanism underlying this effect is the ability of the AMP-activated protein kinase (AMPK) to block the activation of the mammalian target of rapamycin complex 1 (TORC1) by phosphorylating and activating the tuberous sclerosis complex-2 (TSC2) (Inoki et al. 2002). Since the activity of TORC1 correlates with skeletal muscle hypertrophy (Baar & Esser 1999) and AMPK is activated in response to endurance exercise (Winder & Hardie 1996), this is an attractive mechanism to explain the reduced hypertrophy during concurrent training for strength and endurance.
AMPK is a heterotrimeric serine/threonine kinase comprised of a catalytic α and regulatory β and γ subunits. When cellular energy is decreased, causing a drop in the ATP: ADP ratio, the consequent rise in the AMP: ATP ratio produced by adenylate kinase results in increased binding of AMP to two sites on the γ subunit of AMPK (Scott et al. 2004). This causes a conformational change that inhibits dephosphorylation of Thr172 on the α subunit (Davies et al. 1995; Sanders et al. 2007), causing a net increase in phosphorylation at this site and thus activation (Hawley et al. 1996). A number of upstream kinases have now been identified, including the LKB1 complex (Hawley et al. 2003; Woods et al. 2003), the calmodulin-dependent kinase kinases CaMKKα and CaMKKβ (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005) and TGFβ-activated protein kinase-1 (TAK1) (Momcilovic et al. 2006), although in the latter case there is as yet no evidence that it phosphorylates AMPK in vivo. In extensor digitorum longus and tibialis anterior muscles, LKB1 appears to be the primary upstream kinase, since neither contraction nor AICAR activate AMPK in these muscles in mice with a muscle-specific LKB1 knockout (Sakamoto et al. 2005).
AMPK activation has both short- and long-term effects on muscle. Acute effects include increasing glucose transport and activating fatty acid oxidation, whereas repeated activation of AMPK is associated with increases in the expression of enzymes involved in glucose and lipid oxidation as well as proteins of the mitochondrial electron transport chain (Towler & Hardie 2007). Interestingly, all of these metabolic adaptations seem to preferentially occur as a result of activating the α2 and not the α1 isoform of the enzyme (Jorgensen et al. 2004), suggesting that the two isoforms might have distinct roles within the cell.
Over the past 10 years it has become clear that the mammalian target of rapamycin (mTOR) is a major regulator of cell size (Fingar et al. 2002). More recently, two distinct mTOR-containing complexes have been identified with different cellular functions. TORC1 contains mTOR, the regulatory associated protein of mTOR (raptor) and mlST8/GβL and controls translation initiation via its downstream targets the ribosomal S6 protein kinase (S6K1) and the eukaryotic initiation factor 4E binding protein (4E-BP). TORC2 also contains mTOR and mlST8/GβL but, instead of raptor, it contains the rapamycin-insensitive companion of mTOR (rictor) and mitogen-activated-protein kinase-associated protein 1 (mSin1). TORC2 is required for the activation of protein kinase B (PKB/Akt), which mediates insulin signalling and cell survival (Sarbassov et al. 2005). Activation of TORC1, either transiently as a result of resistance exercise or constitutively by the over-expression of an activated form of PKB, results in muscle hypertrophy (Baar & Esser 1999; Bodine et al. 2001). In non-muscle cells it has been found that AMPK activation inhibits the activation of TORC1 via phosphorylation of its upstream regulator, TSC2, at Ser1345 (Inoki et al. 2003). Consistent with this, activation of AMPK using AICAR in vivo is associated with inhibition of muscle protein synthesis, with a concomitant reduction of phosphorylation of S6K1 and 4E-BP1 at the sites phosphorylated by TORC1 (Bolster et al. 2002). During overload-induced hypertrophy of plantaris muscle, increased phosphorylation of Thr172 on AMPK in ageing rats correlated with reduced muscle hypertrophy (Thomson & Gordon 2005). This was taken as evidence for the hypothesis that increased AMPK activation restrains hypertrophy.
To directly test this hypothesis, we studied the hypertrophic response in mice lacking the primary upstream kinase for AMPK in muscle, LKB1. We hypothesized that decreased activation of AMPK in the LKB1 knockout mice would lead to a greater degree of muscle hypertrophy in response to chronic overload.
Methods
Materials
Reagents for enhanced chemiluminescence were obtained from Pierce Biotechnology (Cramlington, UK). Protein G beads were purchased from Amersham (Little Chalfont, UK). Peptides were from the Dundee Division of Signal Transduction Therapy. All other reagents were purchased from Sigma (Dorset, UK).
Antibodies
Antibodies recognizing the α1 and α2 subunits were made in-house (Hawley et al. 1996). The phosphospecific antibody against Ser1345 on TSC2 was made using the peptide CPLSKSSSpSPELQT as previously described for another phosphopeptide (Sugden et al. 1999). Polyclonal antibodies against phosphorylated forms of TAK1 (Thr184/187), Akt (Thr473), S6K1 (Thr389), rS6 (Ser240/244) and 4E-BP1 (Thr37/46) were purchased from Cell Signalling (Danvers, MA, USA), and total GLUT4 and PGC-1 were purchased from Chemicon (Hampshire, UK). Mouse monoclonal antibodies to succinate ubiquinone oxidoreductase and cytochrome oxidase (COX) subunit I were purchased from Molecular Probes (Eugene, OR, USA). A mouse anti-cytochrome c (CytC) monoclonal antibody was purchased from Pharmingen International (Oxford, UK). For CaMKKβ, two antibodies were used. Both were generated based on non-conserved regions between CaMKKβ and CaMKKα; specifically an N-terminal region of CaMKKβ (antibody 1 – amino acid residues 28–49 and antibody 2 – amino acids 4–19 and 34–48 of human CamKKβ). Antibody 1 was generated in rabbit while antibody 2 was generated in sheep by injecting the keyhole limpet hemocyanin-coupled peptides. For pCAMKI and CAMKI, antibodies were generated in sheep against the peptides KPGSVLST(P)ACGTPGY and CAEDKRTQKLVAIK, respectively. Antisera were harvested and purified by standard immunological techniques. Horseradish peroxidase-conjugated secondary antibodies were obtained from Pierce Biotechnology (Cramlington, UK).
Animals
All animal breeding and experiments were approved by the Home Office and the University of Dundee Ethical Review Committee. LKB1 floxed (LKB1fl/fl) mice were crossed with mice expressing Cre recombinase driven by the muscle creatine kinase promoter on a C57BL/6J background as previously described (Sakamoto et al. 2005), to produce mice lacking LKB1 specifically in striated muscle, testis, kidney and lung (LKB1−/−). LKB1fl/fl× MCK Cre−/− animals were used as controls. Genotyping was performed by PCR using genomic DNA isolated from tails as previously described (Sakamoto et al. 2005).
Animal care and surgical intervention
Animals were housed in 12: 12 h light–dark cycles and fed ad libitum at all times. On the day of surgery, animals were anaesthetized and maintained in the surgical plane using 2.5% isoflurane vaporized in oxygen. The synergist ablation surgery consisted of isolating and removing the gastrocnemius (GTN) and the soleus (SOL) muscles at the Achilles tendon while leaving plantaris (PLN) in tact. The overlying fascia and skin were closed separately using sterile suture and the mice were allowed to recover in a temperature-controlled area prior to being returned to their cages. Animals were monitored daily for signs of pain or post-operative infection. However, none of the study animals demonstrated any undue discomfort and returned to full activity within 1 h of the completion of the procedure.
Tissue collection
Following 7 or 28 days of overload, animals were anaesthetized as described above and the PLN muscles from both the experimental and contralateral leg were removed, trimmed of any excess lipid or connective tissue using a Wild Heerbrugg stereomicroscope, blotted dry, and weighed using an A&R HR120 analytical balance. The muscles were then frozen in liquid nitrogen and stored at −80°C until processed. Animals were killed by cervical dislocation. Seven days of overload of the PLN muscle was selected to study the role of AMPK on acute growth because previous studies have shown that AMPK is active at this time point and that the activity of AMPK at this time is inversely related to muscle hypertrophy (Thomson & Gordon 2005). Twenty-eight days of overload was selected as an indicator of maximal muscle growth because at this time point PLN muscle mass stabilizes (Ianuzzo et al. 1976).
Western blotting
Muscles were homogenized in lysis buffer (50 mm Tris pH 7.5, 250 mm sucrose, 1 mm EGTA, 1 mm EDTA, 50 mm NaF, 5 mm Na2PO7, 1% Triton X-100, with fresh 1 mm NaVO4, 0.1% DTT, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, and 0.5 mm phenyl methane sulfonyl fluoride (PMFS)) and the protein concentration was determined using the DC protein assay (Bio-Rad, Hercules, CA, USA). Equal aliquots of protein were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blocked with 5% milk. Membranes were exposed to the primary antibodies overnight at 4°C, washed, and probed with the appropriate HRP-conjugated secondary antibodies. Bound antibody was detected chemiluminescently using a Chemigenius 2 bioimaging system (Syngene, Cambridge, UK).
AMPK activity assays
PLN muscles were rapidly dissected out and frozen in liquid nitrogen. The frozen tissue was ground to a fine powder under liquid nitrogen using a pestle and mortar. The ground tissue was resuspended in 100 μl homogenization buffer (50 mm Tris/HCl, 0.25 m mannitol, 50 mm NaF, 5 mm sodium pyrophosphate, 150 mm NaCl, 5 μg ml−1 soybean trypsin inhibitor, 1 mm DTT, 0.1 mm PMSF, 1% (v/v) Triton X-100) per 20 mg of tissue and further homogenized using a hand-held motor driven pestle. The samples were left on ice for 30 min and then cell debris was pelleted by centrifugation at 17 600 g for 5 min. The supernatants were removed and protein concentration determined. AMPK α1 and α2 antibodies (5 μg) were used independently to immunoprecipitate AMPK from 200 μg of muscle lysate for 2 h at 4°C. The immunoprecipitates were washed in homogenization buffer and then resuspended in assay buffer (50 mm Hepes, 1 mm DTT, 0.02% (v/v) Brij-35). AMP,[32-γP]-ATP (200 cpm pmol−1) and the peptide substate (AMARAASAAALARRR) were added to the immunoprecipitate at a final concentration of 200 μm. The assays were carried out for 15 min at 30°C and terminated by applying 30 μl of the reaction mixture to P81 papers (Whatman, Maidstone, UK). ATP incorporation into the peptide substrate was inhibited by placing the filter paper in 1% orthophosphoric acid. Phosphate incorporation into the peptide was measured as previously described (Hardie et al. 2000).
CamKKα and β activity
Tissues were homogenized as above for AMPK activity and protein concentrations determined. CamKKα and β were independently immunoprecipitated from 200 μg of protein for 2 h at 4°C. The immunoprecipitates were washed and then resuspended in 50 mm Hepes, 1 mm DTT, 0.02% (v/v) Brij-35 (Hepes assay buffer). Calcium and calmodulin were added to a final concentration of 1 mm and 1 μm, respectively, ATP was added to a final concentration 200 μm, and 0.4 μg of purified recombinant human CAMKI was added and incubated on an orbital shaker at 30°C for 10 min. The reactions were stopped by addition of sample loading dye. CAMKI phosphorylation and total CAMKI protein were analysed individually by Western blotting using the 4–12% Bis–Tris gel and transfer system (Invitrogen). Nitrocellulose was blocked for 30 min in Odyssey blocking buffer (Li-Cor). Primary antibodies were diluted in Odyssey blocking buffer containing 0.2% Tween-20. The nitrocellulose membranes were incubated with the primary antibody solution overnight at room temperature. Nitrocellulose was washed in 20 mm Tris HCl, 140 mm NaCl pH 7.4 (TBS) containing 0.2% Tween-20 6 × 5 min. Secondary antibodies (anti-sheep IRDYE 680; Molecular Probes) were diluted in Odyssey blocking buffer containing 0.2% Tween-20. The nitrocellulose membranes were incubated with the secondary antibody solution for 30 min. Nitrocellulose was washed in TBS containing 0.2% Tween-20 6 × 5 min. Membranes were scanned using the Odyssey infrared imaging system and analysed with the corresponding analysis software (http://www.li-cor.com).
TSC2 phosphorylation
Muscles were homogenized in lysis buffer and the protein concentration was determined using the DC protein assay (Bio-Rad, Hercules, CA, USA). TSC2 was immunoprecipitated from 100 μg of protein for 2 h at 4°C, the precipitated protein was separated by SDS-PAGE and Western blotted as above.
Statistical analyses
All values are expressed as means ± s.e.m. Statistical analyses were performed using either a Student's paired t test or two-way ANOVA with post hoc Tukey–Kramer HSD. The level of significance was set at P < 0.05.
Results
Change in muscle mass following overload
Contrary to our hypothesis, the degree of muscle hypertrophy produced by chronic overload was not significantly different between the wild type and LKB1−/− mice after either 1 week (wt, 38.8 ± 7.75%; LKB1−/−, 27.8 ± 12.98%, Table 1) or 4 weeks (wt, 75.8 ± 15.2%; LKB1−/−, 85.0 ± 22.6%).
Table 1.
Effect of 1 or 4 weeks overload on muscle mass in wt and LKB1 KO mice
| Overload | Bodyweight (g) | CTL (mg) | OVL (mg) | % Difference |
|---|---|---|---|---|
| 1 week | ||||
| wt | 25.4 ± 2.40 | 17.7 ± 1.28 | 24.4 ± 1.68* | 38.8 ± 7.75 |
| LKB1 KO | 26.1 ± 3.56 | 16.0 ± 0.79 | 20.3 ± 1.89* | 27.8 ± 12.98 |
| 4 weeks | ||||
| wt | 27.9 ± 1.08 | 16 ± 1.17 | 28.6 ± 4.09* | 75.8 ± 15.2 |
| LKB1 KO | 26.9 ± 0.49 | 15.9 ± 1.51 | 28.7 ± 2.93* | 85.0 ± 22.6 |
Mean body weight and muscle mass from control (CTL) and overloaded (OVL) legs ± s.e.m. Average percentage difference in muscle mass from the CTL and OVL legs of the same animal ± s.e.m.
indicates different from control, P < 0.05.
Effect of overload on AMPK phosphorylation
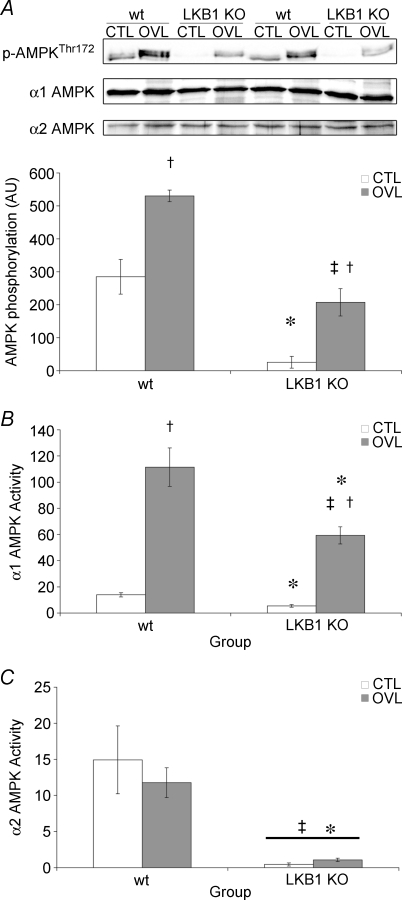
Since a number of upstream kinases for AMPK other than LKB1 have recently been identified, we examined the phosphorylation of AMPK in the wild type and LKB1−/− mice. Although the basal level of phosphorylation was significantly reduced in the knockout mice, in both the wt and the LKB1−/− animals there was a significant increase in the phosphorylation of AMPK in the overloaded muscles, with no change in total AMPK expression (Fig. 1A).
Figure 1. AMPK activation in response to 7 days of overload in wt and LKB1−/− mice.
A, AMPK α subunit phosphorylation at Thr172. B, AMPK α1 subunit activity. C, AMPK α2 subunit activity. All values are reported as the means ± s.e.m. (n = 4 per group). *Significantly different from wt CTL (P < 0.05). ‡Significantly different from wt OVL (P < 0.05). †Significantly different from corresponding CTL (P < 0.05).
Activity of α1 and α2AMPK following overload
The AMPK α subunits detected using anti-pT172 antibody in plantaris muscle migrated as a closely spaced doublet on SDS–polyacrylamide gel electrophoresis (Fig. 1A). In mice, the molecular mass of the α1 subunit calculated from the sequence is slightly larger than that of the α2 subunit (63.9 versus 62 kDa), suggesting that the upper and lower bands in the doublet may correspond to α1 and α2, respectively. While the lower band detected using anti-pT172 antibody was not increased in response to overload and was completely absent in the LKB1−/− mice, the upper band increased markedly in response to overload and was reduced in intensity, but not eliminated, in the LKB1−/− mice. This suggested that the increased phosphorylation of Thr172 was entirely accounted for by the α1 isoform, and that this occurred via a mechanism that was LKB1 independent. To confirm this, the activity of the α1 and α2 isoforms of AMPK were determined specifically using immunoprecipitate kinase assays. In the plantaris muscle of the LKB1−/− mice, the α2 activity decreased by 97% and the α1 activity by 60% (Fig. 1). Following 1 week of overload, the activity of the α2 isoform was not changed in the wild type mice, and the α2 activity in the LKB1−/− mice in both control and overloaded muscle was reduced almost to zero (Fig. 1). By contrast, the activity of the α1 isoform was markedly increased in the overloaded muscle in both the wild type (6.9 ± 1.29-fold) and the LKB1−/− mice (11.9 ± 2.82-fold). Although there was a reduction in the basal α1 activity in LKB1−/− mice compared with wild type mice (2.3 ± 0.93%), the degree of activation in response to chronic overload was essentially unchanged.
Effect of overload on other potential upstream kinases for AMPK
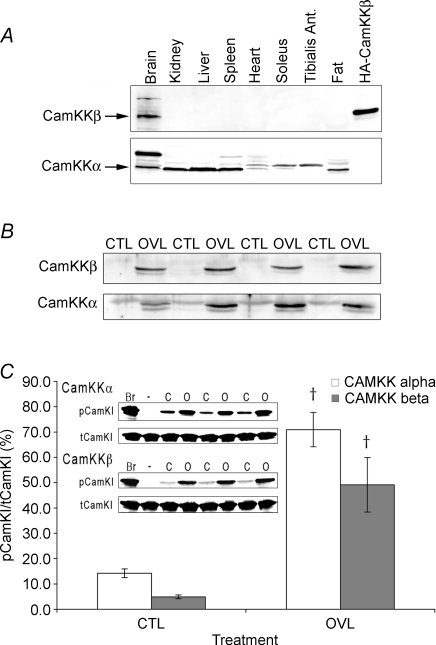
Since increased phosphorylation of the α1 isoform of AMPK in response to overload could not be explained by LKB1, we examined other potential upstream kinases. By Western blotting, CaMKKβ expression was readily detectable in the brain, but we could not detect it in several other tissues, including cardiac, soleus and tibialis anterior muscles (Fig. 2A). By contrast, CaMKKα was expressed in brain and a number of other tissues, including kidney, liver, spleen and fat. It was also detectable in cardiac and slow soleus muscles, where it migrated as a doublet, as well as the fast tibialis anterior where only the slower migrating band was detected (Fig. 2). Intriguingly, following 1 week of overload the expression of both CaMKKα and CaMKKβ in plantaris muscle increased dramatically (Fig. 2B). Because of the low basal level of CaMKKβ, the relative increase in the β isoform was greater (50.9 ± 6.3-fold) than the α isoform (3.4 ± 0.37-fold). To be certain that the polypeptides detected using our antibodies truly represented CaMKK, we also determined the activities of CaMKKα and CaMKKβ using immunoprecipitate kinase assays. As with the Western blots, there was a marked increase in CaMKK activity following 1 week of overload. CamKKα activity increased 5.05 ± 0.86-fold whereas CamKKβ activity increased 10.1 ± 2.59-fold. The increase in CaMKK activity was also observed in the LKB1 animals, with a 4.0 ± 1.53-fold increase in CaMKKα activity in the overloaded muscles.
Figure 2. Expression and activity of CaMKKs in mouse skeletal muscle and other tissues.
A, CaMKKβ and CaMKKα expression analysed by Western blotting in mouse brain, kidney, liver, spleen, heart, slow and fast skeletal muscle, and adipose tissue. B, CaMKKβ and CaMKKα levels analysed by Western blotting in plantaris muscle before and after 1 week of overload (CTL and OVL). C, CaMKKβ and CamKKα activities measured using immunprecipitate kinase assays in extracts of plantaris muscle following 1 week of overload. The inset shows the raw data on phosphorylation of CaMKI from which the bar chart was made (Br-Brain). All values are reported as the means ± s.e.m. (n = 3–4 per group). †Significantly different from corresponding CTL (P < 0.05).
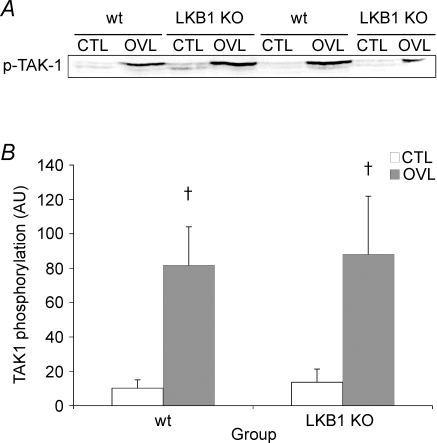
Another potential upstream kinase for AMPK is TAK-1, whose activity is acutely regulated by phosphorylation at Thr184 and Thr187. In both the wild type and the LKB1−/− mice, the phosphorylation of TAK-1 was significantly increased in the overloaded muscle (Fig. 3). Overload increased TAK-1 phosphorylation by 10.2 ± 3.39-fold and 10.4 ± 3.95-fold in the wt and the LKB1 knockout, respectively.
Figure 3. TAK-1 phosphorylation at Thr184/187 in wt and LKB1−/− mice following 7 days of overload.
†Significantly different from corresponding CTL (P < 0.05).
Expression of GLUT4 and mitochondrial enzymes
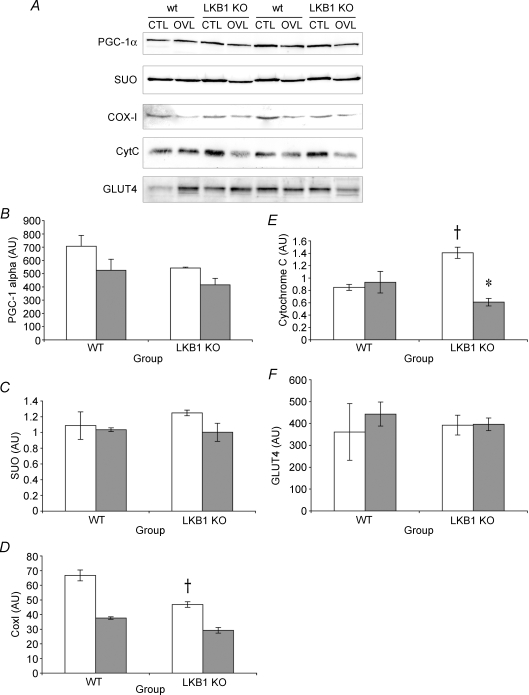
One of the long-term effects of activating AMPK is an increase in GLUT4 and mitochondrial enzymes (Bergeron et al. 2001). Since overload results in an α1-specific increase in AMPK activity, we sought to determine whether this was associated with mitochondrial biogenesis. The relative quantity of all of the mitochondrial proteins tended to decrease following overload, reaching significance for CytC (Fig. 4). However, when normalized to muscle mass, the content of the mitochondrial proteins and GLUT4 was unaffected by overload.
Figure 4. Metabolic enzyme expression in response to 7 days of overload in wt and LKB1−/− mice.
A, representative immunoblots of metabolic enzyme expression. B, PGC-1 expression. C, succinate ubiquinone oxidoreductase expression. D, CoxI expression. E, CytC expression. F, GLUT4 expression. All values are reported as the means ± s.e.m. (n = 2 per group). *Significantly different from wt CTL (P < 0.05). †Significantly different from corresponding CTL (P < 0.05).
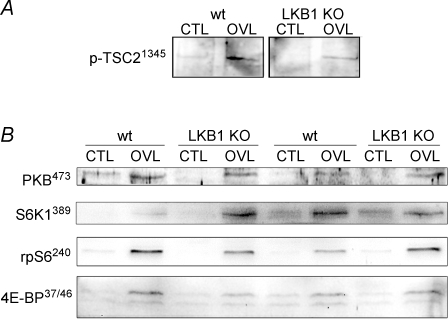
Activation of TORC1 by overload in the LKB1−/− mice
Since activation of the α1 isoform of AMPK during overload did not seem to regulate metabolic adaptations, we hypothesized that it may be involved instead in restricting growth. Since the ability of AMPK to limit growth is thought to be mediated by phosphorylation and activation of TSC2, we examined phosphorylation of TSC2 at the AMPK site Ser1345. We could indeed detect an increase in Ser1345 phosphorylation in response to overload, and this was not altered in the LKB1−/− mice (Fig. 5). Similarly, we observed increases in the phosphorylation of several downstream targets for TORC1, i.e. S6K1 (Thr389), ribosomal protein S6 (Ser–240/–244) and 4E-BP1 (Thr–37/–46), and none of these appeared to be affected by knockout of LKB1 either (Fig. 5).
Figure 5. Growth response and signalling in response to 7 days of overload in wt and LKB1−/− mice.
A, TSC2 phosphorylation at Ser1345. B, PKB Thr473 phosphorylation, S6K1 Thr389 phosphorylation, rpS6 Ser240/244 phosphorylation and 4E-BP Thr37/46 phosphorylation. Data are representative of n = 3.
Discussion
Overload of the plantaris muscle results in the activation of the α1 but not the α2 isoform of AMPK in both wild type and LKB1−/− muscles. The fact that α1 was activated after 1 week of overload, without any changes in mitochondrial mass or GLUT4 expression, suggests that this isoform of AMPK is not involved in driving metabolic adaptations within skeletal muscle. If α1 AMPK is not driving muscle towards a more aerobic phenotype, one possible consequence is the prevention of excess muscle growth.
It is becoming increasingly clear that the α1 and α2 isoforms of AMPK have distinct regulatory properties and functions in vivo. For example, in this study we have shown that LKB1 is essential for α2 activity in plantaris muscle, but that a substantial amount of α1 activity remains and that this isoform can be markedly activated by chronic overload in LKB1-deficient muscle. These findings are consistent with previous findings in other muscle types (Sakamoto et al. 2005) and data from a number of studies showing discordant regulation of α1 and α2 in response to different stimuli. For example, in response to aerobic exercise, α2 activity is preferentially increased (Fujii et al. 2000), while low-frequency muscle contraction increases α1 but not α2 activity in the absence of changes in the AMP/ATP ratio (Toyoda et al. 2006). During aerobic exercise, the AMP: ATP ratio increases and activates the α2 isoform through the ‘classical’ mechanism, i.e. binding of AMP to the γ subunit, promoting net phosphorylation and further allosteric activation (Sakamoto et al. 2005). During low-frequency contraction, the α1 isoform may be activated by an LKB1-independent mechanism. If this is the case, what is the mechanism and what is the upstream kinase? One potential mechanism is that an increase in Ca2+ results in an increase in the activity of calcium-activated upstream kinases. Both CaMKKα and CaMKKβ are known to be upstream kinases for AMPK (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005) and might be regulated by an increase in calcium as would occur during compensatory hypertrophy. Jensen et al. (Jensen et al. 2007) recently reported that, although CaMKKs were difficult to detect in mouse skeletal muscle by Western blotting, they could detect polypeptides of the expected size using a pan-CaMKK antibody following enrichment of mouse quadriceps extracts on calmodulin–agarose. They also reported that inhibiting CaMKK using STO-609 resulted in a decrease in AMPK activity following a short period of contraction. We were unable to detect any CaMKKβ in control mouse skeletal muscle, consistent with previous studies showing that calmodulin-dependent protein kinases-I and -IV, regarded as the primary targets for the CaMKKs, are not expressed in adult skeletal muscle (Akimoto et al. 2004; McGee & Hargreaves 2004; Rose et al. 2006). However, an important finding of this study was that following 1 week of chronic overload, both the expression and the activity of CaMKKα and CaMKKβ increased markedly, with the increase in CaMKKβ being relatively larger. The increase in CaMKK expression is potentially significant since Witczak et al. (2007) have shown that over-expression of constitutively active CaMKKα in skeletal muscle results in the activation of both the α1 and α2 isoforms of AMPK. This differs from our results, where chronic overload induced increased expression of endogenous CaMKKα and CaMKKβ, but this was associated only with activation of the α1 isoform of AMPK. One explanation of these different results is that the localization of the endogenous CaMKK and AMPK isoforms produces an isoform-specific AMPK activation that is not mimicked by over-expression of CaMKKα.
Another potential mechanism for activation of AMPK is through the TGFβ-activated protein kinase, TAK-1, which we showed to be markedly activated during chronic overload. One TGFβ family member, myostatin, is well known for its ability to restrict muscle growth. TAK-1 is activated by myostatin in myoblasts (Philip et al. 2005), although whether this mechanism operates in adult skeletal muscle remains to be determined. Furthermore, while myostatin decreases the activation of TORC1 and the rate of protein synthesis (Suryawan et al. 2006), whether this requires AMPK has yet to be determined. Interestingly, during the early phase of overload hypertrophy, myostatin mRNA decreases (Yamaguchi et al. 2006), suggesting that the local concentration of this growth inhibitor would be lower at the time points used in the current study. However, the expression of the activin IIb receptor and the myostatin competitive inhibitor follistatin need to be investigated before any final conclusions about myostatin signalling can be drawn. It should also be pointed out that, while it has been shown that CaMKKβ can phosphorylate Thr172 and activate AMPK in intact cells (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005), to date TAK-1 has only been shown to do this in cell-free assays (Momcilovic et al. 2006). At present, we therefore favour the CaMKKs as the most likely upstream kinases responsible for the activation of α1 during chronic overload.
In the absence of LKB1, there was greater CytC and less CoxI when compared with the wt controls. Since CoxI is mitochondrially encoded, this might indicate a baseline deficit in either mitochondrial transcription factor A (tFAM) or mitochondrial translation in the LKB1−/− mice. CytC levels appear to be regulated by the PGC-1-related coactivator (PRC; Vercauteren et al. 2006), suggesting that there might be greater levels of PRC in the absence of LKB1.
From data presented in this study, it appears that α1 is not involved in skeletal muscle metabolic adaptations, given that mechanical overload induced 6- and 12-fold increases in α1 activity in wild type and LKB1−/− mice, without any alterations in metabolic phenotype. This can be compared with the effects of a relatively modest activation of AMPK using AICAR, which results in potent mitochondrial biogenesis over the same time scale (Winder et al. 2000; Bolster et al. 2002; Lee et al. 2006). The proposal that α2 and not α1 is the primary isoform of AMPK involved in metabolic adaptations is supported by a number of different lines of evidence. First, mice that over-express a dominant negative form of α2 do not undergo mitochondrial biogenesis in response to chronic energy deprivation induced by feeding β-guanidinopropionic acid (Zong et al. 2002). Second, the α2 but not the α1 isoform is responsible for AS160 phosphorylation and increased glucose uptake in response to AICAR (Treebak et al. 2006). Third, knockout of α2 decreased basal and AICAR-stimulated expression of several metabolic genes in skeletal muscle (Jorgensen et al. 2005, 2007). Finally, global knockout of α1 in mice did not result in a detectable metabolic phenotype, whereas a knockout of α2 exhibited insulin resistance (Viollet et al. 2003; Jorgensen et al. 2004). All of these studies did, however, use genetic modifications that might result in compensatory adaptations (for example, expression of α1 was increased in skeletal muscle of the α2 knockout (Viollet et al. 2003)). Such compensatory mechanisms can affect the interpretation of results obtained with knockout mice. In the present study we utilized a model that selectively activates the endogenous α1 AMPK isoform, which clearly demonstrates a dissociation between α1 activation and metabolic adaptation. Interestingly, AICAR, a drug used previously to activate AMPK and induce mitochondrial biogenesis in vivo, selectively activates the α2 isoform in adult skeletal muscle (Bolster et al. 2002). Taken together, these data suggest that activation of the endogenous α2 isoform results in metabolic adaptations, while activation of the α1 isoform does not.
If activation of α1 does not increase mitochondrial biogenesis and glucose transport, what are the consequences of its LKB1-independent activation following 1 week of chronic overload? We propose that activation of α1 is limiting the growth of the muscle in response to the overload. The increase in muscle mass is well correlated with the activity of TORC1 (Baar & Esser 1999). In C2C12 muscle cells, activation of AMPK using AICAR caused inhibition of the TORC1 signalling pathway and of protein synthesis (Williamson et al. 2006). In the muscles of old rats, increased AMPK activity correlates with decreased skeletal muscle hypertrophy in response to the same 1 week overload protocol used in this study (Thomson & Gordon 2005). One way that AMPK potentially modulates the activity of TORC1 is through the phosphorylation and activation of TSC2 on Ser1345 (Inoki et al. 2002). In this study we show that the increased phosphorylation of TSC2 at Ser1345, and the activity of TORC1 as determined by the phosphorylation of S6K1 and 4E-BP, in response to overload, were not affected by a deficiency in LKB1. These results show that the AMPK-mediated inhibition of TORC1 activity remains intact in the muscles of the LKB1-deficient mice. Since the activity of the α2 isoform is abolished in the LKB1−/− mice, whereas the activation of α1 is similar in the wild type and LKB1−/− mice, this suggests that α1 and not α2 provides the feedback regulation required to limit TORC1 activity and thus prevent excessive muscle growth. Without the α1 knockout mice we cannot fully prove this hypothesis. However, the current work does demonstrate that α2 activation is not required, decreasing the work required to ultimately prove/disprove this hypothesis.
In conclusion, results from the present study show that skeletal muscle hypertrophy is normal in response to chronic mechanical overload in the absence of LKB1. The hypertrophy produced by overload was associated with a marked activation of the α1 isoform of AMPK that still occurred in the LKB1-deficient mice. This activation was associated with a marked increase in expression of the known upstream kinases CaMKKα and CaMKKβ, and with activation of another potential upstream kinase, TAK-1. The increased phosphorylation of TSC2 and activation of the TORC1 pathways in response to overload was not affected by LKB1 deficiency. Chronic overload also did not produce a change in the metabolic phenotype of the muscle. Our results suggest a role for the α1 isoform of AMPK in the regulation of skeletal muscle growth, but not in metabolic adaptation.
Acknowledgments
K.B. was supported by grants from the Wellcome Trust (077426) and the United States Defense Advanced Research Projects Agency (DARPA), Navy contract no. N66001–02-C-8034. S.L.M. is a NHMRC Peter Doherty Fellow (400446). D.G.H. was supported by EXGENESIS, an Integrated Project (LSHM-CT-2004–005272) funded by the European Commission, and by a Programme Grant (080982) from the Wellcome Trust.
References
- Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–74. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45:255–263. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Ianuzzo CD, Gollnick PD, Armstrong RB. Compensatory adaptations of skeletal muscle fiber types to a long-term functional overload. Life Sci. 1976;19:1517–1523. doi: 10.1016/0024-3205(76)90096-5. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. Faseb J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- McGee SL, Hargreaves M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes. 2004;53:1208–1214. doi: 10.2337/diabetes.53.5.1208. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Philip B, Lu Z, Gao Y. Regulation of GDF-8 signaling by the p38 MAPK. Cell Signal. 2005;17:365–375. doi: 10.1016/j.cellsig.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903. doi: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J. 1999;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Frank JW, Nguyen HV, Davis TA. Expression of the TGF-β family of ligands is developmentally regulated in skeletal muscle of neonatal rats. Pediatr Res. 2006;59:175–179. doi: 10.1203/01.pdr.0000196718.47935.6e. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 2005;98:557–564. doi: 10.1152/japplphysiol.00811.2004. [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Sato K, Fushiki T, Nakao K, Hayashi T. Low-intensity contraction activates the α1-isoform of 5′-AMP-activated protein kinase in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E583–E590. doi: 10.1152/ajpendo.00395.2005. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- Vercauteren K, Pasko RA, Gleyzer N, Marino VM, Scarpulla RC. PGC-1-related coactivator. immediate early expression and characterization of a CREB/NRF-1 binding domain associated with cytochrome C promoter occupancy and respiratory growth. Mol Cell Biol. 2006;26:7409–7419. doi: 10.1128/MCB.00585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006;291:E80–E89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-α regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Fujikawa T, Shimada S, Kanbayashi I, Tateoka M, Soya H, Takeda H, Morita I, Matsubara K, Hirai T. Muscle IGF-I Ea, MGF, and myostatin mRNA expressions after compensatory overload in hypophysectomized rats. Pflugers Arch. 2006;453:203–210. doi: 10.1007/s00424-006-0127-9. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]